の 世界の子宮内避妊器具市場 子宮内避妊器具は著しい増加を見せており、安全で効果的な家族計画ソリューションで女性に力を与えています。これらの器具は、高い有効性と利便性を備えた長期避妊法を提供します。市場は、生殖に関する健康に関する意識の高まりと、信頼できる避妊法の必要性から恩恵を受けています。その結果、より多くの女性が、その可逆性と最小限の副作用から子宮内避妊器具を採用しています。支援的な規制枠組みと医療へのアクセスの拡大により、市場は世界中の女性の生殖に関する選択肢に明るい未来を約束します。
完全なレポートにアクセスする:https://www.databridgemarketresearch.com/reports/global-intra-uterine-contraceptive-devices-market
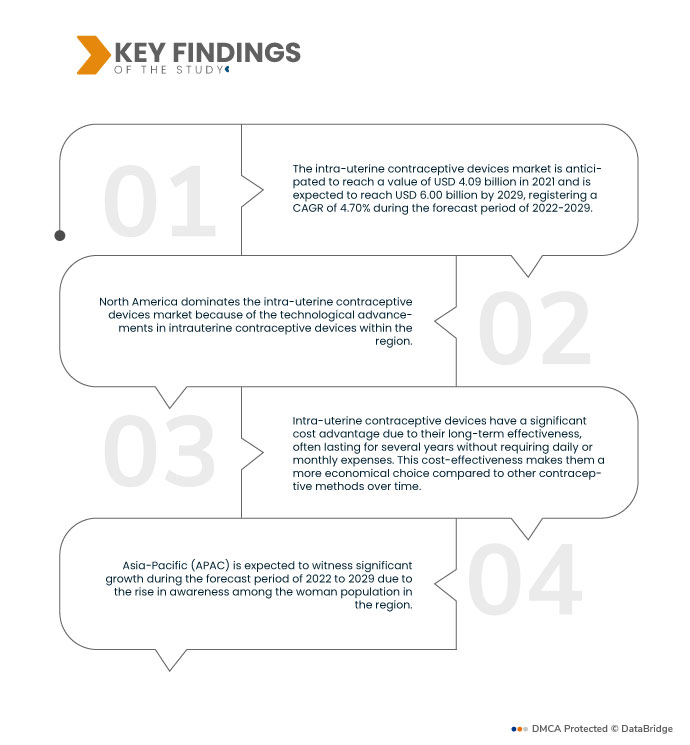
データブリッジマーケットリサーチの分析によると、子宮内避妊器具市場は2021年に40億9,000万米ドルに成長し、2029年には60億米ドルに達し、2022年から2029年の予測期間中に4.70%のCAGRを記録すると予想されています。非ホルモン性子宮内避妊器具は、ホルモンの副作用のない避妊を求める女性に魅力的であり、従来のホルモン法の代替手段を提供します。
研究の主な結果
女性の労働参加率の上昇が市場の成長率を押し上げると予想される
女性の労働参加率が高まるにつれ、女性はキャリアと家族計画を効果的に管理するために、便利で信頼できる避妊方法を求めています。子宮内避妊具は、毎日服用しなくても長期の避妊ができるため、これらの要件を満たしています。実用的で信頼できる避妊方法に対するニーズが高まると、働く女性の間で子宮内避妊具の需要が高まります。生殖に関する選択肢を維持しながら専門職としての成長に集中できる柔軟性を提供するこれらの器具は、市場で好まれる選択肢になります。
レポートの範囲と市場セグメンテーション
|
レポートメトリック
|
詳細
|
|
予測期間
|
2022年から2029年
|
|
基準年
|
2021
|
|
歴史的な年
|
2020 (2014 - 2019 にカスタマイズ可能)
|
|
定量単位
|
売上高(10億米ドル)、販売数量(個数)、価格(米ドル)
|
|
対象セグメント
|
タイプ(ホルモン性子宮内避妊器具、銅製子宮内避妊器具)、製品タイプ(ミレーナ、スカイラ、パラガード、エシュール、レボサート、その他)、エンドユーザー(病院、婦人科クリニック、地域医療センター、その他)
|
|
対象国
|
北米では米国、カナダ、メキシコ、ヨーロッパではドイツ、フランス、英国、オランダ、スイス、ベルギー、ロシア、イタリア、スペイン、トルコ、ヨーロッパではその他のヨーロッパ、中国、日本、インド、韓国、シンガポール、マレーシア、オーストラリア、タイ、インドネシア、フィリピン、アジア太平洋地域 (APAC) ではその他のアジア太平洋地域 (APAC)、中東およびアフリカ (MEA) の一部としてサウジアラビア、UAE、南アフリカ、エジプト、イスラエル、中東およびアフリカ (MEA) の一部としてその他の中東およびアフリカ (MEA)、南米の一部としてブラジル、アルゼンチン、南米のその他の地域
|
|
対象となる市場プレーヤー
|
テバ製薬工業株式会社(エルサレム)、バイエル(ドイツ)、ユーロジン(スペイン)、クーパーサージカル株式会社(米国)、ファイザー株式会社(米国)、HLLライフケア株式会社(ティルヴァナンタプラム)、オコンメディカル株式会社(イスラエル)、プロサンインターナショナル株式会社(オランダ)、メルベアイノベーションズ株式会社(ハンガリー)、アラガン・メルク株式会社(米国)、DKTインターナショナル株式会社(米国)、メディセーフディストリビューション株式会社(カナダ)、メディシンズ360株式会社(米国)、プレグナインターナショナル株式会社(インド)、エゲメンインターナショナル株式会社(トルコ)など
|
|
レポートで取り上げられているデータポイント
|
Data Bridge Market Research がまとめた市場レポートには、市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、専門家による詳細な分析、患者の疫学、パイプライン分析、価格分析、規制の枠組みも含まれています。
|
セグメント分析:
子宮内避妊器具市場は、タイプ、製品タイプ、エンドユーザーに基づいて分類されています。
- タイプに基づいて、子宮内避妊器具市場は、ホルモン子宮内避妊器具と銅子宮内避妊器具に分類されます。
- 製品タイプに基づいて、子宮内避妊器具市場は、ミレーナ、スカイラ、パラガード、エシュール、レボサートなどに分類されます。
- 子宮内避妊器具市場のエンドユーザーセグメントは、病院、婦人科クリニック、コミュニティヘルスケアセンター、その他に分類されます。
主要プレーヤー
データブリッジマーケットリサーチは、以下の企業を世界の主要な子宮内避妊器具メーカーとして認識しています。 世界の子宮内避妊器具市場の市場プレーヤーは、CooperSurgical Inc.(米国)、Pfizer Inc.(米国)、HLL Lifecare Limited(ティルヴァナンタプラム)、OCON Medical Ltd.(イスラエル)、Prosan International BV(オランダ)、Melbea Innovations(ハンガリー)、Allergan, Merck & Co., Inc.(米国)、DKT International(米国)、Medisafe Distribution Inc.(カナダ)、Medicines360(米国)です。
市場動向
- 2022年、ファイザー社は、片頭痛発作の急性治療と予防の両方に承認されている新しい片頭痛治療薬NURTEC ODTの開発会社であるバイオヘイブン・ファーマシューティカル・ホールディング・カンパニー社の買収を完了しました。
- 2022年にCytoReasonとファイザーの協力契約は拡大され、以下の条項が追加されました。 人工知能 医薬品の発見と開発のため。
- 2021年6月、Sebela Pharmaceuticals Inc.はPRA Health Sciencesと提携し、避妊の安全性、忍容性、有効性を評価するための長期作用型可逆性子宮内避妊システムであるLevoCeptの第3相臨床試験を実施すると発表しました。2021年6月に開始されたこの試験は、2027年6月に完了する予定です。
地域分析
地理的に見ると、世界の子宮内避妊器具市場レポートでカバーされている国は、北米では米国、カナダ、メキシコ、ヨーロッパではドイツ、フランス、英国、オランダ、スイス、ベルギー、ロシア、イタリア、スペイン、トルコ、その他のヨーロッパ諸国、中国、日本、インド、韓国、シンガポール、マレーシア、オーストラリア、タイ、インドネシア、フィリピン、アジア太平洋地域 (APAC) ではその他のアジア太平洋地域 (APAC)、サウジアラビア、UAE、南アフリカ、エジプト、イスラエル、中東およびアフリカ (MEA) の一部としてその他の中東およびアフリカ (MEA)、南米の一部としてブラジル、アルゼンチン、その他の南米です。
Data Bridge Market Research の分析によると:
北米は世界的に支配的な地域である 子宮内避妊器具市場 予測期間2022~2029年
子宮内避妊器具市場における北米の優位性は、この分野における技術の進歩に継続的に注力していることに起因します。この地域の強力な研究開発イニシアチブにより、ユーザーエクスペリエンスと有効性が向上した革新的で改良された子宮内避妊器具が導入されています。これらの進歩により、北米のメーカーは競争上の優位性を獲得し、国内外の顧客を引き付け、世界市場におけるこの地域のリーダー的地位に貢献しています。
アジア太平洋地域は、世界の子宮内避妊器具市場において最も急速に成長する地域であると推定されている。 予測期間2022-2029年
アジア太平洋地域では、子宮内避妊器具市場が大幅な成長を遂げると予想されています。この成長は、この地域の女性の間で生殖に関する健康と家族計画に対する意識が高まっていることに起因しています。避妊の選択肢に関する意識が広がり、教育が向上するにつれて、より多くの女性が子宮内避妊器具を信頼性が高く便利な避妊ソリューションと考えるようになり、アジア太平洋地域の市場拡大が促進されるでしょう。
子宮内避妊器具に関する詳しい情報については 市場 レポートはこちらをクリックしてください – https://www.databridgemarketresearch.com/reports/global-intra-uterine-contraceptive-devices-market