ヨーロッパの G-CSF/PEG-G-CSF 市場、適応症別 (好中球減少症、腫瘍学、慢性および自己免疫疾患、血液疾患、成長調和不全症など)、投与量別 (単剤および併用)、投与経路別 (静脈内、皮下)、包装別 (使い捨てバイアルおよびプレフィルドシリンジ)、エンドユーザー別 (病院および診療所、研究および学術機関、外来手術センターなど)、流通チャネル別 (病院薬局、オンライン薬局、小売薬局など) - 2030 年までの業界動向および予測。
ヨーロッパの G-CSF/PEG-G-CSF 市場分析と洞察
先進国におけるがん予防の増加により、政府は早期治療を啓発するための意識的な取り組みを導入し、バイオシミラーG-CSFのヨーロッパでの販売市場を促進しています。承認されたバイオシミラーであり、費用対効果が高く、発展途上国でも容易に入手できるGrafeel、Colstim、Neukine、Filcadは、市場の大幅な成長につながるでしょう。中国とインドは、がん患者数が増加している国であり、ヨーロッパ市場を押し上げる可能性があります。したがって、バイオシミラーの使用は、オリジナルの生物学的製剤の使用と比較して患者の医療費を削減するのに役立ち、ヨーロッパのG-CSFバイオシミラー販売市場での需要が増加します。個々のバイオシミラーの複雑な生物学的製造プロセスのため、バイオシミラーのコストはジェネリックほど安くはありません。自己免疫疾患とまれな慢性疾患の有病率の増加は、セグメント市場の成長を促進すると予想されます。
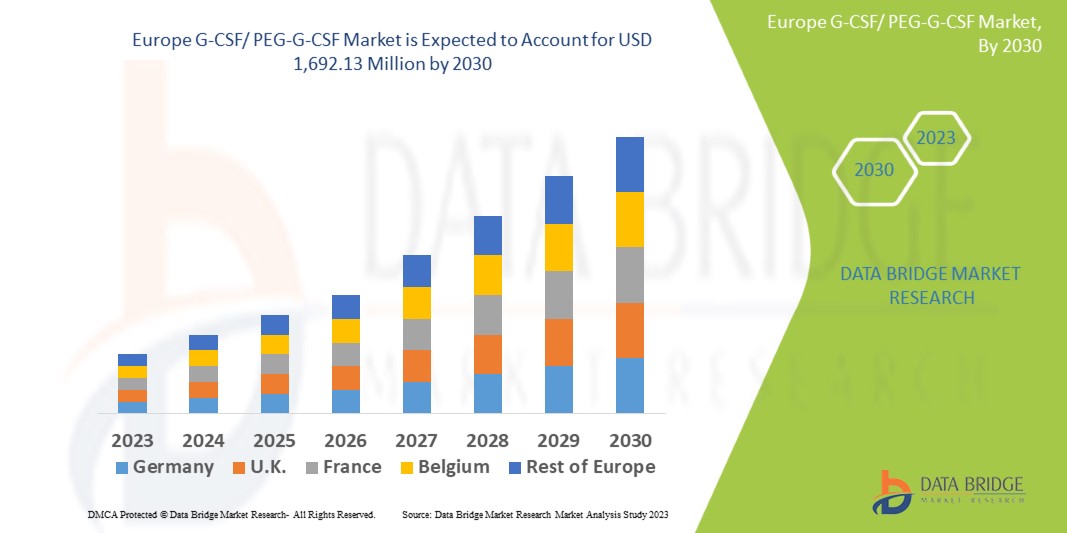
Data Bridge Market Research は、ヨーロッパの G-CSF/PEG-G-CSF 市場は、予測期間中に 5.1% の CAGR で成長し、2030 年までに 16 億 9,213 万米ドルに達すると予測しています。この市場レポートでは、価格分析、特許分析、技術進歩についても詳細に取り上げています。
|
レポートメトリック |
詳細 |
|
予測期間 |
2023年から2030年 |
|
基準年 |
2022 |
|
歴史的な年 |
2021 (2020~2015年にカスタマイズ可能) |
|
定量単位 |
売上高は百万米ドル、価格は米ドル |
|
対象セグメント |
適応症(好中球減少症、腫瘍学、慢性および自己免疫疾患、血液疾患、成長調和不全症など)、用量(単剤および併用)、投与経路(静脈内、皮下)、包装(使い捨てバイアルおよびプレフィルドシリンジ)、エンドユーザー(病院および診療所、研究および学術機関、外来手術センターなど)、流通チャネル(病院薬局、オンライン薬局、小売薬局など) |
|
対象国 |
ドイツ、イギリス、フランス、イタリア、ロシア、オランダ、スペイン、スウェーデン、ポーランド、ベルギー、スイス、デンマーク、ノルウェー、フィンランド、トルコ、その他のヨーロッパ諸国 |
|
対象となる市場プレーヤー |
USV Private Limited、Viatris Inc.、Biocon、Fresenius Kabi AG、Hangzhou Jiuyuan Gene Engineering Co., Ltd.、Amgen Inc.、Pfizer Inc.、Sandoz International GmbH、Apotex Inc.、Cadila Pharmaceuticals、Dr. Reddy's Laboratories Ltd.、Amneal Pharmaceuticals LLC.、Coherus BioSciences、Accord Healthcare、NAPP PHARMACEUTICALS LIMITED.、Intas Pharmaceuticals Ltd.、Mundipharma International、Teva Pharmaceutical Industries Ltd.、Spectrum Pharmaceuticals, Inc.、Kyowa Kirin Co., Ltd.、Jiangsu Hengrui Pharmaceuticals Co., Ltd. など。 |
欧州 G-CSF/PEG-G-CSF 市場の定義
顆粒球コロニー刺激因子 (G-CSF) は、好中球減少症の治療に使用される薬剤です。これは白血球数が平均より少なくなる疾患で、ある種の化学療法によって引き起こされます。G-CSF の主な種類は、レノグラスチム (Granocyte)、フィルグラスチム (Neupogen、zarzio、nivestim、accofil)、長時間作用型 (ペグ化) フィルグラスチム (pegfilgrastim、neulasta、pelmeg、ziextenco)、およびリペグフィルグラスチム (lonquex) です。レノグラスチムは、天然のヒト顆粒球コロニー刺激因子 (G-CSF) と化学的に類似または同一のグリコシル化組み換え治療薬です。さまざまな製品には錠剤やカプセル剤があり、がん、血液疾患、成長ホルモン欠乏症、慢性疾患および自己免疫疾患を治療します。
欧州のG-CSF/PEG-G-CSF市場の動向
このセクションでは、市場の推進要因、利点、機会、制約、課題について理解します。これらについては、以下で詳しく説明します。
ドライバー
- 血液がんやがん性疾患の発生率増加
がんは、体のあらゆる部位に影響を及ぼす可能性のある多くの病気の総称です。がんの他の用語には、悪性腫瘍や新生物などがあります。がんの特徴の 1 つは、異常な細胞が急速に形成され、正常範囲を超えて増殖し、体の隣接部位を侵略して他の臓器に広がることです。後者のプロセスは転移と呼ばれます。広範囲の転移は、がんによる死亡の主な原因です。
フィルグラスチムは、血液中の好中球数の増加を助ける顆粒球コロニー刺激因子 (GCSF) です。フィルグラスチムとペグフィルグラスチムは、がん化学療法または放射線療法後の白血球数の増加によく使用されます。
したがって、血液がんやがん疾患の症例数の増加は、今後数年間、ヨーロッパの G-CSF / PEG-G-CSF 市場を牽引することになります。
- 発熱性好中球減少症の症例増加
発熱性好中球減少症とは、著しい好中球減少症中に起こる発熱を指します。患者が好中球減少症の場合、感染リスクが通常よりも高くなる可能性があり、特定の感染症の重症度も高くなる可能性があります。発熱性好中球減少症は、がん治療における最も一般的な生命を脅かす合併症であり、その治療はしばしば腫瘍学的緊急事態となります。
Febrile neutropenia is neutropenia accompanied by fever. Neutropenia refers to a decrease in the concentration of neutrophils in the blood. Neutrophils are a type of white blood cell that help fight infections as part of the immune system. The Infectious Diseases Society of America defines neutropenia as an absolute neutrophil count (ANC) of less than 1500 cells/mm3. The risk of infection and neutropenic fever increases dramatically with severe neutropenia, defined as an absolute neutrophil count (ANC) of less than 500 cells/mm3. Fever is defined as a single oral temperature greater than or equal to 101° Fahrenheit (38.3° Celsius) or a persistent temperature greater than or equal to 100.4° Fahrenheit (38.0° Celsius) or greater for one hour or longer.
The latest guidelines from three international cancer organizations, the European Organization for Research and Treatment of Cancer, the American Society of Clinical Oncology, and the US National Cancer Network, agree that filgrastim or pegfilgrastim should be given prophylactically in febrile neutropenia with chemotherapy. > or = 20% or if the risk is 10-20% and the patient has other risk factors for febrile neutropenia.
Thus, the increasing cases of febrile neutropenia are further driving the Europe G-CSF / PEG-G-CSF market in the coming years.
Restraint
- Stringent Governmental Regulations
Pharmaceutical companies developing biosimilars such as filgrastim face a major challenge in the approval process for their products. Each country has a different approval process for all drugs, treatments, vaccines, and medical devices. However, these approval procedures are difficult to follow. This is due to the various regulations and evidence required to prove the effectiveness and safety of the product.
European Medicines Agency regulatory requirements ensure the same high quality, safety, and efficacy standards for biosimilars as for originator biologicals. They also include a rigorous comparability exercise with the reference product but are not universally accepted by regulatory bodies outside the European Union (EU). It should be noted that 'similar biologics' approved in India, 'biogenerics' approved in Iran, 'medicamento biológico similares' approved in Argentina, and non-originator biologicals approved in South Africa might not have been authorized if they had been subjected to the strict regulatory processes required for approval of biosimilars in the EU.
Due to this strict regulation by the government that has to be followed for the production and manufacturing of G-CSF / PEG-G-CSF by the various regulatory body, which impedes the market growth of the Europe G-CSF / PEG-G-CSF market.
Opportunity
- The use of biosimilars helps reduce healthcare costs for patients
Biosimilars have the potential to fundamentally change healthcare by providing more affordable, equally effective treatments for patients and providing more treatment options for physicians. Developing biosimilars requires rigorous analysis to demonstrate their equivalence to the reference product and ensure no clinically meaningful differences in their safety, efficacy, and purity. As a result, health systems can channel long-term savings into overall improvements in patient care. To help create a thriving biosimilar market and ensure patient access, policymakers can take steps to reduce or eliminate the cost of biosimilars and encourage physicians to prescribe biosimilars compared to Europe.
Biosimilars have the potential to expand treatment options and increase health equity. At the same time, they can save money for individual patients and health systems. Through legislative and advocacy efforts and future initiatives, access to these effective medicines can expand and fundamentally change the health services for patients who need them.
Thus, the use of biosimilars helps reduce healthcare costs for patients. This factor creates opportunities for market growth.
Challenge
- Multiple side effects of G-CSF
Granulocyte colony-stimulating factor (G-CSF) is a drug used to treat neutropenia, a disorder in which certain forms of chemotherapy cause a lower-than-average number of white blood cells. G-CSF is a type of growth factor that makes the bone marrow produce more white blood cells to reduce the risk of infection after some types of cancer treatment. But there are multiple side effects of G-CSF, such as bone or muscle pain, Bruising, bleeding gums or nosebleeds, diarrhea, high temperature (fever), breathlessness and looking pale, sore mouth, throat, gut and back passage, and, among others. These side effects can be seen in more than 10 in 100 people (more than 10%).
According to a study performed by NCBI, most normal donors receiving G-CSF experience side effects, but these are mild to moderate in degree. Ninety percent of donors experienced some side effects of G-CSF. The most frequent effects noted were bone pain (83%), headache (39%), body aches (23%), fatigue (14%), and nausea and vomiting (12%), which is expected to act as a challenge for market growth.
Thus, the increasing side effects of G-CSF are challenging market growth.
Post-COVID-19 Impact on Europe G-CSF/ PEG-G-CSF Market
The COVID-19 pandemic has had a somewhat positive impact on the G-CSF/ PEG-G-CSF market. The pandemic has imposed new norms and regulations, such as social distancing and lockdowns, to prevent the spread of the virus. As a result, people all over the world were forced to stay at home, which led to new trends such as work at home. This stay at home has led decrease in diagnosis and prognosis of diseases. The increased focus on self-care, exercise and health has helped fitness apps and platforms gain significant traction in the wake of the pandemic.
Manufacturers are making various strategic decisions to bounce back post-COVID-19. The players are conducting multiple R&D activities and product launch and strategic partnerships to improve the technology and test results involved in the transplant diagnostics market.
Recent Developments
- In July 2018, Accord Healthcare, a subsidiary of Intas Pharmaceuticals Ltd., launched a pegfilgrastim biosimilar across Europe after being given Green Light for Pelgraz® (pegfilgrastim) by CHMP (Committee for Medicinal Products for Human Use). This product launched helped the company to expand their business across Europe.
- In March 2022, Kashiv Biosciences announced the approval of its Biologics License Application (BLA) for filgrastim-ayow, a biosimilar referencing Neupogen by U.S. Food and Drug Administration (FDA). The product is marketed under the proprietary name RELEUKO.
Europe G-CSF/ PEG-G-CSF Market Scope
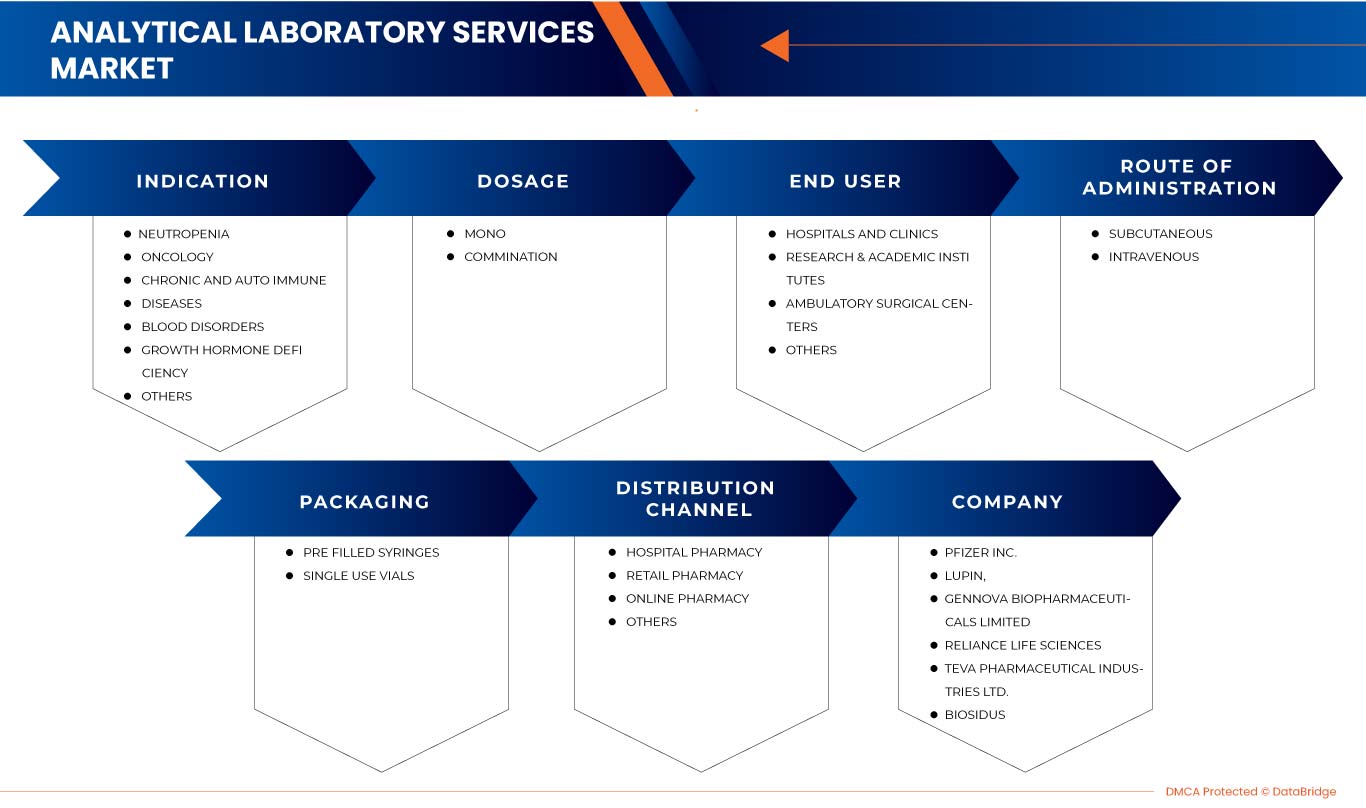
Europe G-CSF/ PEG-G-CSF market is segmented into indication, dosage, route of administration, packaging, end user, and distribution channel. The growth among segments helps you analyze niche pockets of growth and strategies to approach the market and determine your core application areas and the difference in your target markets.
EUROPE G-CSF / PEG-G-CSF MARKET, BY INDICATION
- NEUTROPENIA
- CHEMOTHERAPY INDUCED FEBRILE NEUTROPENIA (MYELOSUPPRESSIVE CHEMOTHERAPY TREATMENT)
- SEVERE CHRONIC NEUTROPENIA
- RADIOTHERAPY INDUCED NEUTROPENIA
- NEUTROPENIA IN HIV PATIENTS
- CLOZAPINE INDUCED NEUTROPENIA
- NEUTROPENIA IN HEPATITIS C PATIENTS
- CONGENITAL NEUTROPENIA
- ONCOLOGY
- ACUTE MYELOID LEUKEMIA RECEIVING CONSOLIDATION CHEMOTHERAPY
- OTHERS
- CHRONIC AND AUTOIMMUNE DISEASES
- BLOOD DISORDERS
- GROWTH HORMONE DEFICIENCY
- OTHERS
On the basis of indication the Europe G-CSF/PEG-G-CSF is further segmented into neutropenia, oncology, chronic and autoimmune diseases, blood disorders, growth hormone deficiency and others.
EUROPE G-CSF / PEG-G-CSF MARKET, BY DOSAGE
- MONO
- COMBINATION
On the basis of dosage the Europe G-CSF/PEG-G-CSF is further segmented into mono and combination.
EUROPE G-CSF / PEG-G-CSF MARKET, BY ROUTE OF ADMINISTRATION
- INTRAVENOUS
- SUBCUTANEOUS
On the basis of route of administration the Europe G-CSF/PEG-G-CSF is further segmented into intravenous and subcutaneous.
EUROPE G-CSF / PEG-G-CSF MARKET, BY PACKAGING
- SINGLE USE VIALS
- PRE FILLED SYRINGES
On the basis of packaging the Europe G-CSF/PEG-G-CSF is further segmented into single use vials and pre filled syringes.
EUROPE G-CSF / PEG-G-CSF MARKET, BY END USER
- HOSPITALS & CLINICS
- RESEARCH & ACADEMIC INSTITUTES
- AMBULATORY SURGICAL CENTERS
- OTHERS
On the basis of end user the Europe G-CSF/PEG-G-CSF is further segmented into hospitals and clinics, research & academic institutes, ambulatory surgical centers and others.
EUROPE G-CSF / PEG-G-CSF MARKET, BY DISTRIBUTION CHANNEL
- HOSPITAL PHARMACY
- ONLINE PHARMACY
- RETAIL PHARMACY
- OTHERS
流通チャネルに基づいて、ヨーロッパの G-CSF/PEG-G-CSF はさらに病院薬局、オンライン薬局、小売薬局、その他に分類されます。
ヨーロッパの G-CSF/PEG-G-CSF 市場の地域分析/洞察
ヨーロッパの G-CSF/PEG-G-CSF 市場が分析され、国、適応症、投与量、投与経路、パッケージ、エンドユーザー、流通チャネルに基づいて市場規模の情報が提供されます。
ヨーロッパの G-CSF/PEG-G-CSF 市場は、ドイツ、イギリス、フランス、イタリア、ロシア、オランダ、スペイン、スウェーデン、ポーランド、ベルギー、スイス、デンマーク、ノルウェー、フィンランド、トルコ、その他のヨーロッパ諸国で構成されています。
ドイツは、G-CSF/PEG-G-CSFにおける最新の高度な技術と発明により、成長が期待されています。
レポートの国別セクションでは、市場の現在および将来の傾向に影響を与える国内市場における個別の市場影響要因と規制の変更も提供しています。新規販売、交換販売、国の人口統計、規制行為、輸出入関税などのデータ ポイントは、各国の市場シナリオを予測するために使用される主要な指標の一部です。また、国別データの予測分析を提供する際には、ヨーロッパ ブランドの存在と可用性、地元および国内ブランドとの競争が激しいか少ないために直面する課題、販売チャネルの影響も考慮されます。
競争環境と欧州の G-CSF/PEG-G-CSF 市場シェア分析
ヨーロッパの G-CSF/PEG-G-CSF 市場の競争状況は、競合他社ごとに詳細を提供します。含まれる詳細には、会社概要、会社の財務状況、収益、市場の可能性、研究開発への投資、新しい市場への取り組み、生産拠点と施設、会社の強みと弱み、製品の発売、製品試験パイプライン、製品の承認、特許、製品の幅と幅、アプリケーションの優位性、技術ライフライン曲線などがあります。提供されている上記のデータ ポイントは、ヨーロッパの G-CSF/PEG-G-CSF 市場への会社の重点にのみ関連しています。
この市場で取引を行っている大手企業としては、USV Private Limited、Viatris Inc.、Biocon、Fresenius Kabi AG、Hangzhou Jiuyuan Gene Engineering Co., Ltd.、Amgen Inc.、Pfizer Inc.、Sandoz International GmbH、Apotex Inc.、Cadila Pharmaceuticals、Dr. Reddy's Laboratories Ltd.、Amneal Pharmaceuticals LLC.、Coherus BioSciences、Accord Healthcare、NAPP PHARMACEUTICALS LIMITED.、Intas Pharmaceuticals Ltd.、Mundipharma International、Teva Pharmaceutical Industries Ltd.、Spectrum Pharmaceuticals, Inc.、Kyowa Kirin Co., Ltd.、Jiangsu Hengrui Pharmaceuticals Co., Ltd. などがあります。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE G-CSF / PEG-G-CSF MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 EUROPE G-CSF/PEG-G-CSF MARKET: MERGERS AND ACQUISITION
4.4 EUROPE G-CSF / PEG-G-CSF MARKET
4.5 STRATEGIES TO THE ENTER THE MARKET
4.5.1 JOINT VENTURE (PARTNERSHIPS):
4.5.2 ACQUISITION:
4.5.3 LINE EXPANSION VIA COLLABORATION:
4.5.4 PRODUCT APPROVAL:
4.5.5 PRODUCT LAUNCH:
4.5.6 GEOGRAPHIC EXPANSION:
4.5.7 COST LEADERSHIP:
4.5.8 PRODUCT DEVELOPMENT:
4.6 EUROPE G-CSF / PEG-G-CSF MARKET, INDUSTRY INSIGHTS
4.6.1 PATENT ANALYSIS
4.6.2 DRUG TREATMENT RATE BY MATURED MARKETS
4.6.3 DEMOGRAPHIC TRENDS: IMPACTS ON ALL INCIDENCE RATES
4.6.4 THERAPEUTIC ASSESSMENT
4.6.5 KEY PRICING STRATEGIES
4.6.6 KEY PATIENT ENROLLMENT STRATEGIES
4.6.7 CONCLUSION
4.7 PIPELINE ANALYSIS FOR EUROPE G-CSF / PEG-G-CSF MARKET
5 EPIDEMIOLOGY
6 EUROPE G-CSF / PEG-G-CSF MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 GROWING INCIDENCES OF BLOOD CANCERS AND CANCER DISEASES
7.1.2 RISING INCIDENCES OF AUTOIMMUNE DISORDERS
7.1.3 INCREASING CASES OF FEBRILE NEUTROPENIA
7.1.4 INCREASING AWARENESS ABOUT FILGRASTIM AND PEGFILGRASTIM
7.2 RESTRAIN
7.2.1 STRINGENT GOVERNMENTAL REGULATIONS
7.2.2 AVAILABILITY OF ALTERNATIVES FOR THE CHEMOTHERAPY
7.3 OPPORTUNITIES
7.3.1 THE USE OF BIOSIMILARS HELPS REDUCE HEALTHCARE COSTS FOR PATIENTS
7.3.2 COST-EFFECTIVENESS AND PATENT EXPIRY OF BIOLOGICAL PRODUCTS
7.4 CHALLENGES
7.4.1 THE HIGH COST ASSOCIATED WITH BRANDED BIOLOGICS AND IMPROVED CHEMOTHERAPY
7.4.2 THE MULTIPLE SIDE EFFECTS OF G-CSF
8 EUROPE G-CSF/ PEG-G-CSF MARKET, BY INDICATION
8.1 OVERVIEW
8.2 NEUTROPENIA
8.2.1 CHEMOTHERAPY INDUCED FEBRILE NEUTROPENIA (MYELOSUPPRESSIVE CHEMOTHERAPY TREATMENT)
8.2.2 SEVERE CHRONIC NEUTROPENIA
8.2.3 RADIOTHERAPY INDUCED NEUTROPENIA
8.2.4 NEUTROPENIA IN HIV PATIENTS
8.2.5 CLOZAPINE INDUCED NEUTROPENIA
8.2.6 NEUTROPENIA IN HEPATITIS C PATIENTS
8.2.7 CONGENITAL NEUTROPENIA
8.3 ONCOLOGY
8.3.1 ACUTE MYELOID LEUKEMIA RECEIVING CONSOLIDATION CHEMOTHERAPY
8.3.2 OTHERS
8.4 CHRONIC AND AUTO IMMUNE DISEASES
8.5 BLOOD DISORDERS
8.6 GROWTH HORMONE DEFICIENCY
8.7 OTHERS
9 EUROPE G-CSF/ PEG-G-CSF MARKET, BY DOSAGE
9.1 OVERVIEW
9.2 MONO
9.3 COMBINATION
10 EUROPE G-CSF/ PEG-G-CSF MARKET, BY ROUTE OF ADMINISTRATION
10.1 OVERVIEW
10.2 SUBCUTANEOUS
10.3 INTRAVENOUS
11 EUROPE G-CSF/ PEG-G-CSF MARKET, BY PACKAGING
11.1 OVERVIEW
11.2 PRE FILLED SYRINGES
11.3 SINGLE USE VIALS
12 EUROPE G-CSF/ PEG-G-CSF MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS AND CLINICS
12.3 RESEARCH & ACADEMIC INSTITUTES
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 EUROPE G-CSF/ PEG-G-CSF MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 HOSPITALS PHARMACY
13.3 RETAIL PHARMACY
13.4 ONLINE PHARMACY
13.5 OTHERS
14 EUROPE G-CSF/PEG-G-CSF MARKET, BY REGION
14.1 EUROPE
14.1.1 RUSSIA
14.1.2 TURKEY
15 EUROPE G-CSF / PEG-G-CSF MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 PFIZER INC.
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENTS
17.2 VIATRIS INC.
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENT
17.3 AMGEN INC.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.4 STADA ARZENEIMITTEL AG
17.4.1 COMPANY SNAPSHOT
17.4.2 COMPANY SHARE ANALYSIS
17.4.3 PRODUCT PORTFOLIO
17.4.4 RECENT DEVELOPMENT
17.5 TEVA PHARMACEUTICAL INDUSTRIES LTD.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENTS
17.6 JIANGSU HENGRUI PHARMACEUTICALS CO., LTD.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENTS
17.7 ACCORD HEALTHCARE
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.8 AMNEAL PHARMACEUTICALS LLC.
17.8.1 COMPANY SNAPSHOT
17.8.2 REVENUE ANALYSIS
17.8.3 PRODUCT PORTFOLIO
17.8.4 RECENT DEVELOPMENTS
17.9 APOTEX INC.
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENT
17.1 BIOCON
17.10.1 COMPANY SNAPSHOT
17.10.2 REVENUE ANALYSIS
17.10.3 PRODUCT PORTFOLIO
17.10.4 RECENT DEVELOPMENT
17.11 BIO SIDUS
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENTS
17.12 CADILA PHARMACEUTICALS
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENTS
17.13 COHERUS BIOSCIENCES
17.13.1 COMPANY SNAPSHOT
17.13.2 REVENUE ANALYSIS
17.13.3 PRODUCT PORTFOLIO
17.13.4 RECENT DEVELOPMENT
17.14 DR. REDDY’S LABORATORIES LTD
17.14.1 COMPANY SNAPSHOT
17.14.2 REVENUE ANALYSIS
17.14.3 PRODUCT PORTFOLIO
17.14.4 RECENT DEVELOPMENTS
17.15 FRESENIUS KABI AG
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENT
17.16 GENNOVA BIOPHARMACEUTICALS LIMITED
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENTS
17.17 HANGZHOU JIUYUAN GENE ENGINEERING CO., LTD.
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENTS
17.18 INTAS PHARMACEUTICALS LTD.
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENT
17.19 KASHIV BIOSCIENCES, LLC.
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 KYOWA KIRIN CO., LTD.
17.20.1 COMPANY SNAPSHOT
17.20.2 REVENUE ANALYSIS
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENT
17.21 LUPIN
17.21.1 COMPANY SNAPSHOT
17.21.2 REVENUE ANALYSIS
17.21.3 PRODUCT PORTFOLIO
17.21.4 RECENT DEVELOPMENT
17.22 MUNDIPHARMA INTERNATIONAL.
17.22.1 COMPANY SNAPSHOT
17.22.2 PRODUCT PORTFOLIO
17.22.3 RECENT DEVELOPMENT
17.23 NAPP PHARMACEUTICALS LIMITED
17.23.1 COMPANY SNAPSHOT
17.23.2 PRODUCT PORTFOLIO
17.23.3 RECENT DEVELOPMENT
17.24 RELIANCE LIFE SCIENCES
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENTS
17.25 SANDOZ INTERNATIONAL GMBH
17.25.1 COMPANY SNAPSHOT
17.25.2 REVENUE ANALYSIS
17.25.3 PRODUCT PORTFOLIO
17.25.4 RECENT DEVELOPMENTS
17.26 SPECTRUM PHARMACEUTICALS, INC.
17.26.1 COMPANY SNAPSHOT
17.26.2 PRODUCT PORTFOLIO
17.26.3 RECENT DEVELOPMENT
17.27 USV PRIVATE LIMITED
17.27.1 COMPANY SNAPSHOT
17.27.2 PRODUCT PORTFOLIO
17.27.3 RECENT DEVELOPMENTS
18 QUESTIONNAIRE
19 RELATED REPORTS
表のリスト
TABLE 1 BELOW ARE THE RULES AND REGULATIONS TO GET APPROVAL FOR USE IN THE MARKET:
TABLE 2 EUROPE G-CSF/ PEG-G-CSF MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 3 EUROPE NEUTROPENIA IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE NEUTROPENIA IN G-CSF/ PEG-G-CSF MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 5 EUROPE ONCOLOGY IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE ONCOLOGY IN G-CSF/ PEG-G-CSF MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 7 EUROPE CHRONIC AND AUTO IMMUNE DISEASES IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 EUROPE BLOOD DISORDERS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE GROWTH HORMONE DEFICIENCY IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE OTHERS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE G-CSF/ PEG-G-CSF MARKET, BY DOSAGE, 2020-2029 (USD MILLION)
TABLE 12 EUROPE MONO IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE COMBINATION IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE G-CSF/ PEG-G-CSF MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE SUBCUTANEOUS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 EUROPE INTRAVENOUS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE G-CSF/ PEG-G-CSF MARKET, BY PACKAGING, 2020-2029 (USD MILLION)
TABLE 18 EUROPE PRE FILLED SYRINGES IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE SINGLE USE VIALS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE G-CSF/ PEG-G-CSF MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 21 EUROPE HOSPITALS AND CLINICS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE RESEARCH & ACADEMIC INSTITUTES IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE AMBULATORY SURGICAL CENTERS IN G-CSF/ PEG-G-CSFMARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE OTHERS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE G-CSF/ PEG-G-CSF MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 26 EUROPE HOSPITALS PHARMACY IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE RETAIL PHARMACY IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE ONLINE PHARMACY IN G-CSF/ PEG-G-CSFMARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE OTHERS IN G-CSF/ PEG-G-CSF MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE G-CSF / PEG-G-CSF MARKET, 2020-2030 (USD MILLION)
TABLE 31 EUROPE G-CSF / PEG-G-CSF MARKET, BY COUNTRY, 2020-2030 (USD MILLION)
TABLE 32 EUROPE G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 33 EUROPE NEUTROPENIA IN G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 34 EUROPE ONCOLOGY IN G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 35 EUROPE G-CSF / PEG-G-CSF MARKET, BY DOSAGE, 2020-2030 (USD MILLION)
TABLE 36 EUROPE G-CSF / PEG-G-CSF MARKET, BY ROUTE OF ADMINISTRATION, 2020-2030 (USD MILLION)
TABLE 37 EUROPE G-CSF / PEG-G-CSF MARKET, BY PACKAGING, 2020-2030 (USD MILLION)
TABLE 38 EUROPE G-CSF / PEG-G-CSF MARKET, BY END USER, 2020-2030 (USD MILLION)
TABLE 39 EUROPE G-CSF / PEG-G-CSF MARKET, BY DISTRIBUTION CHANNEL, 2020-2030 (USD MILLION)
TABLE 40 RUSSIA G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 41 RUSSIA NEUTROPENIA IN G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 42 RUSSIA ONCOLOGY IN G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 43 RUSSIA G-CSF / PEG-G-CSF MARKET, BY DOSAGE, 2020-2030 (USD MILLION)
TABLE 44 RUSSIA MONO IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 45 RUSSIA G-CSF / PEG-G-CSF MARKET, BY ROUTE OF ADMINISTRATION, 2020-2030 (USD MILLION)
TABLE 46 RUSSIA SUBCUTANEOUS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 47 RUSSIA G-CSF / PEG-G-CSF MARKET, BY PACKAGING, 2020-2030 (USD MILLION)
TABLE 48 RUSSIA PRE FILLED SYRINGES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 49 RUSSIA G-CSF / PEG-G-CSF MARKET, BY END USER, 2020-2030 (USD MILLION)
TABLE 50 RUSSIA HOSPITAL AND CLINICS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 51 RUSSIA HOSPITAL AND CLINICS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 52 RUSSIA HOSPITAL AND CLINICS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 53 RUSSIA RESEARCH AND ACADEMIC INSTITUTES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 54 RUSSIA RESEARCH AND ACADEMIC INSTITUTES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 55 RUSSIA RESEARCH AND ACADEMIC INSTITUTES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 56 RUSSIA AMBULATORY SURGICAL CENTERS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 57 RUSSIA AMBULATORY SURGICAL CENTERS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 58 RUSSIA AMBULATORY SURGICAL CENTERS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 59 RUSSIA G-CSF / PEG-G-CSF MARKET, BY DISTRIBUTION CHANNEL, 2020-2030 (USD MILLION)
TABLE 60 RUSSIA HOSPITAL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 61 RUSSIA HOSPITAL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 62 RUSSIA HOSPITAL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 63 RUSSIA RETAIL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 64 RUSSIA RETAIL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 65 RUSSIA RETAIL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 66 RUSSIA ONLINE PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 67 RUSSIA ONLINE PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 68 RUSSIA ONLINE PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 69 TURKEY G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 70 TURKEY NEUTROPENIA IN G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 71 TURKEY ONCOLOGY IN G-CSF / PEG-G-CSF MARKET, BY INDICATION, 2020-2030 (USD MILLION)
TABLE 72 TURKEY G-CSF / PEG-G-CSF MARKET, BY DOSAGE, 2020-2030 (USD MILLION)
TABLE 73 TURKEY MONO IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 74 TURKEY G-CSF / PEG-G-CSF MARKET, BY ROUTE OF ADMINISTRATION, 2020-2030 (USD MILLION)
TABLE 75 TURKEY SUBCUTANEOUS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 76 TURKEY INTRAVENOUS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 77 TURKEY G-CSF / PEG-G-CSF MARKET, BY PACKAGING, 2020-2030 (USD MILLION)
TABLE 78 TURKEY PRE FILLED SYRINGES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 79 TURKEY SINGLE USE VIALS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 80 TURKEY G-CSF / PEG-G-CSF MARKET, BY END USER, 2020-2030 (USD MILLION)
TABLE 81 TURKEY HOSPITAL AND CLINICS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 82 TURKEY HOSPITAL AND CLINICS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 83 TURKEY HOSPITAL AND CLINICS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 84 TURKEY RESEARCH AND ACADEMIC INSTITUTES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 85 TURKEY RESEARCH AND ACADEMIC INSTITUTES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 86 TURKEY RESEARCH AND ACADEMIC INSTITUTES IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 87 TURKEY AMBULATORY SURGICAL CENTERS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 88 TURKEY AMBULATORY SURGICAL CENTERS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 89 TURKEY AMBULATORY SURGICAL CENTERS IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 90 TURKEY G-CSF / PEG-G-CSF MARKET, BY DISTRIBUTION CHANNEL, 2020-2030 (USD MILLION)
TABLE 91 TURKEY HOSPITAL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 92 TURKEY HOSPITAL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 93 TURKEY HOSPITAL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 94 TURKEY RETAIL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 95 TURKEY RETAIL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 96 TURKEY RETAIL PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
TABLE 97 TURKEY ONLINE PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 (USD MILLION)
TABLE 98 TURKEY ONLINE PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 ASP (USD)
TABLE 99 TURKEY ONLINE PHARMACY IN G-CSF / PEG-G-CSF MARKET, BY BRAND, 2020-2030 VOLUME (UNITS)
図表一覧
FIGURE 1 EUROPE G-CSF / PEG-G-CSF MARKET: SEGMENTATION
FIGURE 2 EUROPE G-CSF / PEG-G-CSF MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE G-CSF / PEG-G-CSF MARKET: DROC ANALYSIS
FIGURE 4 EUROPE G-CSF / PEG-G-CSF MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE G-CSF / PEG-G-CSF MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE G-CSF / PEG-G-CSF MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE G-CSF / PEG-G-CSF MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE G-CSF / PEG-G-CSF MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE G-CSF / PEG-G-CSF MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE G-CSF / PEG-G-CSF MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN CANCER PROPHYLAXIS IN DEVELOPED COUNTRIES AND INITIATIVES TAKEN BY GOVERNMENTS ARE TO DRIVE THE EUROPE G-CSF / PEG-G-CSF MARKET FROM 2023 TO 2030
FIGURE 12 NEUTROPENIA SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE G-CSF / PEG-G-CSF MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE G-CSF / PEG-G-CSF MARKET
FIGURE 14 EUROPE G-CSF/ PEG-G-CSF MARKET: BY INDICATION CATEGORY, 2022
FIGURE 15 EUROPE G-CSF/ PEG-G-CSF MARKET: BY INDICATION CATEGORY, 2021-2030 (USD MILLION)
FIGURE 16 EUROPE G-CSF/ PEG-G-CSF MARKET: BY INDICATION CATEGORY, CAGR (2022-2029)
FIGURE 17 EUROPE G-CSF/ PEG-G-CSF MARKET: BY INDICATION CATEGORY, LIFELINE CURVE
FIGURE 18 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DOSAGE, 2022
FIGURE 19 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DOSAGE, 2021-2030 (USD MILLION)
FIGURE 20 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DOSAGE, CAGR (2023-2030)
FIGURE 21 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DOSAGE, LIFELINE CURVE
FIGURE 22 EUROPE G-CSF/ PEG-G-CSF MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 23 EUROPE G-CSF/ PEG-G-CSF MARKET: BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
FIGURE 24 EUROPE G-CSF/ PEG-G-CSF MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 25 EUROPE G-CSF/ PEG-G-CSF MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 EUROPE G-CSF/ PEG-G-CSF MARKET: BY PACKAGING, 2022
FIGURE 27 EUROPE G-CSF/ PEG-G-CSF MARKET: BY PACKAGING, 2021-2030 (USD MILLION)
FIGURE 28 EUROPE G-CSF/ PEG-G-CSF MARKET: BY PACKAGING, CAGR (2023-2030)
FIGURE 29 EUROPE G-CSF/ PEG-G-CSF MARKET: BY PACKAGING, LIFELINE CURVE
FIGURE 30 EUROPE G-CSF/ PEG-G-CSF MARKET: BY END USER, 2022
FIGURE 31 EUROPE G-CSF/ PEG-G-CSF MARKET: BY END USER, 2021-2030 (USD MILLION)
FIGURE 32 EUROPE G-CSF/ PEG-G-CSF MARKET: BY END USER, CAGR (2023-2030)
FIGURE 33 EUROPE G-CSF/ PEG-G-CSF MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 35 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
FIGURE 36 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 37 EUROPE G-CSF/ PEG-G-CSF MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 EUROPE G-CSF/PEG-G-CSF MARKET: SNAPSHOT (2022)
FIGURE 39 EUROPE G-CSF/PEG-G-CSF MARKET: BY COUNTRY (2022)
FIGURE 40 EUROPE G-CSF/PEG-G-CSF MARKET: BY COUNTRY (2022 & 2030)
FIGURE 41 EUROPE G-CSF/PEG-G-CSF MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 EUROPE G-CSF/PEG-G-CSF MARKET: BY INDICATION (2023-2030)
FIGURE 43 EUROPE G-CSF / PEG-G-CSF MARKET: COMPANY SHARE 2022 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。