アジア太平洋地域における多発性遺伝性骨腫市場の規模、シェア、動向分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
22.90 Million
USD
33.33 Million
2024
2032
USD
22.90 Million
USD
33.33 Million
2024
2032
| 2025 –2032 | |
| USD 22.90 Million | |
| USD 33.33 Million | |
|
|
|
|
アジア太平洋地域における多発性遺伝性骨腫市場の細分化:種類別(無柄性および有茎性)、治療(手術、薬物療法、その他)、診断(X線、コンピューター断層撮影(CT)スキャン、磁気共鳴画像(MRI)、遺伝子検査、その他)、部位別(脚、腕、肩、骨盤、指、つま先)、年齢層別(小児および成人)、エンドユーザー別(病院、専門クリニック、外来手術センター、その他) - 2032年までの業界動向および予測
アジア太平洋地域の多発性遺伝性骨腫市場規模
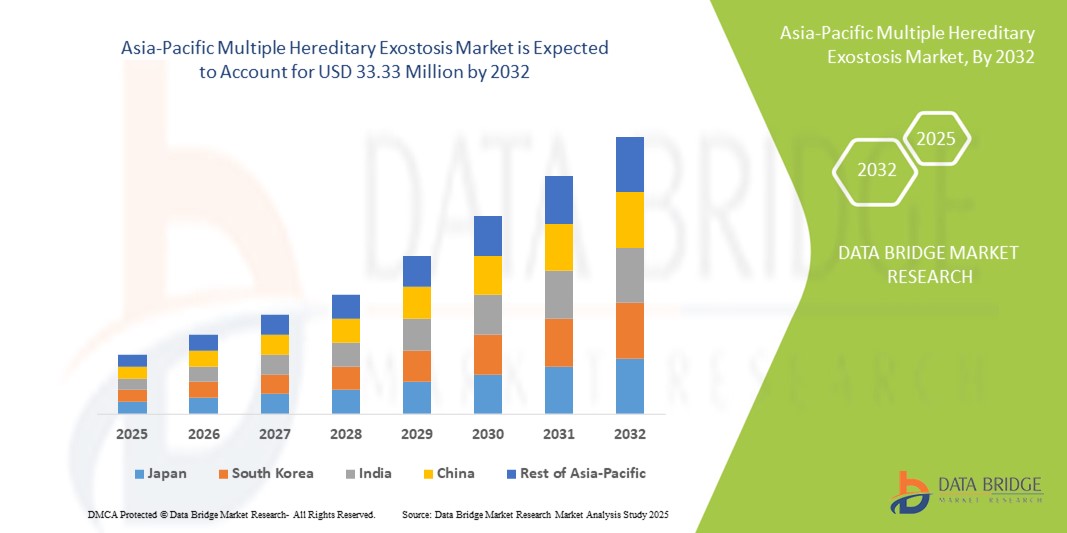
- アジア太平洋地域の多発性遺伝性骨腫市場規模は2024年に2,290万米ドルと評価され、予測期間中に4.80%のCAGRで成長し、2032年には3,333万米ドル に達すると予想されています。
- 市場の成長は、希少遺伝性疾患に対する認識の高まりと早期診断、専門医療へのアクセスの改善、および地域の新興経済国における遺伝子検査プログラムの増加によって主に推進されています。
- さらに、精密医療への投資の増加、政府の支援策、そして小児患者数の増加により、高度な治療オプションに対する需要が高まっています。これらの要因が重なり、診断および治療ソリューションの普及が加速し、業界の成長を大きく押し上げています。
アジア太平洋地域における多発性遺伝性骨腫市場分析
- 多発性遺伝性骨腫症(MHE)は、良性の骨腫瘍を特徴とする稀な遺伝性疾患ですが、アジア太平洋地域では、認知度の高まり、診断能力の向上、都市部における専門的な整形外科および遺伝学的サービスへのアクセスの増加により、臨床的な注目を集めています。
- 早期診断と高度な治療介入に対する需要の高まりは、主に医療インフラの改善、希少疾患管理に対する政府の支援、中国、日本、インドなどの国における遺伝子検査プログラムの拡大によって促進されている。
- 中国は、小児患者数の増加、ゲノム医療の進歩、骨異形成症に特化した地域の卓越したセンターの存在に支えられ、2024年にはアジア太平洋地域の多発性遺伝性骨腫市場で39.9%という最大の収益シェアを獲得して優位に立つだろう。
- インドは、希少疾患政策の強化、臨床試験活動の拡大、患者擁護団体の役割の拡大により、予測期間中にアジア太平洋地域の多発性遺伝性骨腫市場で最も急速に成長する国になると予想されています。
- 2024年には、アジア太平洋地域の多発性遺伝性骨腫市場では、関節付近での発生率が高く、神経圧迫を引き起こす可能性が高く、早期の臨床検出と介入を促すことから、無柄骨腫が59.1%の市場シェアを占め、市場を支配しました。
レポートの範囲とアジア太平洋地域の多発性遺伝性骨腫市場のセグメンテーション
|
属性 |
アジア太平洋地域における多発性遺伝性骨腫症の主要市場分析 |
|
対象セグメント |
|
|
対象国 |
アジア太平洋
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
アジア太平洋地域の多発性遺伝性骨腫市場の動向
「遺伝子診断と個別化治療アプローチの進歩」
- アジア太平洋地域の多発性遺伝性骨腫(MHE)市場において、重要な加速トレンドとして、高度な遺伝子診断ツールの導入が進み、小児骨異形成症患者の個々のニーズに合わせた個別化治療プロトコルが登場しています。このトレンドにより、早期発見が向上し、標的を絞った介入戦略が可能になります。
- 例えば、日本や中国などの国では、EXT1およびEXT2遺伝子変異の特定に次世代シークエンシング(NGS)技術の利用が拡大しており、臨床医はMHEを早期かつ正確に診断できるようになっています。また、整形外科や小児科の専門クリニックでは、遺伝カウンセリングも一般的になりつつあります。
- 低放射線CTや高解像度MRIといった非侵襲性画像診断技術の普及により、骨腫の進行をより正確にモニタリングし、手術計画の策定に役立てることができます。AIを活用した放射線学的解析と組み合わせることで、これらのツールはより正確な病変追跡と治療のカスタマイズを可能にします。
- これらの診断の進歩は、都市部での公衆衛生啓発キャンペーンやスクリーニングプログラムの拡大と相まって、合併症が発生した後にのみ外科的介入を行うのではなく、病気の積極的な管理への移行を促進している。
- 患者登録と国境を越えた連携は地域全体で増加しており、韓国やオーストラリアなどの国々は、MHEに関する臨床研究とデータ共有を促進する希少疾患ネットワークに参加しています。これらの取り組みは、地域特有の疫学的知見を生み出し、エビデンスに基づくケアを促進するのに役立っています。
- 早期発見と個別化ケアへの関心が高まるにつれ、アジア太平洋地域におけるMHE治療の状況は変化し、医療イノベーションと患者中心の整形外科ソリューションの提供のためのプラットフォームが生まれています。
アジア太平洋地域の多発性遺伝性骨腫市場の動向
ドライバ
「早期診断の促進と小児整形外科サービスの拡大」
- アジア太平洋地域の主要経済圏における小児整形外科専門医療および診断インフラの整備は、MHE市場の成長を大きく牽引しています。遺伝子スクリーニングによる早期発見と高度な画像診断へのアクセス向上により、より早期の介入とより良い転帰が可能になっています。
- 例えば、インドと中国では、希少疾患を国家保健プログラムに含めるという政府主導の取り組みにより、三次医療機関の診断パネルにMHEが統合され、症例発見率が向上しました。また、長期的な転帰をモニタリングするための国家希少疾患登録簿も整備されています。
- 臨床医や家族の間で認識が広がるにつれ、変形の外科的矯正や疼痛管理サービスに対する需要が高まっており、特に小児科専門病院や整形外科センターで顕著です。ロボット支援手術プラットフォームや骨モデリングのための3Dプリンターは、外骨腫の精密切除にますます活用されています。
- この地域における希少疾患支援団体の台頭は、患者とその家族がリソースにアクセスしやすくし、より良いケアプロトコルを推進し、専門医とのつながりを築くことにも役立っています。これらの取り組みは、疾患への早期の関与と包括的な治療を促進しています。
抑制/挑戦
「地方における認知度の低さと専門治療へのアクセスの制限」
- 都市部の医療拠点の発展にもかかわらず、一般開業医の間でMHEに対する認識が乏しく、地方や遠隔地では遺伝子診断施設が不足していることが、市場への広範な浸透を阻む大きな障壁となっています。この疾患の希少性と複雑な症状のため、多くの症例が未診断または誤診されています。
- 東南アジアの一部など、医療インフラがそれほど整っていない国では、患者は整形外科専門医の診察を受けるのに時間がかかったり、遺伝子検査や経過観察のための画像診断を受けるための経済的余裕がなかったりする可能性があり、その結果、治療が不十分になったり、避けられるはずの合併症が生じたりする可能性がある。
- さらに、標準化された治療ガイドラインの欠如と分断された紹介システムにより、患者が多科医療を受けることが困難になっています。アジア太平洋地域のいくつかの国では希少疾患に関する政策枠組みの改善に向けた取り組みが進められていますが、その実施状況は依然として不均一です。
- これらの課題を克服するには、農村部の医療への投資拡大、整形外科診療のための遠隔医療プラットフォーム、遺伝子スクリーニングプログラムの補助、希少疾患に関する臨床医の研修が不可欠です。地域全体のMHE患者へのアクセス格差を埋め、公平なケアを確保するためには、政府、NGO、医療機関間の連携が不可欠です。
アジア太平洋地域の多発性遺伝性骨腫市場の展望
市場は、タイプ、治療、診断、部位、年齢層、エンドユーザーに基づいてセグメント化されています。
- タイプ別
アジア太平洋地域の多発性遺伝性骨腫市場は、病型別に無柄型と有柄型の2つに分類されます。無柄型セグメントは、2024年には59.1%という最大の収益シェアで市場を席巻しました。これは、その広範な病変基盤と神経や関節などの重要な構造物への近接性による臨床的認知度の高さが要因です。これらの特徴は、症状の早期発現、診断率の向上、そして外科的介入の必要性の増加につながります。無柄型病変は機能障害や変形を伴うことが多く、迅速な整形外科的評価が求められます。
有茎性骨腫セグメントは、長柄性骨腫の検出を向上させる画像診断能力の向上と、非侵襲性モニタリング技術の利用増加に支えられ、2032年まで最も高いペースで成長すると予想されています。認知度が高まり、外来診療において放射線機器がより利用しやすくなるにつれて、症状の軽い有茎性病変の検出率も上昇すると予想されます。
- 治療別
アジア太平洋地域の多発性遺伝性骨腫市場は、治療方法に基づいて、外科手術、薬物療法、その他に分類されます。外科手術分野は、神経圧迫、四肢変形、関節可動域制限などの合併症の管理において重要な役割を果たすため、2024年には46.7%という最大の市場シェアを獲得しました。小児患者は、成長異常や慢性疼痛の予防のため、骨腫の外科的切除を受けることがよくあります。日本、中国、韓国などの国では、熟練した整形外科医や、ロボット支援手術や3Dモデリングなどの高度なツールが利用可能であり、この分野の成長を支えています。
医薬品セグメントは、主に術後疼痛管理と炎症抑制のサポートにおいて、2025年から2032年にかけて最も高いCAGRを達成すると予想されています。また、骨芽腫の増殖を抑制することを目的とした標的療法への臨床的関心も高まっていますが、これらの選択肢は依然として研究段階にあります。
- 診断別
アジア太平洋地域の多発性遺伝性骨腫市場は、診断方法に基づいて、X線、コンピュータ断層撮影(CT)、磁気共鳴画像(MRI)、遺伝子検査、その他に分類されます。X線は、その費用対効果の高さ、広範な入手性、そしてMHEに特徴的な骨増殖の特定における効率性により、2024年には診断セグメントで34.2%という最大のシェアを占めました。X線は、多くの臨床現場において依然として第一選択の画像診断ツールとなっています。
遺伝子検査は、EXT1およびEXT2遺伝子解析による遺伝パターンの確認という役割から、2032年まで最も急速に成長すると予想されています。特に都市部の医療センターにおいて、次世代シーケンシング(NGS)への認知度とアクセスが高まるにつれ、遺伝子検査は早期診断と家族カウンセリングに不可欠なものになりつつあります。
- サイト別
アジア太平洋地域の多発性遺伝性骨腫市場は、病変部位に基づいて、脚、腕、肩、骨盤、指、つま先に区分されています。脚セグメントは、2024年には36.9%と最も高いシェアを占めました。これは、小児において機械的ストレスや成長障害が生じやすい大腿骨、脛骨、腓骨付近に骨腫が発生することが多いためです。これらの病変は、歩行異常や四肢長差などの問題を引き起こすため、整形外科的評価と矯正手術が必要となることがよくあります。
腕と肩は、変形により正常な機能や可動域が妨げられる可能性のある小児患者にとって特に一般的な解剖学的部位であるため、予測期間中に最も急速に成長すると予想されます。
- 年齢別
アジア太平洋地域の多発性遺伝性骨腫市場は、年齢層別に小児と成人に分類されます。MHEは骨の成長が活発で、目に見える隆起、関節の制限、四肢の変形などの症状が現れ始める幼少期に診断されることが多いため、2024年には小児セグメントが72.1%のシェアを占め、市場をほぼ独占しました。
成人の症例は、特に診断の遅れや再発により、2025年から2032年にかけて最も速いCAGRを示すことが予想されますが、介入、検査、手術の大部分は小児期および青年期に行われます。
- エンドユーザー別
アジア太平洋地域の多発性遺伝性骨腫市場は、エンドユーザーに基づいて、病院、専門クリニック、外来手術センター、その他に分類されます。病院は、整形外科、放射線科、遺伝カウンセリングを含む包括的な診断・手術機能を一元的に提供していることから、2024年には48.3%という最大のシェアを獲得し、このセグメントを牽引しました。中国、日本、インドなどの国では、小児病院や大学付属医療センターがMHEの診断と治療の主要拠点となっています。
外来手術センターは、低侵襲手術や日帰り手術の好ましさが高まっているため、予測期間中に大幅な成長を示すことが予想されます。
アジア太平洋地域の多発性遺伝性骨腫市場の地域分析
- 中国は、小児患者数の増加、ゲノム医療の進歩、骨異形成症に特化した地域の卓越したセンターの存在に支えられ、2024年にはアジア太平洋地域の多発性遺伝性骨腫市場で39.9%という最大の収益シェアを獲得して優位に立つだろう。
- 中国や日本、インドなどの国の患者と臨床医は、早期介入戦略、高度な外科技術、個別化されたケアプランを積極的に採用しており、患者の転帰の改善と市場の拡大につながっています。
- この成長は、国の希少疾患政策、医療における官民パートナーシップ、そして進行中の研究協力によってさらに促進され、アジア太平洋地域はMHEの診断と治療における革新と臨床進歩の先導的な地域となっています。
中国多発性遺伝性骨腫市場の洞察
中国の多発性遺伝性骨腫市場は、2024年にアジア太平洋地域のMHE市場において最大の収益シェア(39.9%)を獲得しました。これは、小児人口の多さ、先進画像技術の導入拡大、そして都市部の病院における遺伝子検査へのアクセス拡大に支えられています。国家の希少疾患戦略とゲノム医療への投資増加は、遺伝性骨疾患の早期診断と治療を支えています。政府支援によるスクリーニングプログラムと専門整形外科センターの存在は、引き続き市場の成長を牽引しています。
日本における多発性遺伝性骨腫市場の洞察
日本の多発性遺伝性骨腫市場は、先進的な医療インフラと精密医療への注力に支えられ、著しい成長を遂げています。遺伝子検査を標準的な診断経路に統合し、日本の積極的な希少疾患政策の枠組みと相まって、MHEのより正確で早期の発見が可能になっています。さらに、研究機関と医療提供者の緊密な連携が、治療プロトコルと患者の転帰の改善に貢献しています。
インドにおける多発性遺伝性骨腫市場の洞察
インドの多発性遺伝性骨腫市場は、整形外科専門医の確保、三次医療機関における診断ツールの改善、そして国民の意識向上に牽引され、2024年にはアジア太平洋地域のMHE市場において大きなシェアを占めると予測されます。希少疾患に関する国家政策をはじめとする希少疾患の特定を目的とした政府の取り組みにより、早期診断と外科的治療へのアクセスが向上しています。小児人口の多さと都市部における医療インフラの拡大も、市場の成長を支えています。
アジア太平洋地域の多発性遺伝性骨腫市場シェア
アジア太平洋地域の多発性遺伝性骨腫業界は、主に以下の企業を含む定評ある企業によって牽引されています。
- イルミナ社(米国)
- メドトロニック(アイルランド)
- ジンマー・バイオメット・ホールディングス(米国)
- ストライカー(米国)
- シーメンス・ヘルシニアーズAG(ドイツ)
- GEヘルスケア・テクノロジーズ(米国)
- キヤノンメディカルシステムズ株式会社(日本)
- Esaote SpA(イタリア)
- サーモフィッシャーサイエンティフィック社(米国)
- ロシュ・ホールディングAG(スイス)
- Koninklijke Philips NV (オランダ)
- B. ブラウン メルズンゲン AG (ドイツ)
- アジレント・テクノロジーズ社(米国)
- パーキンエルマー社(米国)
- 富士フイルム株式会社(日本)
- 北京ゲノミクス研究所 (BGI Genomics) (中国)
- 深セン・ミンドレイ・バイオメディカル・エレクトロニクス株式会社(中国)
- 江蘇トラウテックメディカルテクノロジー株式会社(中国)
- 東芝株式会社(日本)
- タカラバイオ株式会社(日本)
アジア太平洋地域の多発性遺伝性骨腫市場の最近の動向は何ですか?
- 中国保健省は2024年5月、国家希少疾患登録簿を多発性遺伝性骨腫症(MHE)まで拡大し、主要省における患者の早期診断、治療アクセス、長期モニタリングの改善を目指しました。この取り組みは、中国が希少疾患管理に戦略的投資を行い、集中型データシステムを活用して個別化された患者ケアと臨床転帰の改善を目指す姿勢を反映しています。
- 2024年3月、国立成育医療研究センターは、骨芽細胞腫(MHE)におけるEXT1およびEXT2遺伝子変異を標的とした遺伝子編集療法に焦点を当てた共同研究プロジェクトを開始しました。このプロジェクトには、一流大学やバイオテクノロジー企業が参加しており、国内における希少骨疾患に対する精密医療と遺伝子治療の革新において大きな前進となるでしょう。
- 2024年2月、インド医学研究評議会(ICMR)は、骨異形成症(MHEを含む)が疑われる小児患者の遺伝子スクリーニングに焦点を当てた全国規模のパイロットプログラムへの資金提供を承認しました。このプログラムは、早期診断の促進、公立病院の診断能力の向上、そして遺伝カウンセリングサービスを通じて家族を支援することを目的としています。
- 2024年1月、韓国のソウル国立大学病院は、MRIおよびCTスキャンの読影によるMHE診断の精度向上を目的としたAI支援画像アルゴリズムの臨床試験を開始しました。この開発は、韓国が整形外科診断へのAIの統合を推進し、効率性を高め、診断の遅延を短縮しようとしていることを示しています。
- 2023年12月、オーストラリア希少疾患ネットワーク(ARDN)は、MHEおよびその他の骨疾患の治療プロトコルの標準化を目指し、複数の小児病院との提携を発表しました。この連携には、医療従事者向けの研修モジュールや、研究、臨床意思決定、政策立案を促進するための一元化されたデジタル患者登録システムの開発が含まれます。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
目次
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.3 PIPELINE ANALYSIS - OBSERVATORY DATA
4.4 EPIDEMIOLOGY
5 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: REGULATIONS
5.1 REGULATION IN UNITED STATES: U.S. FOOD AND DRUG ADMINISTRATION (FDA)
5.1.1 REGULATION IN EUROPE: EUROPEAN MEDICINES AGENCY (EMA)
5.2 REGULATION IN ASIA-PACIFIC (JAPAN): PHARMACEUTICAL AND MEDICAL DEVICES AGENCY (PMDA)
5.3 MEDICAL DEVICE STANDARDS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF GENETIC DISORDERS
6.1.2 GROWING PEDIATRIC POPULATION
6.1.3 DEVELOPMENT OF NOVEL THERAPIES
6.2 RESTRAINTS
6.2.1 HIGH COST OF ADVANCED THERAPIES
6.2.2 LIMITED AVAILABILITY OF THERAPIES
6.3 OPPORTUNITIES
6.3.1 INCREASE IN PATIENT CARE AND SUPPORT SYSTEMS
6.3.2 INCREASE IN THE NUMBER OF COLLABORATIONS AND PARTNERSHIPS
6.4 CHALLENGES
6.4.1 LIMITED AWARENESS ABOUT THE DISORDER
6.4.2 LACK OF DRUG APPROVALS ASSOCIATED WITH THE DISORDER
7 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE
7.1 OVERVIEW
7.2 SESSILE
7.3 PEDUNCULATED
8 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT
8.1 OVERVIEW
8.2 SURGERY
8.2.1 REMOVE THE TUMOR
8.2.2 LENGTHEN LIMBS
8.3 MEDICATION
8.3.1 HOSPITAL PHARMACIES
8.3.2 DRUGS STORES AND RETAIL PHARMACIES
8.3.3 ONLINE PHARMACIES
8.4 OTHERS
9 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS
9.1 OVERVIEW
9.2 X-RAY
9.2.1 SESSILE
9.2.2 PEDUNCULATED
9.3 COMPUTED TOMOGRAPHY (CT) SCAN
9.3.1 SESSILE
9.3.2 PEDUNCULATED
9.4 MAGNETIC RESONANCE IMAGING (MRI)
9.4.1 SESSILE
9.4.2 PEDUNCULATED
9.5 GENETIC TESTS
9.5.1 SESSILE
9.5.2 PEDUNCULATED
9.6 OTHERS
9.6.1 SESSILE
9.6.2 PEDUNCULATED
10 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE
10.1 OVERVIEW
10.2 LEGS
10.3 ARMS
10.4 SHOULDERS
10.5 PELVIS
10.6 FINGERS
10.7 TOES
11 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 PEDIATRIC
11.3 ADULT
12 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.2.1 PRIVATE
12.2.2 GOVERNMENT
12.3 SPECIALTY CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 OTHERS
13 ASIA-PACIFIC MULTIPLE HEREDITY EXOSTOSIS MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 JAPAN
13.1.3 INDIA
13.1.4 AUSTRALIA
13.1.5 SOUTH KOREA
13.1.6 INDONESIA
13.1.7 NEW ZEALAND
13.1.8 MALAYSIA
13.1.9 THAILAND
13.1.10 TAIWAN
13.1.11 SINGAPORE
13.1.12 PHILIPPINES
13.1.13 VIETNAM
13.1.14 REST OF ASIA-PACIFIC
14 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILES
16.1 BAYERS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT UPDATES
16.2 HALEON GROUP OF COMPANIES
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 BASF
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT UPDATES
16.4 VIATRIS INC.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT UPDATES
16.5 ACTIZAPHARMA
16.5.1 COMPANY SNAPSHOT
16.5.2 PRODUCT PORTFOLIO
16.5.3 RECENT UPDATES
16.6 ADVACARE PHARMA
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT UPDATES
16.7 AUROBINDO PHARMA
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT TABLETS
16.8 HALEON GROUP OF COMPANIES
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT UPDATES
16.9 IPSEN BIOPHARMACEUTICALS, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT UPDATES
16.1 MALLINCKRODT
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT UPDATES
16.11 TEVA PHARMACEUTICAL INDUSTRIES LTD.
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT UPDATES
16.12 TAJ PHARMACEUTICALS LIMITED
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT UPDATES
17 QUESTIONNAIRE
18 RELATED REPORTS
表のリスト
TABLE 1 PIPELINE ANALYSIS - OBSERVATORY DATA
TABLE 2 PIPELINE ANALYSIS - INTERVENTIONAL DATA
TABLE 3 SALES DATA - 2024
TABLE 4 SALES DATA - 2023
TABLE 5 SALES DATA - 2022
TABLE 6 SALES DATA - 2021
TABLE 7 SALES DATA - 2020
TABLE 8 SALES DATA - 2019
TABLE 9 SALES DATA - 2018
TABLE 10 COST OF PALOVAROTENE
TABLE 11 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 12 ASIA-PACIFIC SESSILE IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 13 ASIA-PACIFIC PEDUNCULATED IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 14 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 15 ASIA-PACIFIC SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 16 ASIA-PACIFIC SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 17 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 18 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 19 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 20 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 21 ASIA-PACIFIC X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 22 ASIA-PACIFIC X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 23 ASIA-PACIFIC COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 24 ASIA-PACIFIC COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 25 ASIA-PACIFIC MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 26 ASIA-PACIFIC MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 27 ASIA-PACIFIC GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 28 ASIA-PACIFIC GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 29 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 30 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 31 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 32 ASIA-PACIFIC LEGS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 33 ASIA-PACIFIC ARMS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 34 ASIA-PACIFIC SHOULDERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 35 ASIA-PACIFIC PELVIS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 36 ASIA-PACIFIC FINGERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 37 ASIA-PACIFIC TOES IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 38 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 39 ASIA-PACIFIC PEDIATRIC IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 40 ASIA-PACIFIC ADULT IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 41 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 42 ASIA-PACIFIC HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 43 ASIA-PACIFIC HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 44 ASIA-PACIFIC SPECIALTY CLINICS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 45 ASIA-PACIFIC AMBULATORY SURGICAL CENTERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 46 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY REGION, 2022-2031 (USD THOUSAND)
TABLE 47 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY COUNTRY, 2022-2031 (USD THOUSAND)
TABLE 48 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 49 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 50 ASIA-PACIFIC SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 51 ASIA-PACIFIC MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 52 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 53 ASIA-PACIFIC X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 54 ASIA-PACIFIC COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 55 ASIA-PACIFIC MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 56 ASIA-PACIFIC GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 57 ASIA-PACIFIC OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 58 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 59 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 60 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 61 ASIA-PACIFIC HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 62 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 63 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 64 CHINA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 65 CHINA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 66 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 67 CHINA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 68 CHINA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 69 CHINA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 70 CHINA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 71 CHINA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 72 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 73 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 74 CHINA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 75 CHINA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 76 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 77 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 78 JAPAN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 79 JAPAN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 80 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 81 JAPAN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 82 JAPAN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 83 JAPAN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 84 JAPAN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 85 JAPAN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 86 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 87 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 88 JAPAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 89 JAPAN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 90 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 91 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 92 INDIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 93 INDIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 94 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 95 INDIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 96 INDIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 97 INDIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 98 INDIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 99 INDIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 100 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 101 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 102 INDIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 103 INDIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 104 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 105 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 106 AUSTRALIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 107 AUSTRALIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 108 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 109 AUSTRALIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 110 AUSTRALIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 111 AUSTRALIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 112 AUSTRALIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 113 AUSTRALIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 114 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 115 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 116 AUSTRALIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 117 AUSTRALIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 118 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 119 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 120 SOUTH KOREA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 121 SOUTH KOREA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 122 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 123 SOUTH KOREA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 124 SOUTH KOREA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 125 SOUTH KOREA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 126 SOUTH KOREA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 127 SOUTH KOREA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 128 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 129 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 130 SOUTH KOREA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 131 SOUTH KOREA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 132 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 133 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 134 INDONESIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 135 INDONESIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 136 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 137 INDONESIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 138 INDONESIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 139 INDONESIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 140 INDONESIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 141 INDONESIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 142 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 143 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 144 INDONESIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 145 INDONESIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 146 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 147 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 148 NEW ZEALAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 149 NEW ZEALAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 150 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 151 NEW ZEALAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 152 NEW ZEALAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 153 NEW ZEALAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 154 NEW ZEALAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 155 NEW ZEALAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 156 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 157 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 158 NEW ZEALAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 159 NEW ZEALAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 160 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 161 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 162 MALAYSIA SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 163 MALAYSIA MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 164 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 165 MALAYSIA X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 166 MALAYSIA COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 167 MALAYSIA MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 168 MALAYSIA GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 169 MALAYSIA OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 170 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 171 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 172 MALAYSIA MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 173 MALAYSIA HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 174 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 175 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 176 THAILAND SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 177 THAILAND MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 178 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 179 THAILAND X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 180 THAILAND COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 181 THAILAND MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 182 THAILAND GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 183 THAILAND OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 184 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 185 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 186 THAILAND MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 187 THAILAND HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 188 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 189 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 190 TAIWAN SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 191 TAIWAN MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 192 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 193 TAIWAN X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 194 TAIWAN COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 195 TAIWAN MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 196 TAIWAN GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 197 TAIWAN OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 198 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 199 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 200 TAIWAN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 201 TAIWAN HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 202 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 203 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 204 SINGAPORE SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 205 SINGAPORE MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 206 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 207 SINGAPORE X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 208 SINGAPORE COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 209 SINGAPORE MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 210 SINGAPORE GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 211 SINGAPORE OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 212 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 213 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 214 SINGAPORE MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 215 SINGAPORE HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 216 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 217 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 218 PHILIPPINES SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 219 PHILIPPINES MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 220 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 221 PHILIPPINES X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 222 PHILIPPINES COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 223 PHILIPPINES MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 224 PHILIPPINES GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 225 PHILIPPINES OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 226 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 227 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 228 PHILIPPINES MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 229 PHILIPPINES HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 230 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 231 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TREATMENT, 2022-2031 (USD THOUSAND)
TABLE 232 VIETNAM SURGERY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 233 VIETNAM MEDICATION IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DISTRIBUTION CHANNEL, 2022-2031 (USD THOUSAND)
TABLE 234 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY DIAGNOSIS, 2022-2031 (USD THOUSAND)
TABLE 235 VIETNAM X-RAY IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 236 VIETNAM COMPUTED TOMOGRAPHY (CT) SCAN IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 237 VIETNAM MAGNETIC RESONANCE IMAGING (MRI) IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 238 VIETNAM GENETIC TESTS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 239 VIETNAM OTHERS IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 240 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY SITE, 2022-2031 (USD THOUSAND)
TABLE 241 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY AGE GROUP, 2022-2031 (USD THOUSAND)
TABLE 242 VIETNAM MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY END USER, 2022-2031 (USD THOUSAND)
TABLE 243 VIETNAM HOSPITAL IN MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
TABLE 244 REST OF ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET, BY TYPE, 2022-2031 (USD THOUSAND)
図表一覧
FIGURE 1 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: ASIA-PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 PRODUCT LIFELINE CURVE
FIGURE 9 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: DBMR MARKET POSITION GRID
FIGURE 10 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: SEGMENTATION
FIGURE 12 EXECUTIVE SUMMARY
FIGURE 13 STRATEGIC DECISIONS
FIGURE 14 TWO SEGMENTS COMPRISE THE ASIA-PACIFIC MULTIPLE HEREDITY EXOSTOSIS MARKET, BY TYPE
FIGURE 15 RISING PREVALENCE OF GENETIC DISORDERS IS DRIVING THE GROWTH OF THE ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET FROM 2024 TO 2031
FIGURE 16 THE TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET IN 2024 AND 2031
FIGURE 17 MARKET OVERVIEW
FIGURE 18 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2023
FIGURE 19 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, 2024-2031 (USD THOUSAND)
FIGURE 20 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, CAGR (2024-2031)
FIGURE 21 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2023
FIGURE 23 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, 2024-2031 (USD THOUSAND)
FIGURE 24 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, CAGR (2024-2031)
FIGURE 25 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY TREATMENT, LIFELINE CURVE
FIGURE 26 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2023
FIGURE 27 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, 2024-2031 (USD THOUSAND)
FIGURE 28 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, CAGR (2024-2031)
FIGURE 29 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY DIAGNOSIS, LIFELINE CURVE
FIGURE 30 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2023
FIGURE 31 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, 2024-2031 (USD THOUSAND)
FIGURE 32 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, CAGR (2024-2031)
FIGURE 33 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY SITE, LIFELINE CURVE
FIGURE 34 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2023
FIGURE 35 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, 2024-2031 (USD THOUSAND)
FIGURE 36 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, CAGR (2024-2031)
FIGURE 37 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 38 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2023
FIGURE 39 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, 2024-2031 (USD THOUSAND)
FIGURE 40 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, CAGR (2024-2031)
FIGURE 41 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: BY END USER, LIFELINE CURVE
FIGURE 42 ASIA-PACIFIC MULTIPLE HEREDITY EXOSTOSIS MARKET: SNAPSHOT (2023)
FIGURE 43 ASIA-PACIFIC MULTIPLE HEREDITARY EXOSTOSIS MARKET: COMPANY SHARE 2023 (%)

調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。