欧州デジタルセラピューティクス(DTx)市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
862.86 Million
USD
4,639.40 Million
2024
2032
USD
862.86 Million
USD
4,639.40 Million
2024
2032
| 2025 –2032 | |
| USD 862.86 Million | |
| USD 4,639.40 Million | |
|
|
|
|
欧州デジタルセラピューティクス(DTx)市場セグメンテーション、製品別(ソリューション/ソフトウェア、ハードウェア製品、サービス)、アプリケーション別(治療、予防、その他)、エンドユーザー別(病院、専門クリニック、在宅医療、その他)、流通チャネル別(直接入札、小売販売、その他) - 2032年までの業界動向と予測
欧州デジタルセラピューティクス(DTx)市場規模
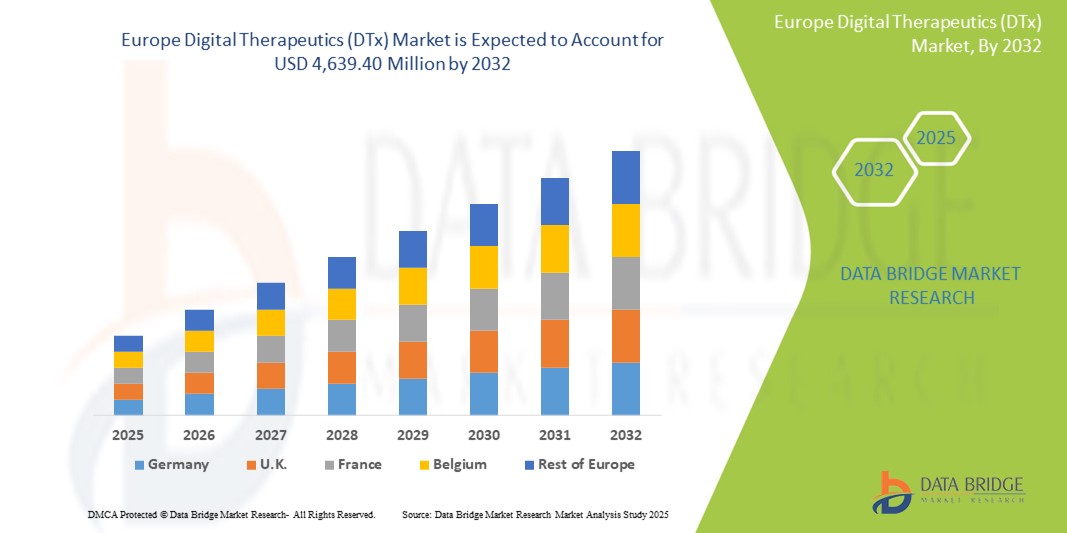
- ヨーロッパのデジタル治療薬(DTx)市場規模は2024年に8億6,286万米ドルと評価され、予測期間中に23.4%のCAGRで成長し、2032年には46億3,940万米ドル に達すると予想されています。
- 市場の成長は、主に医療のデジタル化の進展、慢性疾患の負担の増加、そして地域全体でのエビデンスに基づいた個別化治療ソリューションの需要の増加によって推進されています。
- さらに、好ましい規制枠組み、ヘルステック・イノベーションへの投資増加、そして医療従事者や患者によるデジタルセラピューティクスの受容拡大は、補完的または代替的な治療ツールとしてのDTxの役割を強化しています。これらのトレンドが相まって市場での普及を加速させ、欧州全域におけるこの分野の持続的な拡大を後押ししています。
欧州デジタルセラピューティクス(DTx)市場分析
- デジタルセラピューティクスは、ソフトウェアを介してエビデンスに基づいた治療介入を提供し、病状の予防、管理、または治療を行うもので、その拡張性、費用対効果、慢性疾患の管理と行動健康をサポートする能力により、進化するヨーロッパの医療環境においてますます重要になっています。
- DTxの需要の高まりは、糖尿病、心血管疾患、精神疾患などの慢性疾患の罹患率の増加と、患者と医療提供者の両方におけるデジタルヘルスツールの受け入れの増加によって推進されています。
- ドイツは、処方されたDTxの償還を可能にするデジタルヘルスアプリケーション(DiGA)イニシアチブ、支援的な規制環境、およびデジタルセラピューティクスの臨床ケアパスウェイへの強力な統合により、2024年に31.7%の最大の収益シェアでヨーロッパのデジタルセラピューティクス(DTx)市場を支配しました。
- スペインは、国家のデジタルヘルスイニシアチブ、医療のデジタル化の進展、遠隔の患者中心の治療ソリューションに対する需要の高まりに支えられ、予測期間中にヨーロッパのデジタル治療(DTx)市場で最も急速に成長する国になると予想されています。
- ソリューション/ソフトウェアセグメントは、導入の容易さ、拡張性、そしてモバイルアプリやクラウドベースのプラットフォームを通じた慢性疾患や行動疾患の管理における幅広い使用により、2024年には65.2%の市場シェアでヨーロッパのデジタル治療薬(DTx)市場を席巻しました。
レポートの範囲とヨーロッパのデジタルセラピューティクス(DTx)市場のセグメンテーション
|
属性 |
欧州デジタルセラピューティクス(DTx)主要市場インサイト |
|
対象セグメント |
|
|
対象国 |
ヨーロッパ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
欧州デジタルセラピューティクス(DTx)市場動向
「国家保健システムと償還経路との広範な統合」
- 欧州のDTx市場における重要かつ加速的なトレンドは、体系化された規制および償還の枠組みを通じて、デジタルセラピューティクスを公的医療制度に統合することです。ドイツ、英国、フランスなどの国々は、デジタルヘルスツールを従来の治療法と同様に処方および償還するための経路を正式に定めることで、この移行を主導しています。
- 例えば、ドイツのデジタルヘルスアプリケーション(DiGA)フレームワークでは、認定されたデジタル治療薬を医師が処方し、法定医療保険会社が償還できるようにすることで、迅速な導入とイノベーションを促進しています。フランスも、慢性疾患ケアのためのデジタルソリューションを公的システムに統合することを目指したPECANプログラムを開始しました。
- この変化により、DTx開発者は医師と患者の信頼を高めながら、臨床検証と商業的持続可能性を獲得することが可能になります。これらのフレームワークは通常、厳格な臨床的エビデンス、データセキュリティのコンプライアンス、そして実世界における有効性を求めており、市場はより高い品質基準へと向かっています。
- さらに、電子医療記録(EHR)や国のデジタルヘルスプラットフォームとの統合機能により、DTxツールの価値提案がさらに強化され、医師にはシームレスなワークフローを提供し、患者には包括的なケア体験を提供します。
- 欧州各国の政府が、DTxが医療費を削減し、慢性疾患の転帰を改善する可能性をますます認識するにつれて、この傾向はさらなる市場や治療領域に拡大すると予想されます。
- 政策支援と制度的支援の拡大は、導入規模の拡大に貢献するだけでなく、欧州の医療システム全体で治療法の提供と償還の方法も変革している。
欧州デジタルセラピューティクス(DTx)市場の動向
ドライバ
「慢性疾患の負担増加とスケーラブルなケアソリューションの需要」
- 糖尿病、高血圧、心血管疾患、精神疾患といった慢性疾患の有病率の上昇は、欧州におけるデジタル治療市場の主要な牽引力となっています。これらの疾患は長期にわたる個別化された管理を必要としますが、従来の医療システムでは大規模に提供することが困難な場合が多くあります。
- 例えば、2024年1月、サイドキック・ヘルスはファイザーと提携し、炎症性疾患に対するDTxプラットフォームを欧州市場に拡大し、スマートフォンやウェアラブルデバイスを通じて標的介入を提供しています。こうした連携は、DTxの実臨床応用への信頼の高まりを浮き彫りにしています。
- デジタルセラピューティクスは、患者に自己管理、行動修正、リアルタイムフィードバックのためのツールを提供し、医療資源の利用を削減するのに役立つ、拡張性のあるエビデンスに基づくソリューションを提供します。
- 価値に基づくケアを提供するという医療システムへの圧力が高まり、従来の治療法の効果的な補完または代替としてDTxの採用がさらに促進されています。
- さらに、遠隔監視、パーソナライゼーション、自動進捗追跡の柔軟性により、DTxは特に高齢化したヨーロッパの人口や、サービスが行き届いていない地域や農村地域の人々に適する。
抑制/挑戦
「データプライバシーの懸念と規制遵守の障壁」
- ヨーロッパの DTx 市場における最も重要な課題の 1 つは、複雑な規制およびデータ プライバシー法、特に患者データの使用、同意、セキュリティを管理する一般データ保護規則 (GDPR) への準拠を確保することです。
- 例えば、ドイツのDiGAは市場へのアクセスを加速させたが、データ保護基準の厳格な遵守と良好な医療成果の証明を要求しており、新興開発者にとってはリソース集約的となる可能性がある。
- 患者や医療提供者は、データの誤用、サイバーセキュリティの脆弱性、長期的な実臨床での有効性データの欠如に対する懸念から、DTxソリューションの導入に躊躇することもある。
- さらに、欧州諸国の規制環境は断片化しており、単一の国内市場を超えて拡大を目指す企業にとって、市場参入や償還手続きが困難になる可能性がある。
- 透明性のあるデータ慣行、高度な暗号化、強力なエビデンスの生成を通じてこれらの課題に対処し、EUレベルの保健機関による調和の取り組みと組み合わせることは、ヨーロッパ全体でのDTxの長期的な信頼と拡張性にとって非常に重要となるでしょう。
欧州デジタルセラピューティクス(DTx)市場展望
市場は、製品、アプリケーション、エンドユーザー、流通チャネルに基づいてセグメント化されています。
- 製品別
欧州のデジタルセラピューティクス(DTx)市場は、製品別に見ると、ソリューション/ソフトウェア、ハードウェア製品、サービスに分類されます。ソリューション/ソフトウェアセグメントは、導入の容易さ、費用対効果、そしてアプリやクラウドプラットフォームを通じた個別化医療の提供における拡張性の高さから、2024年には65.2%という最大の収益シェアで市場を席巻しました。ソフトウェアベースのDTxは、インタラクティブなAI駆動型治療モジュールとリアルタイムの進捗状況追跡を通じて、糖尿病やメンタルヘルスなどの慢性疾患の管理に広く利用されています。
ハードウェア製品セグメントは、患者のモニタリングを強化し、治療成果をサポートするリアルワールドヘルスデータを提供するウェアラブルデバイスやセンサー統合ツールの導入増加に支えられ、2025年から2032年にかけて最も高い成長率を示すと予想されています。これらの製品は、継続的な患者エンゲージメントとデータに基づく調整を確実にするために、ソフトウェアプラットフォームと併用されることが多くなっています。
- アプリケーション別
欧州のデジタルセラピューティクス(DTx)市場は、用途別に治療、予防、その他に分類されます。治療分野は、糖尿病、高血圧、うつ病といった慢性疾患や行動疾患の管理におけるDTxの需要に牽引され、2024年には58.3%と最大の収益シェアを占めました。これらのプラットフォームは、服薬アドヒアランス、行動変容、遠隔症状追跡を支援する、構造化されたエビデンスに基づいた治療法を提供します。
予防セグメントは、ライフスタイル管理、早期介入、長期的な医療費と病気の進行を抑える積極的な健康関与戦略への重点が高まっていることから、予測期間中に最も速い CAGR を記録すると予想されます。
- エンドユーザー別
欧州のデジタルセラピューティクス(DTx)市場は、エンドユーザーに基づいて、病院、専門クリニック、在宅医療、その他に分類されます。2024年には、DTxソリューションが患者の回復、慢性疾患管理、退院後モニタリングを支援するために病院のワークフローに統合されるケースが増えており、病院が市場を牽引し、収益シェアは41.6%に達しました。病院は、組織的な信頼、保険償還パスウェイの可用性、そして価値に基づくケアへの注力といった理由から、デジタルセラピューティクスの導入において主要な役割を担っています。
在宅医療分野は、遠隔医療、患者中心のモデル、そして人口の高齢化の傾向に対する需要の高まりを背景に、予測期間中に最も高い成長率で成長すると予測されています。DTxソリューションは、患者が自宅で病状を管理できるようにすることで、通院回数を減らし、長期的なケア戦略をサポートします。
- 流通チャネル別
欧州のデジタルセラピューティクス(DTx)市場は、流通チャネルに基づいて、直接入札、小売販売、その他に分類されます。直接入札は2024年に49.1%と最大の収益シェアを占め、これは特にドイツや英国などの国の国民保健システムや保険資金によるモデルにおいて、医療提供者や医療機関による大規模な調達が牽引役となっています。こうした構造化された調達により、医療施設全体にわたるDTxプラットフォームの標準化された導入が可能になります。
小売売上高は、アプリストアやD2Cモデルを通じたDTxアプリやプラットフォームの利用拡大に支えられ、予測期間中に最も高いペースで成長すると予想されています。消費者意識の高まりとセルフケアのトレンドは、特にライフスタイルやメンタルヘルスの課題に対し、個人がデジタルセラピーを自主的に導入することを促しています。
ヨーロッパのデジタルセラピューティクス(DTx)市場地域分析
- ドイツは、処方されたDTxの償還を可能にするデジタルヘルスアプリケーション(DiGA)イニシアチブ、支援的な規制環境、およびデジタルセラピューティクスの臨床ケアパスウェイへの強力な統合により、2024年に31.7%の最大の収益シェアでヨーロッパのデジタルセラピューティクス(DTx)市場を支配しました。
- この地域の消費者と医療提供者は、臨床的有効性が実証されていること、リモートアクセスが容易であること、そして特に慢性疾患や精神疾患の長期的な疾患管理をサポートする能力があることから、DTxソリューションを採用するケースが増えています。
- この導入の増加は、人口の高齢化、デジタルヘルスインフラの拡大、医療費の削減と患者の転帰の改善を目的とした政策レベルの取り組みによってさらに後押しされており、デジタル治療はヨーロッパの将来の医療提供モデルの重要な要素として位置付けられています。
欧州デジタルセラピューティクス(DTx)市場インサイト
欧州のデジタルセラピューティクス(DTx)市場は、慢性疾患の有病率上昇、人口の高齢化、そしてデジタルヘルスの統合に対する政府の強力な支援を背景に、予測期間中に大幅な成長が見込まれています。医療費の上昇と価値に基づくケアへの推進は、拡張可能でエビデンスに基づいたデジタルセラピューティクスソリューションの導入を促進しています。複数の国で規制の明確化と償還モデルの導入が進むにつれ、デジタルセラピューティクスは慢性疾患および行動疾患の治療と予防の両方において、臨床パスウェイにおいて注目を集めています。
ドイツにおけるデジタルセラピューティクス(DTx)市場インサイト
ドイツのデジタルセラピューティクス(DTx)は、2024年にヨーロッパのDTx市場において31.7%という最大の収益シェアを獲得しました。これは、処方されたDTxを法定健康保険で償還できるようにする先駆的なDiGAプログラムが牽引しています。同国の強力なデジタルヘルス基盤と厳格な臨床検証要件が相まって、医療提供者と患者の間で信頼が育まれています。規制改革におけるドイツのリーダーシップは、国内外のDTx開発者を惹きつけており、ヨーロッパにおけるデジタルヘルス導入の中心地となっています。
英国デジタルセラピューティクス(DTx)市場インサイト
英国のデジタルセラピューティクス(DTx)市場は、国民保健サービス(NHS)による慢性疾患やメンタルヘルス管理のためのデジタルツールの活用拡大に支えられ、予測期間中に堅調な年平均成長率(CAGR)で成長すると予想されています。英国政府はデジタルファーストケアへの取り組みと、遠隔医療とデジタルプラットフォームの広範な統合を推進しており、英国はDTxの主要導入国としての地位を確立しています。公衆衛生機関と民間開発者の連携も、多様な患者層を対象とした臨床的に検証されたデジタルセラピーの展開を促進しています。
フランスのデジタルセラピューティクス(DTx)市場インサイト
フランスでは、デジタルセラピューティクス(DTx)市場が急速に成長しています。これは、PECANなどの国家的な保険償還パイロットプログラムの開始を背景に、標準的な慢性疾患管理へのデジタルソリューションの統合を目指しています。同国の国民皆保険制度とデジタル変革への積極的な投資は、DTxの導入に有利な条件を整えています。患者中心のケアと予防医療への関心の高まりは、特に糖尿病や心血管疾患などの分野において、デジタル治療介入の導入をさらに加速させています。
スペインのデジタルセラピューティクス(DTx)市場インサイト
スペインでは、医療イノベーションへの投資増加、デジタルヘルスインフラの拡大、そして遠隔医療モデルの普及拡大により、デジタルセラピューティクス(DTx)が着実に成長すると見込まれています。医療システムが高齢化と慢性疾患の管理のための費用対効果の高いソリューションを模索する中、DTxプラットフォームへの関心が高まっています。地方自治体や地域の保健当局は、特に糖尿病管理、メンタルヘルスサポート、ライフスタイルの改善といった分野で、デジタルソリューションの試験導入を進めています。
ヨーロッパのデジタルセラピューティクス(DTx)市場シェア
ヨーロッパのデジタル治療 (DTx) 業界は、主に次のような定評のある企業によって牽引されています。
- サイドキックヘルス(アイスランド)
- カイア・ヘルス(ドイツ)
- HelloBetter(ドイツ)
- MySugr GmbH(オーストリア)
- Voluntis SA(フランス)
- レジリエントデジタルヘルス(フランス)
- メディバイオセンス株式会社(英国)
- シルバークラウドヘルス(英国)
- ハピファイヘルス(英国)
- Zanadio by aidhere GmbH (ドイツ)
- TicTrac Ltd.(英国)
- キュレティ(フランス)
- Oviva AG(スイス)
- Doccla Ltd.(英国)
- Psious(スペイン)
- S3コネクテッドヘルス(アイルランド)
- Qolware GmbH(ドイツ)
- アミコメッド(イタリア)
- ソーマ・オイ(フィンランド)
ヨーロッパのデジタル治療 (DTx) 市場の最近の動向は何ですか?
- 2024年3月、アイスランドを拠点とするデジタルセラピューティクス企業であるSidekick Healthは、ファイザーとの提携を拡大し、英国とドイツを含むヨーロッパの複数の国で炎症性疾患を対象としたデジタルセラピー(DTx)プログラムを開始しました。この提携は、慢性疾患に対するエビデンスに基づいたデジタルセラピーの提供に重点を置き、DTxを主流の医療に統合し、ヨーロッパ全域でパーソナライズされたケアソリューションへのアクセスを拡大するというSidekick Healthの役割を強化します。
- 2024年2月、ドイツを拠点とするメンタルヘルス専門のデジタルセラピー(DTx)プロバイダーであるHelloBetterは、DiGAフレームワークに基づき、複数のデジタルセラピープログラムが恒久的な償還対象となりました。これはドイツのデジタルヘルス戦略における重要な節目であり、デジタルセラピーに対する制度的信頼の高まりを示すとともに、DTxを国の医療システムに長期的に統合するための先例となるものです。
- 2024年1月、ドイツに本社を置くKaia Healthは、英国とフランスにおける筋骨格系(MSK)疼痛管理プラットフォームの拡大を発表しました。非侵襲性でデジタルファーストな治療法への需要の高まりに対応するため、現地の医療機関と連携します。この取り組みには、臨床試験とリアルワールドエビデンスの創出が含まれており、欧州のヘルスケア市場における主要なMSKソリューションプロバイダーとなるという同社のより広範な目標を支えています。
- 2023年12月、英国を拠点とするコネクテッドヘルステクノロジーを専門とするMediBioSenseは、心臓病および呼吸器疾患の患者を対象としたDTx対応ウェアラブルプラットフォームを発表しました。このデバイスはデジタル治療ソフトウェアと統合され、継続的なモニタリングと個別化治療を提供することで、英国の国民保健サービス(NHS)全体で高まる遠隔データ駆動型ケアのニーズに対応します。
- 2023年11月、フランスを拠点とするスタートアップ企業Resilient Digital Healthは、行動介入とリアルタイムの血糖値トラッキング、そしてAIを活用したコーチングを組み合わせた、完全デジタルの糖尿病管理プログラムを導入しました。このプログラムはパリの公立病院と共同で試験運用されており、デジタルファーストのケアモデルを通じて入院再発率の低減と患者エンゲージメントの向上を目指しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE DIGITAL THERAPEUTICS (DTX) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT AND SERVICE TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 RISING TECHNOLOGICAL ADVANCEMENTS IN DIGITAL HEALTHCARE DEVICES
5.1.2 INTEGRATION OF ARTIFICIAL INTELLIGENCE (AI) AND MACHINE LEARNING (ML)
5.1.3 INCREASING GOVERNMENT SUPPORT AND FUNDING
5.2 RESTRAINTS
5.2.1 DATA SECURITY AND PRIVACY CONCERNS RELATED TO DATA
5.2.2 HIGH LITERACY GAP IN DIGITAL HEALTH WITHIN THE POPULATION
5.3 OPPORTUNITIES
5.3.1 GROWING EXPANSION OF TELEHEALTH APPLICATIONS IN EUROPE
5.3.2 INCREASING FOCUS ON PERSONALIZED DEVICES FOR DIGITAL THERAPEUTICS
5.3.3 INCREASING STRATEGIC COLLABORATIONS AMONG THE MARKET PLAYERS
5.4 CHALLENGES
5.4.1 SOFTWARE INCOMPATIBILITY ISSUES DUE TO VARYING DATA STANDARDS
5.4.2 REGULATORY AND LEGAL CHALLENGES RELATED TO DTX DEVICES
6 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT
6.1 OVERVIEW
6.2 SOLUTIONS/SOFTWARE
6.2.1 MOBILE APPLICATIONS
6.2.2 WEB BASED PLATFORMS
6.2.3 ELECTRONIC HEALTH RECORDS
6.2.4 OTHERS
6.3 HARDWARE PRODUCTS
6.3.1 WEARABLE DEVICES
6.3.2 REMOTE PATIENT MONITORING DEVICES
6.4 SERVICES
7 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION
7.1 OVERVIEW
7.2 PREVENTIVE
7.2.1 OBESITY
7.2.2 PREDIABETES
7.2.3 OTHERS
7.3 TREATMENT
7.3.1 CARDIOVASCULAR
7.3.2 MENTAL HEALTH
7.3.3 DIABETES
7.3.4 RESPIRATORY CARE
7.4 OTHERS
8 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL
8.1 OVERVIEW
8.2 DIRECT TENDER
8.3 RETAIL SALES
8.4 OTHERS
9 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER
9.1 OVERVIEW
9.2 HOSPITALS
9.3 SPECIALTY CLINICS
9.4 HOME HEALTHCARE
9.5 OTHER
10 EUROPE DIGITAL THERAPEUTIC (DTX) MARKET, BY COUNTRY
10.1 EUROPE
10.1.1 GERMANY
10.1.2 FRANCE
10.1.3 ITALY
10.1.4 SPAIN
10.1.5 U.K.
10.1.6 SWITZERLAND
10.1.7 NETHERLANDS
10.1.8 RUSSIA
10.1.9 TURKEY
10.1.10 POLAND
10.1.11 SWEDEN
10.1.12 BELGIUM
10.1.13 DENMARK
10.1.14 FINLAND
10.1.15 NORWAY
10.1.16 REST OF EUROPE
11 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, COMPANY LANDSCAPE
11.1 COMPANY SHARE ANALYSIS: EUROPE
12 SWOT ANALYSIS
13 COMPANY PROFILE
13.1 HEALTHHERO
13.1.1 COMPANY SNAPSHOT
13.1.2 SERVICE PORTFOLIO
13.1.3 RECENT DEVELOPMENT
13.2 RESMED
13.2.1 COMPANY SNAPSHOT
13.2.2 REVENUE ANALYSIS
13.2.3 PRODUCT PORTFOLIO
13.2.4 RECENT DEVELOPMENT
13.3 GAIA AG
13.3.1 COMPANY SNAPSHOT
13.3.2 PRODUCT PORTFOLIO
13.3.3 RECENT DEVELOPMENT
13.4 SIDEKICK HEALTH GMBH
13.4.1 COMPANY SNAPSHOT
13.4.2 PRODUCT PORTFOLIO
13.4.3 RECENT DEVELOPMENT
13.5 KAIA HEALTH
13.5.1 COMPANY SNAPSHOT
13.5.2 PRODUCT PORTFOLIO
13.5.3 RECENT DEVELOPMENT
13.6 MINDABLE HEALTH GMBH
13.6.1 COMPANY SNAPSHOT
13.6.2 PRODUCT PORTFOLIO
13.6.3 RECENT DEVELOPMENT
14 QUESTIONNAIRE
15 RELATED REPORTS
表のリスト
TABLE 1 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 2 EUROPE SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTIC (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 3 EUROPE HARDWARE PRODUCTS IN DIGITAL THERAPEUTIC (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 4 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 5 EUROPE PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 6 EUROPE TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 7 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 8 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 9 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY COUNTRY, 2022-2031 (USD MILLION)
TABLE 10 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 11 GERMANY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 12 GERMANY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 13 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 14 GERMANY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 15 GERMANY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 16 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 17 GERMANY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 18 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 19 FRANCE SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 20 FRANCE HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 21 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 22 FRANCE PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 23 FRANCE TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 24 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 25 FRANCE DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 26 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 27 ITALY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 28 ITALY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 29 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 30 ITALY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 31 ITALY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 32 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 33 ITALY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 34 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 35 SPAIN SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 36 SPAIN HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 37 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 38 SPAIN PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 39 SPAIN TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 40 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 41 SPAIN DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 42 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 43 U.K. SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 44 U.K. HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 45 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 46 U.K. PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 47 U.K. TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 48 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 49 U.K. DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 50 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 51 SWITZERLAND SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 52 SWITZERLAND HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 53 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 54 SWITZERLAND PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 55 SWITZERLAND TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 56 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 57 SWITZERLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 58 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 59 NETHERLANDS SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 60 NETHERLANDS HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 61 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 62 NETHERLANDS PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 63 NETHERLANDS TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 64 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 65 NETHERLANDS DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 66 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 67 RUSSIA SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 68 RUSSIA HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 69 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 70 RUSSIA PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 71 RUSSIA TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 72 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 73 RUSSIA DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 74 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 75 TURKEY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 76 TURKEY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 77 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 78 TURKEY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 79 TURKEY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 80 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 81 TURKEY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 82 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 83 POLAND SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 84 POLAND HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 85 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 86 POLAND PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 87 POLAND TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 88 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 89 POLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 90 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 91 SWEDEN SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 92 SWEDEN HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 93 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 94 SWEDEN PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 95 SWEDEN TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 96 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 97 SWEDEN DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 98 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 99 BELGIUM SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 100 BELGIUM HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 101 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 102 BELGIUM PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 103 BELGIUM TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 104 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 105 BELGIUM DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 106 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 107 DENMARK SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 108 DENMARK HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 109 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 110 DENMARK PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 111 DENMARK TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 112 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 113 DENMARK DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 114 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 115 FINLAND SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 116 FINLAND HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 117 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 118 FINLAND PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 119 FINLAND TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 120 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 121 FINLAND DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 122 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
TABLE 123 NORWAY SOLUTIONS/SOFTWARE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 124 NORWAY HARDWARE PRODUCTS IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 125 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY APPLICATION, 2022-2031 (USD MILLION)
TABLE 126 NORWAY PREVENTIVE IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 127 NORWAY TREATMENT IN DIGITAL THERAPEUTICS (DTX) MARKET, BY TYPE, 2022-2031 (USD MILLION)
TABLE 128 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY END USER, 2022-2031 (USD MILLION)
TABLE 129 NORWAY DIGITAL THERAPEUTICS (DTX) MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD MILLION)
TABLE 130 REST OF EUROPE DIGITAL THERAPEUTICS (DTX) MARKET, BY PRODUCT, 2022-2031 (USD MILLION)
図表一覧
FIGURE 1 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: SEGMENTATION
FIGURE 2 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: MULTIVARIATE MODELLING
FIGURE 7 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 10 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: SEGMENTATION
FIGURE 12 RISING TECHNOLOGICAL ADVANCEMENTS IN THE DISEASE DIAGNOSIS AND TREATMENT APPROACH, INCREASING INVESTMENT IN EARLY STAGE VENTURES, REIMBURSEMENT COVERAGE PROVIDED BY THE REGULATORY AGENCIES ARE SOME OF THE FACTORS EXPECTED TO DRIVE THE EUROPE DIGITAL THERAPEUTICS MARKET IN THE FORECAST PERIOD 2024 TO 2031
FIGURE 13 SOLUTIONS/ SOFTWARES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE DIGITAL THERAPEUTICS (DTX) MARKET IN 2024 AND 2031
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE DIGITAL THERAPEUTICS (DTX) MARKET
FIGURE 15 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, 2023
FIGURE 16 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, 2024-2031 (USD MILLION)
FIGURE 17 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, CAGR (2024-2031)
FIGURE 18 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 19 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, 2023
FIGURE 20 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, 2024-2031 (USD MILLION)
FIGURE 21 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, CAGR (2024-2031)
FIGURE 22 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 23 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, 2023
FIGURE 24 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, 2024-2031 (USD MILLION)
FIGURE 25 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2024-2031)
FIGURE 26 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 27 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, 2023
FIGURE 28 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, 2024-2031 (USD MILLION)
FIGURE 29 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, CAGR (2024-2031)
FIGURE 30 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: SNAPSHOT (2023)
FIGURE 32 EUROPE DIGITAL THERAPEUTICS (DTX) MARKET: COMPANY SHARE 2023 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。