アジア太平洋地域の分子診断サービス市場規模、シェア、トレンド分析レポート
Market Size in USD Billion
CAGR :
% 
 USD
25.84 Million
USD
49.99 Million
2024
2032
USD
25.84 Million
USD
49.99 Million
2024
2032
| 2025 –2032 | |
| USD 25.84 Million | |
| USD 49.99 Million | |
|
|
|
|
アジア太平洋地域の分子診断サービス市場の細分化、サービスタイプ別(機器修理サービス、トレーニングサービス、コンプライアンスサービス、 校正 サービス、メンテナンスサービス、スケーラブル自動化サービス、ターンキーサービス、機器移転サービス、ハードウェアカスタマイズ、パフォーマンス保証サービス、設計開発サービス、サプライチェーンソリューション、新製品導入サービス、製造サービス、環境および規制サービス、 医療管理 システム認証および監査、臨床研究サービス、コンサルティングサービス、その他のサービス)、テクノロジー別(PCR、リアルタイムPCR、次世代シーケンシング、その他のテクノロジー)、エンドユーザー別(病院、診断センター、学術研究機関、その他)業界動向と2030年までの予測。
アジア太平洋地域の分子診断サービス市場規模
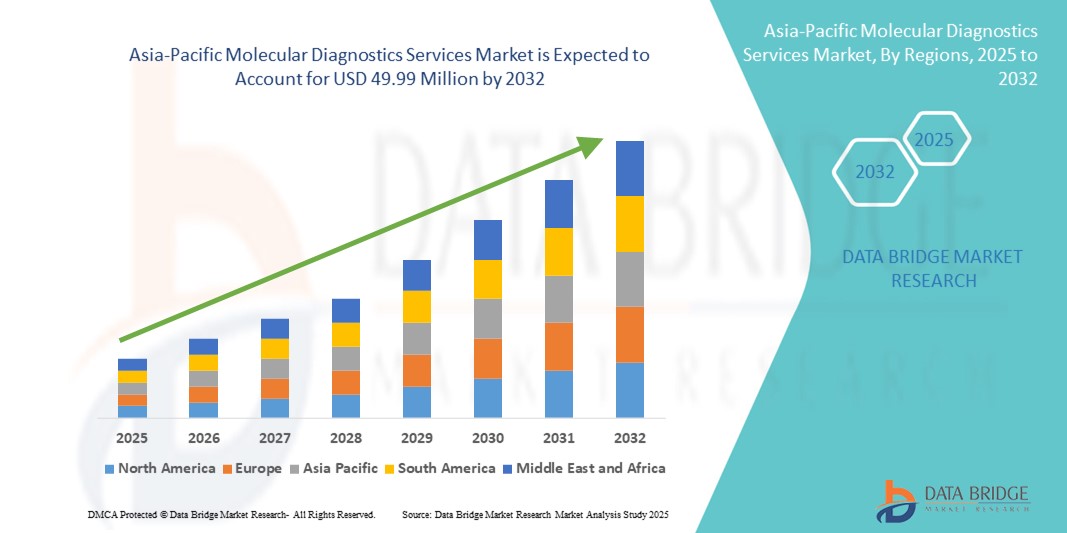
- アジア太平洋地域の分子診断サービス市場規模は2024年に2,584万米ドルと評価され、予測期間中に8.60%のCAGRで成長し、2032年には4,999万米ドル に達すると予想されています。
- 市場の成長は、アジア太平洋地域における認知度の高まり、医療へのアクセス向上、そして診断技術の進歩によって主に推進されており、幅広い遺伝性疾患、感染症、慢性疾患の迅速な検出と管理が可能になっています。インド、中国、インドネシアなどの国々では、医療インフラの整備が急速に進んでおり、分子診断サービスの導入拡大に貢献しています。
- さらに、検査施設への投資の拡大、地方および準都市部における診断サービスの拡大、官民連携の強化が、高度な分子検査技術の革新と普及を促進しています。政府の保健政策に加え、国際的な診断企業の存在感の高まりと現地の製造能力の強化が相まって、アジア太平洋地域の分子診断サービス市場の成長を大きく後押ししています。
アジア太平洋地域の分子診断サービス市場分析
- アジア太平洋地域の分子診断サービス市場は、高度な診断プラットフォームへの投資の増加、感染症および遺伝性疾患の罹患率の上昇、そして精密医療への取り組みの拡大に牽引され、大幅な成長を遂げています。中国、インド、日本、韓国などの国々は、研究施設のインフラと研究能力を拡充しており、高品質な分子診断サービスに対する需要の高まりに貢献しています。
- この地域における個別化医療と臨床研究への関心の高まりは、政府の資金援助の拡充、民間医療への投資の増加、そして専門診断センターの拡大によって支えられています。早期疾患発見への意識の高まりと、PCR、リアルタイムPCR、次世代シーケンシング(NGS)といった分子検査技術の進歩が相まって、病院や研究機関における導入が広がっています。
- 中国は、確立された病院ネットワーク、大規模な患者人口、および高度な分子診断技術の臨床および研究ワークフローへの迅速な統合により、アジア太平洋の分子診断サービス市場を支配し、2024年には35.1%の最大の収益シェアを占めました。
- インドは、医療アクセスの拡大、手頃な価格の分子検査への需要の高まり、検査インフラへの投資の増加に支えられ、予測期間中にアジア太平洋地域の分子診断サービス市場において最も高い年平均成長率(CAGR)13.6%を記録すると予測されています。政府支援の診断プログラムや民間セクターとの連携強化といった取り組みにより、都市部および準都市部における分子診断の導入が加速しています。
- PCRベースのサービスは、感染症、遺伝子検査、日常的な診断アプリケーションにおける分子検査への幅広い導入により、2024年にはアジア太平洋地域の分子診断サービス市場で36.5%のシェアを占め、市場を席巻しました。PCRは、その信頼性、手頃な価格、そして複数の臨床ワークフローへの適応性から、依然として基盤技術となっています。
レポートの範囲とアジア太平洋地域の分子診断サービス市場のセグメンテーション
|
属性 |
アジア太平洋地域の分子診断サービス主要市場分析 |
|
対象セグメント |
|
|
対象国 |
アジア太平洋
|
|
主要な市場プレーヤー |
|
|
市場機会 |
|
|
付加価値データ情報セット |
データブリッジマーケットリサーチがまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、価格設定分析、ブランドシェア分析、消費者調査、人口統計分析、サプライチェーン分析、バリューチェーン分析、原材料/消耗品の概要、ベンダー選択基準、PESTLE分析、ポーター分析、規制の枠組みも含まれています。 |
アジア太平洋地域の分子診断サービス市場動向
アジア太平洋地域における治療法の進歩と臨床研究の拡大
- アジア太平洋地域の分子診断サービス市場における重要な加速トレンドとして、特に腫瘍学、感染症、遺伝性疾患といった分野における治療革新と臨床研究への注目が高まっています。高度な分子検査により、より早期の診断とより的を絞った治療アプローチが可能になっています。
- 例えば、アジア太平洋地域の様々な診断企業や研究機関は、次世代シーケンシング(NGS)、PCRベースのアッセイ、マルチプレックスバイオマーカーパネルに投資しています。これらの開発は、個別化医療や疾患管理に不可欠な、より迅速で正確かつ費用対効果の高い診断結果の提供を目指しています。
- 病院や専門クリニックにおける精密医療モデルの導入が進むにつれ、より効果的な治療介入が可能になっています。これらのモデルは、高度な分子プロファイリングとバイオインフォマティクスを活用し、治療法の選択、疾患の進行のモニタリング、患者の反応予測に役立てられています。
- 診断技術企業、学術研究センター、政府支援プログラム間のパートナーシップは、償還枠組みの改善、検査室の実践の標準化、臨床医の研修の強化を通じて、分子検査へのアクセス拡大にも役立っています。
- アジア太平洋地域が精密医療と価値に基づく成果を優先し続ける中、分子診断サービス市場は、イノベーション、診断精度の向上、早期発見と個別化治療戦略への需要の高まりによって持続的な成長が見込まれています。
アジア太平洋地域の分子診断サービス市場の動向
ドライバ
診断率の上昇と遺伝子研究の進歩によるニーズの高まり
- アジア太平洋地域における複雑性疾患および慢性疾患の罹患率の増加は、認知度の高まりと診断能力の向上に支えられ、市場の成長を大きく牽引しています。中国、インド、日本、韓国などの国々は、医療インフラと診断プログラムを強化し、がん、感染症、遺伝性疾患などの疾患の早期発見と適切な介入を可能にしています。
- 例えば、2024年4月、アナベックス・ライフサイエンスは、精密診断と分子バイオマーカーを用いて神経変性疾患を標的とするアナベックス2-73(ブラルカメシン)の第III相臨床試験において良好な進展があったと報告しました。このようなイノベーションは、高度な分子診断の導入を促進し、予測期間中にアジア太平洋地域の分子診断サービス市場の成長を加速させると期待されます。
- 個別化医療への関心の高まりと、PCR、リアルタイムPCR、次世代シーケンシングなどの次世代分子技術の利用可能性により、従来の診断方法からより正確で患者固有の検査ソリューションへの市場シフトが促進されています。
- 日本の医薬品医療機器総合機構(PMDA)や中国の国家薬品監督管理局(NMPA)など、アジア太平洋地域の規制当局は、迅速な承認、臨床試験のサポート、合理化されたコンプライアンスガイドラインを通じて診断のイノベーションをますます支援しており、高度な分子診断サービスへの迅速な市場アクセスを促進しています。
- アジア太平洋地域のバイオテクノロジー企業、学術研究センター、医療協会間の連携は、イノベーション・エコシステムを強化しています。これらのパートナーシップは、高度な分子診断への患者アクセスの拡大、臨床研究イニシアチブの拡大、そして多様な集団における早期疾患発見と遺伝子検査の認知度向上に大きく貢献しています。
抑制/挑戦
限られたインフラと臨床導入におけるばらつき
- 高度な分子診断サービス(高度な機器、試薬、ハイスループットシーケンシングなど)に伴う高コストは、特に農村部や資金不足の地域では、広く普及するための大きな障壁となっている。
- 政府の補助金を受けても、分子診断検査は通常、複雑なワークフロー、専門の人員、厳格な品質管理を必要とするため、予算が限られている医療システムでは利用しにくい。
- さらに、専門的な検査施設や訓練を受けた人員は都市部に集中していることが多く、遠隔地の患者は長距離の移動を強いられたり、検査や報告に遅れが生じたりしている。
- もう一つの課題は、特定の分子検査や遺伝子検査における標準化されたプロトコルの欠如です。限られた臨床経験と検査室の能力のばらつきが、医療提供者間での導入の一貫性を欠く一因となっています。
- これらの課題を克服するためには、政策改革、政府資金の増強、国境を越えた研究協力、そしてアジア太平洋地域全体にわたる専用の分子診断ハブの設立が、アジア太平洋の分子診断サービス市場へのアクセス拡大と持続可能な成長の達成に不可欠となる。
アジア太平洋地域の分子診断サービス市場の範囲
市場は、サービスタイプ、テクノロジー、エンドユーザーに基づいてセグメント化されています。
- サービスタイプ別
サービスタイプに基づいて、アジア太平洋の分子診断サービス市場は、機器修理サービス、トレーニングサービス、コンプライアンスサービス、校正サービス、保守サービス、スケーラブル自動化サービス、ターンキーサービス、機器移転サービス、ハードウェアカスタマイズ、性能保証サービス、設計開発サービス、サプライチェーンソリューション、新製品導入サービス、製造サービス、環境および規制サービス、医療管理システム認証および監査、臨床研究サービス、コンサルティングサービス、およびその他のサービスに分類されます。保守サービスは、分子診断機器の継続的かつ正確な機能を確保する上で重要な役割を果たすため、2024年には23.7%という最大の収益シェアで市場を支配しました。これらのサービスは、ダウンタイムを最小限に抑え、機器の寿命を延ばし、病院と研究室のネットワーク全体で一貫した診断精度を維持するために不可欠です。
臨床研究サービスは、トランスレーショナル・メディシン(TDM)研究とプレシジョン・メディシン(精密医療)研究への関心の高まりを背景に、2025年から2032年にかけて9.1%という最も高いCAGRを達成すると予測されています。この成長は、バイオマーカー探索、ゲノミクス、パーソナライズド・ヘルスケアへの投資増加、そして研究機関と診断サービスプロバイダー間の連携拡大によって支えられています。
- テクノロジー別
アジア太平洋地域の分子診断サービス市場は、技術に基づいてPCR、リアルタイムPCR、次世代シーケンシング(NGS)、その他の技術に分類されます。PCRベースのサービスは、感染症、遺伝子スクリーニング、日常的な診断アプリケーションにおける分子検査への幅広い導入により、2024年には36.5%のシェアで市場をリードしました。PCRは、その信頼性、手頃な価格、そして多様な臨床ワークフローへの適応性から、依然として基盤技術として重要な位置を占めています。
次世代シーケンシング(NGS)は、ハイスループットゲノム解析、精密腫瘍学、包括的な疾患プロファイリングへの需要の高まりを背景に、2025年から2032年にかけて14.2%という最も高いCAGRで成長すると予測されています。NGSにより、研究者や臨床医は大規模なゲノム研究をより高精度に実施できるようになり、早期診断、治療の層別化、個別化治療アプローチの支援が可能になります。
- エンドユーザー別
アジア太平洋地域の分子診断サービス市場は、エンドユーザーに基づいて、病院、診断センター、学術研究機関、その他に分類されます。病院は、患者数の増加、統合された検査インフラ、そして高度な分子診断プラットフォームの継続的な導入に支えられ、2024年には41.8%と最大のシェアを占めました。病院は、日常的な診断だけでなく、腫瘍学、感染症、遺伝カウンセリングなどの専門部門のサポートにもこれらのサービスを活用しています。
学術研究機関は、分子研究イニシアチブの増加、ゲノミクスおよび臨床研究への政府および民間からの資金提供、そしてトランスレーショナルリサーチのためのハイエンド診断技術の導入に牽引され、予測期間中に10.3%という最も高いCAGRで成長すると予想されています。これらの機関は、イノベーションを推進し、新しい分子診断ソリューションを商業化前に検証する上で重要な役割を果たします。
アジア太平洋地域の分子診断サービス市場地域分析
- アジア太平洋地域は、医療インフラの拡大、慢性疾患および感染症の罹患率の上昇、そして高度な分子診断技術の急速な導入に牽引され、2024年には世界の分子診断サービス市場において32.5%という最大の収益シェアを獲得しました。医療施設への投資、診断センターの増加、そして早期発見を促進する政府の取り組みが、市場の成長をさらに促進しています。
- 強力な規制枠組み、広範な保険適用範囲、そして患者の高い意識は、公的医療セクターと民間医療セクターの両方で成長を促進しています。診断プログラムへの政府資金の増加、官民パートナーシップ、そしてパンデミック後の医療イニシアチブと相まって、高度な分子診断サービスの導入が加速しています。
- さらに、アジア太平洋地域には、いくつかの主要な診断サービスプロバイダー、学術機関、研究開発センターがあり、アッセイ開発、臨床評価、次世代技術の統合における継続的な革新を促進しています。
中国アジア太平洋地域における分子診断サービス市場の洞察
中国の分子診断サービス市場は、人口基盤の巨大さ、感染症および遺伝性疾患の有病率の上昇、そして専門診断センターへのアクセス拡大を背景に、2024年にはアジア太平洋地域で最大の市場シェア(35.1%)を占めると予想されています。政府の医療制度改革、保険適用範囲の拡大、そして有利な償還政策は、国内外のサービスプロバイダーによる分子診断サービスの拡充を後押ししています。また、高度な診断ソリューションに対する需要の高まりに対応するため、中国企業も研究開発に多額の投資を行っています。
日本・アジア太平洋地域における分子診断サービス市場インサイト
日本の分子診断サービス市場は、高度に発達した医療インフラ、充実した保険適用範囲、そして技術的に高度な検査環境を背景に、2024年にはアジア太平洋地域の市場シェアの21.5%を占めました。次世代シーケンシング(NGS)、PCR検査、その他の分子診断技術の導入が、特に病院や研究センターにおいて増加しており、市場需要を押し上げています。日本は、疾患の早期発見と精密医療を重視しており、この地域における主導的地位を強化しています。
インド・アジア太平洋地域における分子診断サービス市場の洞察
インドの分子診断サービス市場は、ヘルスケア意識の高まり、診断サービスへのアクセス性の向上、そして可処分所得の増加を背景に、2025年から2032年にかけて年平均成長率(CAGR)13.6%を記録し、アジア太平洋地域で最も急速に成長する市場になると予測されています。疾病検出のための国家プログラム、第2・第3層都市における診断インフラの拡充、そして民間セクターの参入拡大が、このサービスの導入を加速させています。インドは費用対効果の高い分子診断の拠点としても台頭しており、地域における競争力を高めています。
アジア太平洋地域の分子診断サービス市場シェア
アジア太平洋地域の分子診断サービス業界は、主に以下のような定評のある企業によって牽引されています。
- F. ホフマン・ラ・ロシュ社(スイス)
- ダナハーコーポレーション(米国)
- ビオメリュー(フランス)
- QIAGEN NV(オランダ)
- サーモフィッシャーサイエンティフィック社(米国)
- バイオ・ラッド・ラボラトリーズ社(米国)
- アボット(米国)
- DiaSorin SpA(イタリア)
- ホロジック社(米国)
アジア太平洋地域の分子診断サービス市場の最新動向
- 2025年8月、中国の製薬研究開発企業は、コスト削減と納期短縮のため、上海タイタンサイエンティフィックや南京バザイムバイオテックといった国内サプライヤーから実験用試薬を調達する傾向が強まった。これは、米国との貿易摩擦が続く中で、輸入関税の上昇やサプライチェーンの信頼性に対する懸念に対応したものだった。以前はサーモフィッシャーやメルクといった欧米企業が市場を独占していた57億6000万米ドル規模の中国試薬市場では、現地企業への大きな動きが見られた。
- 2025年3月、キアゲンは、COVID-19パンデミック後の市場動向と顧客ニーズの変化により、NeuMoDx 96および288統合PCR検査システムの生産を中止すると発表しました。同社は、2024年の売上への影響を把握するために顧客との協議を開始し、既存の顧客に対しては2025年までサポートを提供する予定です。
- 2024年2月、プレックスバイオはメドラボドバイで先進的な肺がん検出技術を展示し、腫瘍学における分子診断能力の拡大への取り組みを強調した。
- 2024年1月、パーキンエルマー傘下であったレブビティは、分社化とブランド変更に伴い、研究、ソフトウェア、社内業務への投資を大幅に増加しました。同社はライフサイエンスおよび診断分野の強化に注力し、研究開発費の増額と、新たなeコマースプラットフォームやサプライチェーンの最適化を含む業務効率化への投資を目指しています。
SKU-
世界初のマーケットインテリジェンスクラウドに関するレポートにオンラインでアクセスする
- インタラクティブなデータ分析ダッシュボード
- 成長の可能性が高い機会のための企業分析ダッシュボード
- カスタマイズとクエリのためのリサーチアナリストアクセス
- インタラクティブなダッシュボードによる競合分析
- 最新ニュース、更新情報、トレンド分析
- 包括的な競合追跡のためのベンチマーク分析のパワーを活用
調査方法
データ収集と基準年分析は、大規模なサンプル サイズのデータ収集モジュールを使用して行われます。この段階では、さまざまなソースと戦略を通じて市場情報または関連データを取得します。過去に取得したすべてのデータを事前に調査および計画することも含まれます。また、さまざまな情報ソース間で見られる情報の不一致の調査も含まれます。市場データは、市場統計モデルと一貫性モデルを使用して分析および推定されます。また、市場シェア分析と主要トレンド分析は、市場レポートの主要な成功要因です。詳細については、アナリストへの電話をリクエストするか、お問い合わせをドロップダウンしてください。
DBMR 調査チームが使用する主要な調査方法は、データ マイニング、データ変数が市場に与える影響の分析、および一次 (業界の専門家) 検証を含むデータ三角測量です。データ モデルには、ベンダー ポジショニング グリッド、市場タイムライン分析、市場概要とガイド、企業ポジショニング グリッド、特許分析、価格分析、企業市場シェア分析、測定基準、グローバルと地域、ベンダー シェア分析が含まれます。調査方法について詳しくは、お問い合わせフォームから当社の業界専門家にご相談ください。
カスタマイズ可能
Data Bridge Market Research は、高度な形成的調査のリーダーです。当社は、既存および新規のお客様に、お客様の目標に合致し、それに適したデータと分析を提供することに誇りを持っています。レポートは、対象ブランドの価格動向分析、追加国の市場理解 (国のリストをお問い合わせください)、臨床試験結果データ、文献レビュー、リファービッシュ市場および製品ベース分析を含めるようにカスタマイズできます。対象競合他社の市場分析は、技術ベースの分析から市場ポートフォリオ戦略まで分析できます。必要な競合他社のデータを、必要な形式とデータ スタイルでいくつでも追加できます。当社のアナリスト チームは、粗い生の Excel ファイル ピボット テーブル (ファクト ブック) でデータを提供したり、レポートで利用可能なデータ セットからプレゼンテーションを作成するお手伝いをしたりすることもできます。