欧州抗核抗体検査市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
659.47 Million
USD
1,728.50 Million
2024
2032
USD
659.47 Million
USD
1,728.50 Million
2024
2032
| 2025 –2032 | |
| USD 659.47 Million | |
| USD 1,728.50 Million | |
|
|
|
|
欧州抗核抗体検査市場:抗体タイプ別(抽出核抗原(ENA)、抗DSDNAおよびヒストン、抗DFS70抗体、抗PM-SCL、抗セントロメア抗体、抗SP100など)、製品別(機器、消耗品、試薬、サービス)、技術別(ELISA、間接免疫蛍光法(IIF)、ブロッティング試験、抗原マイクロアレイ、ゲルベース技術、マルチプレックスアッセイ、フローサイトメトリー、受動血球凝集反応(PHA)、その他)、用途別(自己免疫疾患および感染症)、エンドユーザー別(病院、研究所、診断センター、研究機関など)、流通チャネル別(直接入札、小売販売、サードパーティ販売業者など) - 2032年までの業界動向と予測
欧州の抗核抗体検査市場規模
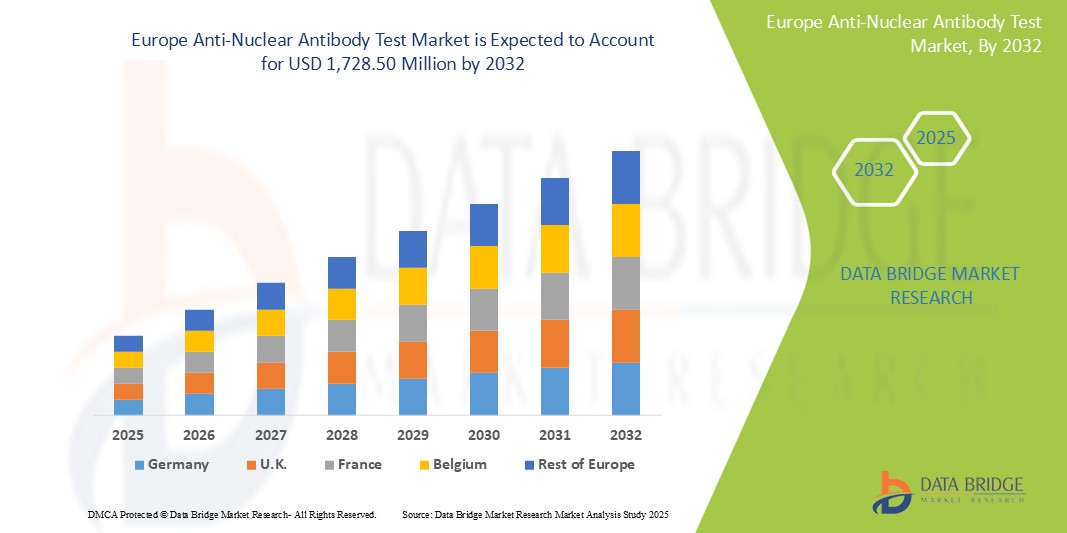
- ヨーロッパの抗核抗体検査市場規模は2024年に6億5,947万米ドルと評価され、予測期間中に12.8%のCAGRで成長し、2032年には17億2,850万米ドルに達すると予想されています。
- この成長は、自己免疫疾患の罹患率の上昇、診断方法の技術的進歩、国民の意識の向上と早期発見の取り組みなどの要因によって推進されている。
欧州における抗核抗体検査市場分析
- 抗核抗体(ANA)検査は、血液中の自己抗体を検出するために用いられる重要な診断ツールであり、全身性エリテマトーデス(SLE)、関節リウマチ、シェーグレン症候群などの自己免疫疾患の診断に役立ちます。これらの検査は、これらの疾患の早期発見と治療管理において重要な役割を果たします。
- ANA検査の需要は、自己免疫疾患の罹患率の上昇、早期診断の意識の高まり、診断技術の進歩によって大きく推進されている。
- ドイツは、先進的な医療システム、高い患者意識、先進的な検査技術の存在により、欧州の抗核抗体検査市場で25.6%のシェアを占めると予想されている。
- イタリアは、ループス、関節リウマチ、シェーグレン症候群といった自己免疫疾患の増加により、抗核抗体検査市場において欧州で最も急速な成長を遂げる国となり、年平均成長率(CAGR)は13.4%と予測されています。こうした疾患負担の増加により、ANA検査を含む早期かつ正確な診断検査の需要が高まっています。
- 間接蛍光抗体法(IIF)は、59.9%の市場シェアで市場を席巻すると予想されています。この優位性は、ANA検査のゴールドスタンダードとしての地位、高い感度、そして幅広い自己抗体の検出能力に起因しています。
レポートの範囲と欧州の抗核抗体検査市場のセグメンテーション
|
属性 |
欧州における抗核抗体検査の主要市場分析 |
|
対象セグメント |
|
|
対象国 |
ヨーロッパ
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、輸出入分析、生産能力概要、生産消費分析、価格動向分析、気候変動シナリオ、サプライチェーン分析、バリューチェーン分析、原材料/消耗品概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制枠組みも含まれています。 |
欧州における抗核抗体検査市場の動向
「自己免疫疾患診断のための抗核抗体検査の技術的進歩」
- ヨーロッパの抗核抗体検査市場における顕著な傾向の一つは、自動化システムやマルチプレックスアッセイなどの高度な診断技術の統合が進み、精度と効率性が向上していることである。
- これらの革新により、複数の自己抗体の同時検出が可能になり、ターンアラウンドタイムが短縮され、人的ミスが最小限に抑えられ、早期診断と個別化治療がサポートされるため、診断精度が向上します。
- 例えば、高度なマルチプレックスアッセイプラットフォームは、1回の検査で幅広い自己抗体を検出できるため、全身性エリテマトーデス(SLE)や関節リウマチなどの複雑な自己免疫疾患の管理に不可欠な包括的な患者プロファイルを提供します。
- これらの進歩は自己免疫診断の状況を変革し、患者の転帰を改善し、感度と特異性を高めた次世代ANA検査ソリューションの需要を促進しています。
欧州における抗核抗体検査市場の動向
ドライバ
「自己免疫疾患の有病率の上昇」
- 全身性エリテマトーデス(SLE)、関節リウマチ、シェーグレン症候群、全身性硬化症などの自己免疫疾患の発生率の増加により、抗核抗体検査の需要が大幅に増加しています。
- 自己免疫疾患は遺伝的、環境的、ライフスタイル的要因の組み合わせにより一般的になりつつあり、これらの複雑な状態を管理するために正確で早期の診断の必要性が高まっています。
- 自己免疫疾患に関する認識が高まるにつれ、医療提供者は患者の転帰を改善し、長期的な医療費を削減するために、日常的な診断の一環としてANA検査を導入するようになっている。
例えば、
- 2024年3月、欧州リウマチ学会が発表した報告書によると、欧州におけるSLEの有病率は人口の約0.1~0.2%と推定されており、これらの患者のかなりの割合が、病気の管理とモニタリングのために定期的なANA検査を必要としている。
- 自己免疫疾患の罹患率が上昇し続けるにつれて、ANA検査の需要は増加し、早期診断、個別化治療、そしてより良い患者転帰をサポートすることが期待されます。
機会
「高度な診断技術の統合」
- マルチプレックスアッセイ、自動化プラットフォーム、人工知能(AI)駆動型診断ツールなど、ANA検査における技術的進歩は、市場に大きな成長機会をもたらしています。
- これらの技術は、複数の自己抗体を同時に検出し、ターンアラウンドタイムを短縮し、人的ミスを最小限に抑えることで、より迅速で正確かつ費用対効果の高い診断を可能にします。
- さらに、AI搭載システムは検査結果をリアルタイムで分析し、病気の進行に関する予測的な洞察を提供し、臨床医がより情報に基づいた意思決定を行うのに役立ちます。
例えば、
- 2025年1月、Journal of Autoimmunity誌に掲載された研究によると、ANA検査用に開発されたAIアルゴリズムは、自己免疫疾患の早期段階の検出精度の向上、偽陽性の減少、そして全体的な診断効率の向上を実証しました。この統合により、早期介入と標的治療を促進することで、患者の転帰を大幅に改善することができます。
- 研究所や医療提供者が診断能力と患者ケアを強化しようとしているため、これらの先進技術の採用は市場の成長を促進すると予想されます。
抑制/挑戦
「高度な診断システムの高コスト」
- 利点があるにもかかわらず、高度なANA検査システムとマルチプレックスプラットフォームの高コストは、特に予算が限られている小規模な研究室や医療施設にとって、市場の成長に対する大きな障壁となっている。
- 自動化システムや特殊な検査機器に必要な初期投資は高額になる可能性があり、高度な診断の費用対効果やアクセス性に影響を与える。
- この財政的負担は、特に医療予算が限られている地域では最先端技術の導入を遅らせ、高度な自己免疫診断の普及を制限している可能性がある。
例えば、
- 2024年11月、欧州診断機器製造業者協会の報告書によると、マルチプレックスアッセイプラットフォームと自動化されたANA検査システムの高コストは依然として重大な課題であり、小規模な検査室は大幅な量の増加や償還サポートがなければ投資を正当化するのに苦労している。
- その結果、このコスト障壁は診断の質の格差につながり、多くの患者にとって早期かつ正確な自己免疫疾患検出へのアクセスを制限する可能性がある。
欧州における抗核抗体検査市場の展望
市場は、抗体の種類、製品、技術、用途、エンドユーザー、流通チャネルに基づいてセグメント化されています。
|
セグメンテーション |
サブセグメンテーション |
|
抗体の種類別 |
|
|
製品別 |
|
|
テクニック別 |
|
|
アプリケーション別 |
|
|
エンドユーザー別 |
|
|
流通チャネル別 |
|
2025年には、間接免疫蛍光法(IIF)が技術セグメントで最大のシェアを占め、市場を支配すると予測されています。
間接蛍光抗体法(IIF)は、2025年には欧州の抗核抗体検査市場において最大のシェア(59.9%)を占めると予想されています。この優位性は、主に自己抗体検査(ANA)のゴールドスタンダードとしての地位に起因しており、高い感度と幅広い自己抗体の検出能力で知られています。IIFは多様な染色パターンを識別できるため、複数の自己免疫疾患の診断に大きく貢献し、臨床医の間で好まれる選択肢となっています。IIF技術の継続的な進歩と自己免疫疾患の有病率の上昇が、IIFが市場をリードする重要な要因となっています。
抽出可能な核抗原(ENA)は、抗体型市場において予測期間中に最大のシェアを占めると予想されます。
2025年には、抽出核抗原(ENA)セグメントが、全身性エリテマトーデス、シェーグレン症候群、全身性硬化症などの全身性自己免疫リウマチ性疾患(SARD)における高い診断的関連性から、34.7%という最大の市場シェアを獲得し、市場を席巻すると予想されています。ENAパネルは複数の自己抗体を同時に検出できるため、診断の精度と効率性が向上します。臨床医の意識の高まり、マルチプレックスアッセイ技術の進歩、そして自己免疫疾患の有病率の増加も、このセグメントの市場における主導的地位をさらに支えています。
欧州抗核抗体検査市場地域分析
- 西ヨーロッパは、確立された医療インフラ、高度な医療技術、そして専門的な診断ソリューションへの高い需要に支えられ、欧州の抗核抗体検査市場において圧倒的なシェアを占めています。西ヨーロッパは、欧州の抗核抗体検査市場の約28%を占めています。
- ドイツは、先進的な医療システム、高い患者意識、先進的な検査技術の存在により、市場シェア25.6%でヨーロッパのトップ国となっている。
- 英国は、強力な医療インフラと専門的な診断検査の需要増加により、欧州の抗核抗体検査市場で大きなシェアを占めています。
- イタリアは、ループス、関節リウマチ、シェーグレン症候群といった自己免疫疾患の増加により、予測期間中に市場で最も高い年平均成長率(CAGR)13.4%を記録すると予測されています。こうした疾患負担の増加により、ANA検査を含む早期かつ正確な診断検査の需要が高まっています。
- ポーランド、ロシア、ハンガリーを含む東欧諸国は、医療投資の増加、意識の高まり、そして近代的な医療ソリューションへの移行により、急速な市場成長を遂げています。東欧は、ヨーロッパのANA検査市場の約5~6%を占めると推定されています。
- ヨーロッパ、特にオランダやスウェーデンなどの国々では、在宅医療の普及がANA検査サービスの需要を押し上げています。慢性疾患の患者が自宅で治療を受けるケースが増えており、高度な診断ツールの導入が進んでいます。ヨーロッパにおける在宅医療関連の診断ソリューションは、ANA検査市場の約4%を占めています。
欧州における抗核抗体検査の市場シェア
市場競争環境は、競合他社ごとに詳細な情報を提供します。企業概要、財務状況、収益、市場ポテンシャル、研究開発投資、新規市場への取り組み、グローバルプレゼンス、生産拠点・設備、生産能力、強みと弱み、製品投入、製品群の幅広さ、アプリケーションにおける優位性などの詳細が含まれます。上記のデータは、各社の市場への注力分野にのみ関連しています。
市場で活動している主要なマーケットリーダーは次のとおりです。
- BioNTech SE(ドイツ)
- Genmab A/S(デンマーク)
- Evotec SE(ドイツ)
- Grifols SA(スペイン)
- CRISPRセラピューティクス (スイス)
- サーモフィッシャーサイエンティフィック社(米国)
- トリニティ・バイオテック・アイルランド(アイルランド)
- EUROIMMUN Medizinische Labordiagnostika AG (ドイツ)
欧州抗核抗体検査市場の最新動向
- 2023年6月、Revvity傘下のEUROIMMUN Medizinische Labordiagnostika AG(ドイツ)は、高度な自動間接蛍光抗体法(IIFT)システムであるUNIQO 160を発売しました。このシステムは、検体調製から画像解析まで、IIFTプロセス全体を自動化し、自己免疫疾患検査における診断効率と信頼性を向上させます。
- 2023年5月、サーモフィッシャーサイエンティフィックは、自己免疫疾患の検出において迅速かつ正確な結果を提供するよう設計された自己免疫アッセイキットを発表しました。この新製品は、診断能力と患者管理の向上を目指しています。
- 2023年3月、トリニティ・バイオテックは、様々な自己免疫疾患の検出において、精度とスピードを向上させた診断アッセイ「Autoimmune Panel Plus」を発売しました。この製品は、臨床検査室における診断ワークフローを大幅に改善します。
- 2022年6月、THERADIAGはQuotient Limited社と提携し、同社のMosaiQプラットフォームを活用した自己免疫診断の発展を目指します。この契約に基づき、TheradiagはQuotient社に自己免疫マイクロアレイ開発のための自己免疫試薬と品質管理を提供し、最初のアプリケーションとして結合組織疾患(CTD)に焦点を当てます。
- ZEUS Scientificは2022年5月、ZEUSのANA HEp-2間接蛍光抗体(IFA)アッセイで使用されるdIFineデジタル免疫蛍光システムのFDA承認を取得しました。この承認には、陽性および陰性の判定と、8つの一般的なANA HEp-2染色パターンが含まれており、診断精度の向上に貢献します。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。