乳房再建市場にはさまざまな技術が組み込まれており、さまざまな外科的アプローチが重要な決定要因となります。乳房下組織の再建には乳房のひだを切開し、インプラントを埋入するための直接アクセスを提供します。乳輪周囲の再建では、乳輪の周囲を切開します。経腋窩再建では脇の下の切開を通して乳房にアクセスしますが、経臍再建ではへその近くの切開が必要です。各テクノロジーは患者の多様な好みや臨床上の考慮事項に応え、特定のニーズや望ましい結果に合わせた再建手術を求める乳がん生存者に選択肢を提供します。
完全なレポートにアクセス @ https://www.databridgemarketresearch.com/reports/europe-breast-reconstruction-market
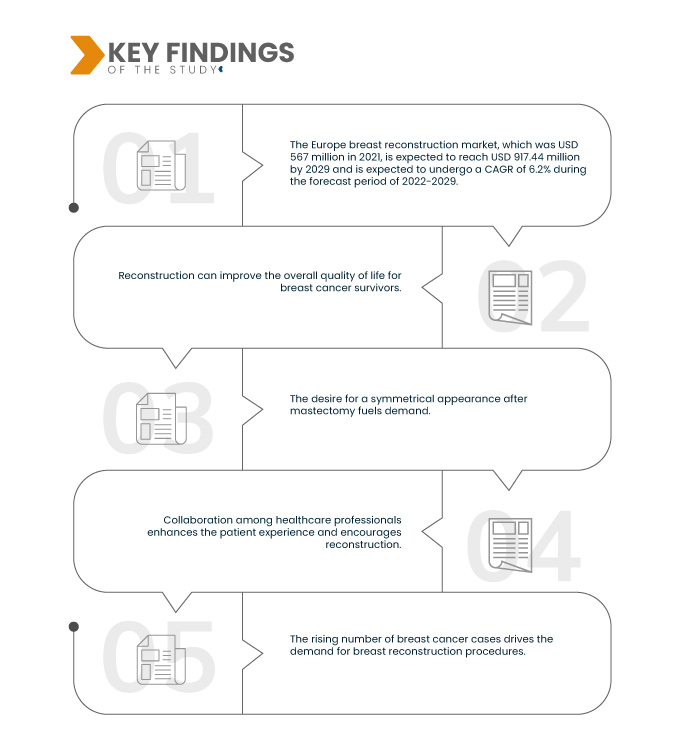
データブリッジ市場調査は次のように分析しています。 ヨーロッパの乳房再建市場、2021年には5億6,700万米ドルでしたが、2029年までに9億1,744万米ドルに達すると予想されており、2022年から2029年の予測期間中に6.2%のCAGRが予想されます。乳房再建手術に対する健康保険適用範囲の拡大により、これらの手術を受けやすくなりました。これにより、経済的な障壁がなくなり、より幅広い乳がん生存者が再建を選択できるようになり、生活の質と回復プロセスが向上します。
研究の主な結果
患者の擁護が市場の成長率を促進すると予想される
患者擁護団体は、乳がんによる乳房切除術を受けた個人にサポート、情報、リソースを提供することで、乳房再建市場で重要な役割を果たしています。これらのグループは、患者が乳房再建の選択肢を検討できるようにし、利用可能な手術、利点、選択の重要性について十分な情報を確実に得られるようにします。擁護団体は、コミュニティの感覚、精神的なサポート、実践的な指導を提供することで、個人が乳房再建に関して十分な情報に基づいた決定を下せるよう支援し、最終的には生活の質を向上させます。
レポートの範囲と市場セグメンテーション
|
レポート指標
|
詳細
|
|
予測期間
|
2022年から2029年
|
|
基準年
|
2021
|
|
歴史的な年
|
2020 (2014 ~ 2019 にカスタマイズ可能)
|
|
量的単位
|
収益(百万米ドル)、数量(単位)、価格(米ドル)
|
|
対象となるセグメント
|
製品 (豊胸手術、組織エキスパンダー、無細胞真皮マトリックス)、タイプ(片側、両側)、形状(円形インプラント、解剖学的インプラント)、技術(乳房下、乳輪周囲、経腋窩、経臍)、配置(デュアルプレーン挿入、腺下挿入、筋肉下挿入)、手順(即時手順、遅延手順、再修正手順)、エンドユーザー(病院、美容クリニック、 外来手術センター、その他)
|
|
対象国
|
ドイツ、フランス、イギリス、オランダ、スイス、ベルギー、ロシア、イタリア、スペイン、トルコ、その他のヨーロッパ。
|
|
対象となる市場関係者
|
POLYTECH Health & Aesthetics GmbH (ドイツ)、CEREPLAS (フランス)、Guangzhou Wanhe Plastic Materials Co. Ltd (中国)、ALLERGAN (アイルランド)、Johnson & Johnson Private Limited (米国)、GC Aesthetics (英国)、Laboratories Arion (フランス) 、Sientra Inc. (米国)、HANSBIOMED CO. LTD (韓国)、Sientra, Inc. (米国)、Deal Implant Incorporated (米国)、Integra LifeSciences (米国)、PMT Corporation (米国)、RTI Surgical (米国)、確立ラボ SA (米国)、シリメド (ブラジル)
|
|
レポートで取り上げるデータポイント
|
市場価値、成長率、セグメンテーション、地理的範囲、主要プレーヤーなどの市場シナリオに関する洞察に加えて、Data Bridge Market Researchが厳選した市場レポートには、専門家の詳細分析、患者疫学、パイプライン分析、価格分析、そして規制の枠組み。
|
セグメント分析:
ヨーロッパの乳房再建市場は、製品、種類、形状、技術、配置、手順、エンドユーザーに基づいて分割されています。
- 製品に基づいて、ヨーロッパの乳房再建市場は、乳房インプラント、 組織拡張器、および無細胞真皮マトリックス。
- タイプに基づいて、ヨーロッパの乳房再建市場は片側乳房再建と両側乳房再建市場に分類されます。
- 形状に基づいて、ヨーロッパの乳房再建市場は円形インプラントと解剖学的インプラントに分類されます。
- 技術に基づいて、ヨーロッパの乳房再建市場は乳房下、乳輪周囲、腋窩横断、臍帯横断に分類されます。
- ヨーロッパの乳房再建市場は、配置に基づいて、デュアルプレーン挿入、腺下挿入、および筋肉下挿入に分類されます。
- ヨーロッパの乳房再建市場は、手術手順に基づいて、即時手術、遅延手術、再手術に分類されます。
- エンドユーザーに基づいて、ヨーロッパの乳房再建市場は病院、美容クリニック、外来手術センターなどに分類されます。
主なプレーヤー
Data Bridge Market Researchは、ヨーロッパの乳房再建市場におけるプレーヤーとして次の企業を認識しています:POLYTECH Health & Aesthetics GmbH (ドイツ)、CEREPLAS (フランス)、Guangzhou Wanhe Plastic Materials Co. Ltd (中国)、ALLERGAN (アイルランド) 、Johnson & Johnson Private Limited (米国)、GC Aesthetics (英国)、Laboratories Arion (フランス)、Sientra Inc. (米国)。
市場の発展
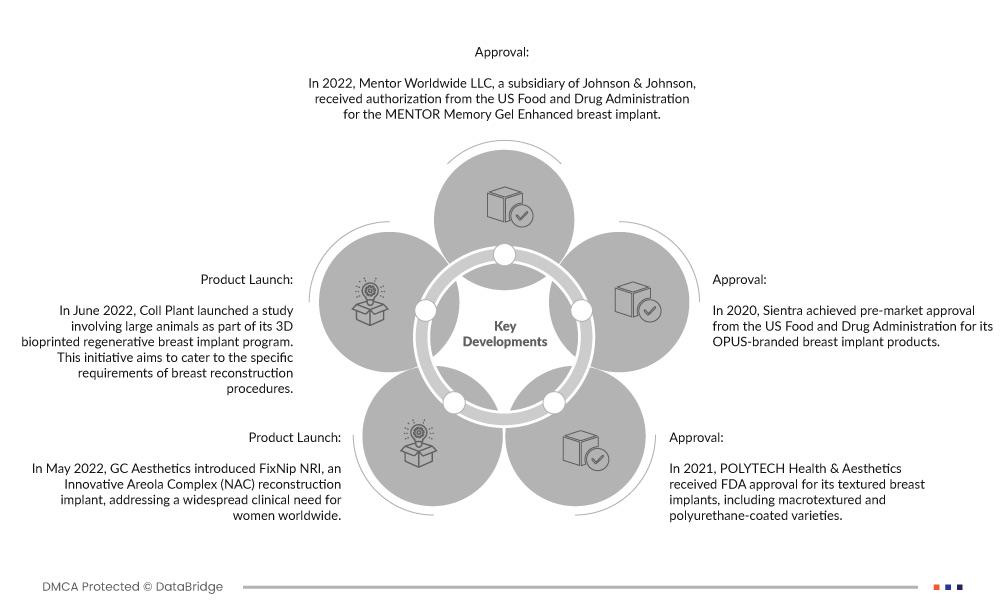
- 2022年、ジョンソン・エンド・ジョンソンの子会社であるメンター・ワールドワイドLLCは、米国食品医薬品局からメンター・メモリー・ジェル・エンハンスド乳房インプラントの認可を取得しました。この承認は、メンターの製品ポートフォリオに新たに追加されたことを意味し、豊胸手術患者に FDA 認可の革新的な選択肢を提供します。 Memory Gel Enhanced インプラントは、高度な乳房再建および乳房強化ソリューションを提供するという同社の取り組みを反映しています。
- 2022 年 6 月、Coll Plant は 3D バイオプリント再生乳房インプラント プログラムの一環として、大型動物を対象とした研究を開始しました。この取り組みは、乳房再建手術の特定の要件に応えることを目的としています。高度な 3D バイオプリンティング技術を活用することで、Coll Plant は、乳房再建の分野を変革する可能性のある革新的で再生的な乳房インプラント ソリューションの開発を目指しており、より自然でパーソナライズされたオプションを患者に提供します。
- 2022 年 5 月、GC Aesthetics は、革新的な乳輪複合体 (NAC) 再建インプラントである FixNip NRI を導入し、世界中の女性の広範な臨床ニーズに応えました。この医療機器は、乳がん生存者や乳房切除術後の乳頭・乳輪複合体再建を求める患者にソリューションを提供します。 GC Aesthetics は、専門的かつ革新的なインプラントを提供することで、多くの女性の生活の質と美的成果の向上に努めています。
- 2021年、POLYTECH Health & Aestheticsは、マクロテクスチャーやポリウレタンコーティングの種類を含むテクスチャード乳房インプラントのFDA承認を取得しました。この規制承認は重要なマイルストーンであり、同社はこれらのインプラントを米国市場で提供できるようになりました。自然な感触と審美的な結果で知られるテクスチャードインプラントは、豊胸手術や再建手術の選択肢を求めるより幅広い患者に利用可能になり、この分野での選択肢と結果が向上しています。
- 2020年、シエントラはOPUSブランドの乳房インプラント製品について米国食品医薬品局から市販前の承認を取得しました。この規制上のマイルストーンは、高品質で安全な乳房インプラントのオプションを米国市場に提供するという同社の取り組みを表しています。 FDAの承認により、シエントラのOPUSインプラントは豊胸と再建のための信頼できる選択肢となり、患者と外科医に審美的および修復的処置のための信頼できる革新的なソリューションを提供します。
ヨーロッパの乳房再建市場レポートの詳細については、ここをクリックしてください –https://www.databridgemarketresearch.com/reports/europe-breast-reconstruction-market