U.S. Acute Respiratory Distress Syndrome (ARDS) Market, By Cause (Coronavirus Disease 2019 (COVID-19), Sepsis, Inhalation Of Harmful Substances, Severe Pneumonia And Others), Type (Diagnosis And Treatment), Route Of Administration (Oral, Parenteral And Others), End User (Hospitals, Specialty Clinics, Home Healthcare And Others), Distribution Channel (Direct Tender, Hospital Pharmacy, Retail Pharmacy, Online Pharmacy And Others) - Industry Trends and Forecast to 2029.
U.S. Acute Respiratory Distress Syndrome (ARDS)Market Analysis and Insights
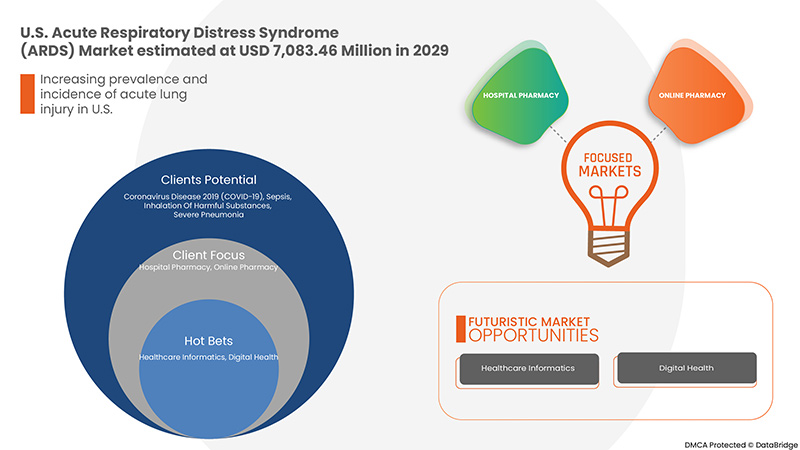
The U.S. acute respiratory distress syndrome (ARDS) market is expected to grow significantly in the forecast period of 2022 to 2029. Data Bridge Market Research analyses that the market is growing with a CAGR of 10.6% in the forecast period of 2022 to 2029 and is expected to reach USD 7,083.46 million by 2029. The major factor driving the growth of the market is the Increasing prevalence and incidence of acute lung injury, a wide range of risk factors for ARDS and acceleration in a patient pool of covid-19 with ARDS, the rising rate of air pollution and lifestyle-related diseases, and increasing accident rates and trauma causing ARDS.
Acute respiratory distress syndrome (ARDS) is a life-threatening lung injury that allows fluid to leak into the lungs. Most people who get ARDS are already hospitalized for trauma or illness like COVID-19. The syndrome usually occurs when fluids build up in the lungs' tiny, elastic air sacs called alveoli. This fluid build-up causes less oxygen reaches the bloodstream. This deprives the organs of getting enough oxygen for their normal function. People with other illness develops ARDS within a few hours to days after the precipitating injury or infection. The risk of death increases with age, and depending on the severity of the illness, patients surviving the syndrome becomes hard. Severe illness or injury causing damage to the membrane sacs of the lungs leads to ARDS. The most common underlying causes for the said diseases include sepsis, inhalation of harmful substances, severe pneumonia, head, chest, or another major injury, coronavirus disease 2019 (COVID-19), and others.
The U.S. acute respiratory distress syndrome (ARDS) market report provides details of market share, new developments, and the impact of domestic and localized market players, analyses opportunities in terms of emerging revenue pockets, changes in market regulations, products approvals, strategic decisions, product launches, geographic expansions, and technological innovations in the market. To understand the analysis and the market scenario, contact us for an Analyst Brief. Our team will help you create a revenue impact solution to achieve your desired goal.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Années historiques |
2020 (personnalisable de 2019 à 2014) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD |
|
Segments couverts |
Par cause (maladie à coronavirus 2019 (COVID-19), septicémie, inhalation de substances nocives, pneumonie grave et autres), type (diagnostic et traitement), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile et autres), canal de distribution (appel d'offres direct, pharmacie hospitalière, pharmacie de détail, pharmacie en ligne et autres) |
|
Pays couverts |
NOUS |
|
Acteurs du marché couverts |
Gilead Sciences, Inc., Terumo Medical Corporation, Getinge AB., Medtronic, LivaNova PLC, Fresenius SE & Co. KGaA, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Fisher & Paykel Healthcare Limited., Hamilton Medical, NIPRO, Pfizer Inc., ResMed, Smiths Medical, WEINMANN Emergency Medical Technology GmbH + Co. KG |
Définition du marché
La prévalence et l'incidence croissantes des lésions pulmonaires aiguës constituent un facteur important pour le marché américain du syndrome de détresse respiratoire aiguë (SDRA). Un large éventail de facteurs de risque pour le SDRA et l'accélération du nombre de patients atteints de COVID-19 atteints de SDRA accélèrent la croissance du marché. Les principales contraintes peuvent avoir un impact négatif sur le marché américain du syndrome de détresse respiratoire aiguë (SDRA). Complications associées aux traitements et coût élevé des appareils et des traitements. La population gériatrique croissante devrait offrir des opportunités de marché. Cependant, des règles et réglementations strictes devraient remettre en cause la croissance du marché américain du syndrome de détresse respiratoire aiguë (SDRA).
Dynamique du marché du syndrome de détresse respiratoire aiguë (SDRA) aux États-Unis
Conducteurs
- Augmentation de la prévalence et de l’incidence des lésions pulmonaires aiguës
Les patients souffrant de lésions pulmonaires aiguës sont largement signalés en raison de nombreux facteurs tels que le vieillissement de la population et le nombre croissant de patients atteints de septicémie et de pneumonie, entre autres. Cependant, la plupart des personnes ne reçoivent un diagnostic de lésions pulmonaires et de syndrome de détresse respiratoire aiguë qu'à un stade avancé. Par conséquent, l'incidence et la prévalence du SDRA continuent d'augmenter et la maladie est largement reconnue comme un problème clinique majeur dans le monde entier, entraînant une charge de morbidité et de mortalité élevée. Par conséquent, l'augmentation des taux de prévalence et d'incidence des lésions pulmonaires aiguës et du syndrome de détresse respiratoire aiguë qui l'accompagne stimule le marché du syndrome de détresse respiratoire aiguë (SDRA) aux États-Unis.
- Large éventail de facteurs de risque pour le SDRA
Un large éventail de facteurs de risque est signalé pour le syndrome de détresse respiratoire aiguë. Des facteurs de risque environnementaux et individuels sont impliqués dans le syndrome. Une certaine consommation chronique d'alcool et le tabagisme actif ou passif sont généralement associés. Par conséquent, l'augmentation de la consommation d'alcool quel que soit l'âge conduit à un SDRA de type syndrome. D'autres facteurs de risque comme la dyspnée, l'hypertension , le diabète et d'autres conduisent au développement du SDRA. Ainsi, ce large éventail de facteurs de risque associés au syndrome de détresse respiratoire aiguë augmente la possibilité de développer la condition clinique, augmentant simultanément les cas de la maladie. Ces facteurs de risque positifs devraient stimuler la croissance du marché.
- Accélération du bassin de patients atteints du Covid-19 et du syndrome de détresse respiratoire aiguë (SDRA)
Alors que le monde souffre énormément de l’infection au COVID-19, il existe également une corrélation directe entre le COVID-19 et le syndrome de détresse respiratoire aiguë. Les cas graves de la maladie COVID-19 finiront par entraîner un SDRA et une pneumonie. Il a été prouvé que cela est mortel pour les personnes infectées. Lorsque le virus responsable du COVID-19 pénètre dans l’organisme, il se fixe aux cellules des voies respiratoires supérieures. Cela déclenche une réponse immunitaire qui provoque une inflammation et entraîne des symptômes tels que la toux, le mal de gorge et la fièvre. Dans certains cas graves, le virus se déplace au-delà des voies respiratoires supérieures, traverse les poumons et se retrouve dans les alvéoles. Ainsi, le nombre croissant de cas de COVID-19 augmente également le risque de syndrome de détresse respiratoire aiguë chez les personnes, ce qui devrait servir de moteur à la croissance du marché du syndrome de détresse respiratoire aiguë.
- L’augmentation de la pollution de l’air et des maladies liées au mode de vie
L'exposition à long terme à une pollution atmosphérique faible à modérée est associée à un facteur de risque plus élevé de développer une infection pulmonaire, y compris le SDRA. Il s'agit d'un nouveau facteur de risque environnemental potentiellement modifiable pour le syndrome de détresse respiratoire aiguë. Selon l'Organisation mondiale de la santé, la pollution de l'air est l'un des plus grands risques environnementaux pour la santé en augmentant la charge de maladies telles que les maladies cardiaques et les infections pulmonaires et respiratoires à des niveaux aigus et chroniques. Par conséquent, l'augmentation du taux de pollution de l'air dans les pays développés et en développement augmente les risques de développer des syndromes tels que le syndrome de détresse respiratoire aiguë. Ainsi, ce facteur devrait stimuler la croissance du marché.
- Augmentation des taux d'accidents et de traumatismes provoquant le syndrome de détresse respiratoire aiguë (SDRA)
Les traumatismes majeurs causés par des accidents sont un facteur de risque bien connu pour le développement du SDRA. Le syndrome de détresse respiratoire aiguë peut résulter d'une blessure corporelle par inflammation du pancréas, inhalation du contenu de l'estomac dans les poumons, transfusion sanguine, incendie domestique et inhalation de fumée, infection grave, noyade et réaction grave aux médicaments. Même les accidents de voiture sont une cause de SDRA. Par conséquent, l'inhalation de fumée libère certains matériaux comme la fibrine, les neutrophiles, le mucus et les débris de cellules épithéliales qui obstruent la lumière des voies respiratoires, provoquant des changements dans la ventilation. Cette condition conduit à une hypoxémie qui est l'une des principales causes du SDRA.
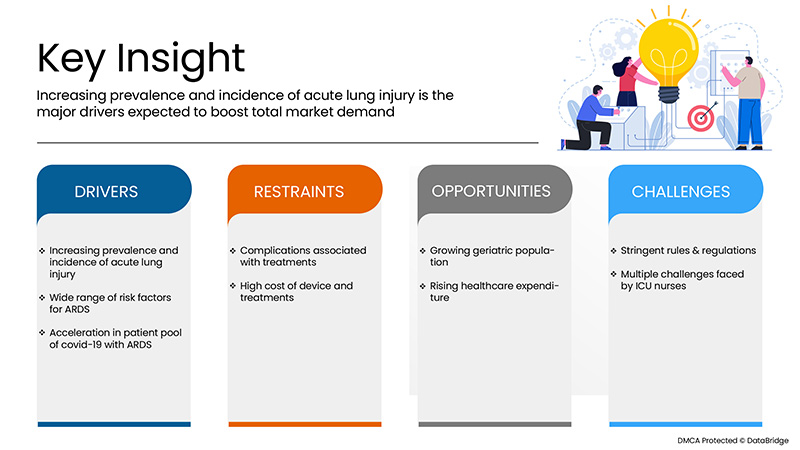
Opportunités
- Croissance de la population gériatrique
La population gériatrique mondiale augmente à un rythme rapide. Les personnes âgées sont devenues une population de plus en plus répandue d'admissions en soins intensifs. Selon un article du National Center for Biotechnology Information (NCBI), le taux de mortalité associé au syndrome de détresse respiratoire aiguë (SDRA) a été signalé de 69 à 80 % parmi la population âgée, ce qui rend le risque plus élevé pour les personnes âgées. Ainsi, le rythme de croissance de la population âgée démontre l'escalade de la demande de traitements contre le syndrome de détresse respiratoire aiguë, car les personnes âgées sont plus vulnérables aux maladies respiratoires graves en raison de leur système immunitaire faible. On prévoit que la population gériatrique croissante, qui devrait augmenter dans les années à venir dans le monde entier, crée une opportunité de croissance du marché au cours de la période de prévision.
- Augmentation des dépenses de santé
Les dépenses de santé ont augmenté dans le monde entier en raison de l'augmentation du revenu disponible des citoyens dans divers pays. De plus, pour répondre aux besoins de la population, les organismes gouvernementaux et les organisations de santé prennent des initiatives en accélérant les dépenses de santé. L'augmentation des dépenses de santé aide simultanément les établissements de santé à améliorer leurs installations de traitement du syndrome de détresse respiratoire aiguë, car ce trouble est très répandu ces dernières années. L'augmentation des dépenses de santé est également bénéfique pour la croissance future de l'économie et du secteur de la santé. Elle est principalement fructueuse car elle affecte de manière significative le développement d'options thérapeutiques meilleures et avancées avec des ventilateurs et d'autres appareils de traitement du SDRA.
- Initiatives stratégiques des acteurs du marché
Avec l'augmentation des taux de maladies infectieuses telles que la COVID-19 et de maladies chroniques comme le cancer du poumon, le fardeau simultané du syndrome de type SDRA est largement observé à l'échelle mondiale. L'augmentation spectaculaire de la qualité de la recherche et l'augmentation des opportunités de recherche sont dues aux diverses initiatives stratégiques prises par ces acteurs. Ils prennent des initiatives telles que des lancements de produits, des collaborations, des fusions, des acquisitions et bien d'autres au fil des ans et devraient être à l'avant-garde et créer davantage d'opportunités sur le marché. Ces lancements de produits stratégiques, ces acquisitions et ces fusions réalisés par de grandes entreprises sur le marché du syndrome de détresse respiratoire aiguë ont ouvert des opportunités pour les entreprises de diverses régions. Cette stratégie permet aux entreprises de renforcer leur présence sur le marché.
- Améliorer la sensibilisation au syndrome de détresse respiratoire aiguë (SDRA)
Étant donné que le syndrome de détresse respiratoire aiguë a de multiples causes différentes, il est généralement ignoré parmi les causes courantes de décès. La nécessité d'un traitement technique avancé et d'une bonne connaissance de la maladie peut réduire considérablement l'incidence du SDRA. Comme un diagnostic et une prévention rapides sont essentiels pour prévenir ou guérir plus rapidement, l'attention du public est primordiale. Le gouvernement et les organisations actuels ont élargi le champ de recherche sur les lésions pulmonaires pour inclure la prévention primaire du SDRA et réduire le taux de morbidité ou de mortalité du syndrome. Ainsi, une sensibilisation accrue au SDRA grâce au soutien de diverses associations améliore les chances de croissance du marché américain du syndrome de détresse respiratoire aiguë à l'avenir.
Contraintes/Défis
- Complications liées aux traitements
Le syndrome de détresse respiratoire aiguë est une affection clinique qui nécessite un traitement et des soins appropriés et opportuns pour prévenir les taux de mortalité et de morbidité. Cependant, pendant le traitement du SDRA, les patients peuvent être confrontés à de nombreuses complications dans les hôpitaux. Bien qu'il existe des traitements pour le SDRA qui aident les personnes à survivre, les complications associées pendant et après le traitement restent un fardeau pour les patients. La plupart des effets potentiels et durables comprennent des problèmes respiratoires, une dépression, des problèmes de mémoire et de réflexion, de la fatigue et une faiblesse musculaire, etc. Ces complications associées au syndrome de détresse respiratoire aiguë et au traitement ultérieur devraient entraver la croissance du marché au cours de notre période de prévision.
- Coût élevé des appareils et des traitements
Bien que le syndrome de détresse respiratoire aiguë bénéficie d'un large éventail d'options de traitement avancées, le coût d'un traitement plus long est assez difficile à supporter pour les personnes à revenu moyen. Le recours aux services de soins intensifs et de réanimation augmente dans le monde entier, et son coût élevé constitue une préoccupation majeure dans le système de santé actuel. Les patients atteints de SDRA doivent généralement subir de longues hospitalisations avec une monétarisation et une utilisation fréquentes de la ventilation, ce qui consomme une quantité importante de ressources de santé. Par conséquent, le coût élevé du traitement et des respirateurs est très difficile à payer pour un remède fiable à long terme jusqu'à ce que le patient se rétablisse complètement. Cela devrait freiner la croissance du marché.
- Manque de main d'oeuvre qualifiée
La crise soudaine du COVID-19 a créé divers défis pour les organisations de soins de santé, notamment une pénurie de main-d'œuvre. La pandémie a frappé massivement les pays développés comme les États-Unis. Ils ont mis en place de multiples moyens pour surmonter ces défis, mais des articles de presse ont rapporté que les pays étaient confrontés à des pénuries de personnel. Il y avait déjà des pénuries d'infirmières avant le début de la pandémie aux États-Unis, car la région compte une forte population souffrant de troubles respiratoires. Par conséquent, le manque d'infirmières correctement qualifiées et formées dans les unités de soins intensifs constituera une contrainte plus remarquable qui devrait entraver la croissance du marché du syndrome de détresse respiratoire aiguë (SDRA).
- Des règles et réglementations strictes
L'utilisation de ventilateurs dans le monde entier augmente rapidement, avec la croissance de la population âgée et plusieurs maladies pulmonaires aiguës, y compris le syndrome de détresse respiratoire aiguë, qui peut être évité par un diagnostic précoce et des traitements rapides. Dans le même temps, les acteurs du marché des fabricants de produits contre le syndrome de détresse respiratoire aiguë doivent suivre des réglementations spécifiques pour obtenir l'approbation des autorités supérieures pour lancer le produit comme les ventilateurs mécaniques ou les humidificateurs. Ces directives strictes doivent être respectées, et c'est l'une des tâches les plus difficiles parmi toutes les étapes. Par exemple, la Food and Drug Administration (FDA) des États-Unis réglemente les produits contre les maladies respiratoires aux États-Unis. Par conséquent, les règles et réglementations strictes pour l'approbation des produits remettent en cause la croissance du marché.
- Les multiples défis auxquels sont confrontées les infirmières des unités de soins intensifs
Les unités de soins intensifs doivent faire face à de nombreux défis pendant la pandémie en raison d'un bassin de patients important et de ressources hospitalières réduites. Depuis la première vague de COVID-19, les hôpitaux ont augmenté la capacité et le potentiel des unités de soins intensifs, ce qui a entraîné une augmentation des heures de travail des prestataires de soins de santé en USI. Les infirmières qui travaillent en USI ont toujours peur en raison de multiples raisons pour lesquelles elles doivent s'occuper de patients infectés. Par conséquent, le défi le plus important est celui des difficultés rencontrées par les infirmières et les autres prestataires de soins de santé qui traitent le syndrome de détresse respiratoire aiguë et le syndrome respiratoire aigu sévère. Les organisations tentent de les surmonter avec de nouvelles idées innovantes et en engageant davantage de personnel.
Développement récent
- En juillet 2020, Gilead Sciences, Inc. a lancé des essais cliniques sur une solution inhalée de remdesivir pour le traitement potentiel en ambulatoire de la COVID-19. Ces essais ont permis à l'entreprise d'améliorer son portefeuille de produits antiviraux, renforçant ainsi sa présence sur le marché
Portée du marché américain du syndrome de détresse respiratoire aiguë (SDRA)
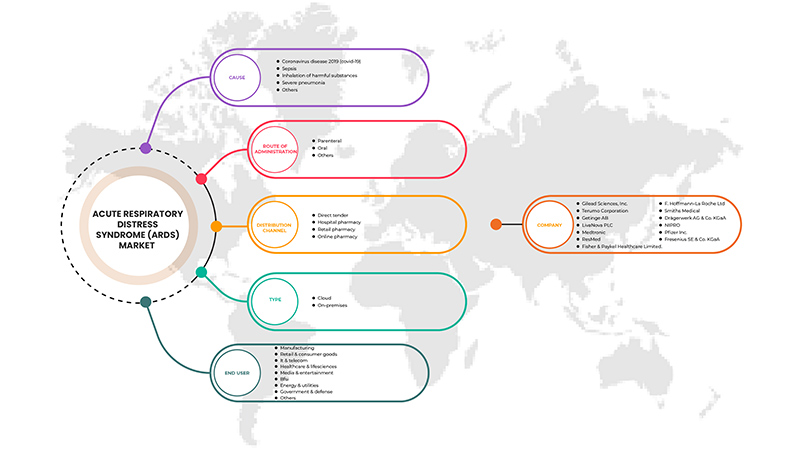
Le marché américain du syndrome de détresse respiratoire aiguë (SDRA) est classé en fonction de la cause, du type, de la voie d'administration, de l'utilisateur final et du canal de distribution. La croissance de ces segments vous aidera à analyser les principaux segments de croissance des industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour prendre des décisions stratégiques afin d'identifier les principales applications du marché.
Cause
- Maladie à coronavirus 2019 (COVID-19)
- État septique
- Inhalation de substances nocives
- Pneumonie grave
- Autres
En fonction de la cause, le marché américain du syndrome de détresse respiratoire aiguë (SDRA) est classé en maladie à coronavirus 2019 (COVID-19), septicémie, inhalation de substances nocives, pneumonie sévère et autres.
Taper
- Diagnostic
- Traitement
En fonction du type, le marché américain du syndrome de détresse respiratoire aiguë (SDRA) est classé en diagnostic et traitement.
Voie d'administration
- Oral
- Parentérale
- Autres
En fonction de la voie d'administration, le marché américain du syndrome de détresse respiratoire aiguë (SDRA) est classé en voie orale, parentérale et autres.
Utilisateur final
- Hôpitaux
- Cliniques spécialisées
- Soins à domicile
- Autres
En fonction de l'utilisateur final, le marché américain du syndrome de détresse respiratoire aiguë (SDRA) est classé en hôpitaux, cliniques spécialisées, soins de santé à domicile et autres.
Canal de distribution
- Appel d'offres direct
- Pharmacie de l'hôpital
- Pharmacie de détail
- Pharmacie en ligne
- Autres
En fonction du canal de distribution, le marché américain du syndrome de détresse respiratoire aiguë (SDRA) est classé en appel d'offres direct, pharmacie hospitalière, pharmacie de détail, pharmacie en ligne et autres.
Analyse du paysage concurrentiel et des parts de marché du syndrome de détresse respiratoire aiguë (SDRA) aux États-Unis
Le paysage concurrentiel du marché américain du syndrome de détresse respiratoire aiguë (SDRA) fournit des détails sur les concurrents. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, les sites et installations de production, les forces et les faiblesses de l'entreprise, le lancement de produits, les pipelines d'essais de produits, les approbations de produits, les brevets, la largeur et l'étendue du produit, la domination des applications, la courbe de survie technologique. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises liée au marché américain du syndrome de détresse respiratoire aiguë (SDRA).
Français Certains des principaux acteurs opérant sur le marché américain du syndrome de détresse respiratoire aiguë (SDRA) sont Gilead Sciences, Inc., Terumo Medical Corporation, Getinge AB., Medtronic, LivaNova PLC, Fresenius SE & Co. KGaA, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Fisher & Paykel Healthcare Limited., Hamilton Medical, NIPRO, Pfizer Inc., ResMed, Smiths Medical, WEINMANN Emergency Medical Technology GmbH + Co. KG.
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. Les données du marché sont analysées et estimées à l'aide de modèles statistiques et cohérents du marché. En outre, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. La principale méthodologie de recherche utilisée par l'équipe de recherche DBMR est la triangulation des données, qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). En dehors de cela, les modèles de données comprennent les grilles de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, les grilles de positionnement des entreprises, l'analyse des parts de marché des entreprises, les normes de mesure, les États-Unis par rapport aux régions et l'analyse des parts des fournisseurs. Veuillez demander un appel d'analyste en cas de demande de renseignements supplémentaires.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 PIPELINE ANALYSIS
5 INSURANCE REIMBURSEMENT
5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
5.3 ABBOTT CODING GUIDE FOR ECMO
5.4 CERN HEALTH INSURANCE SCHEME
5.5 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
5.6 AMERICAN HOSPITAL ASSOCIATION
5.7 CONCLUSION
6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
6.1 REGULATION IN U.S.
6.2 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
6.3 LABELING OF MODIFIED DEVICES
7 COUNTRY SUMMERY
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
8.1.2 WIDE RANGE OF RISK FACTORS FOR ARDS
8.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
8.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
8.1.5 INCREASING ACCIDENT RATES AND TRAUMA CAUSING ARDS
8.2 RESTRAINTS
8.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
8.2.2 HIGH COST OF DEVICE AND TREATMENTS
8.2.3 LACK OF SKILLED WORKFORCE
8.3 OPPORTUNITIES
8.3.1 GROWING GERIATRIC POPULATION
8.3.2 RISING HEALTHCARE EXPENDITURE
8.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
8.3.4 IMPROVING AWARENESS REGARDING ARD SYNDROME
8.4 CHALLENGES
8.4.1 STRINGENT RULES & REGULATIONS
8.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
9 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
9.1 OVERVIEW
9.2 CORONAVIRUS DISEASE 2019 (COVID-19)
9.3 SEPSIS
9.4 INHALATION OF HARMFUL SUBSTANCES
9.5 SEVERE PNEUMONIA
9.6 OTHERS
10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
10.1 OVERVIEW
10.2 DIAGNOSIS
10.2.1 IMAGING TESTS
10.2.1.1 CHEST X-RAY
10.2.1.2 CT SCAN
10.2.1.3 ULTRASOUND
10.2.1.4 OTHERS
10.2.2 BLOOD TEST
10.2.3 RESPIRATORY RATE
10.2.4 SPO2 TEST
10.2.5 OTHERS
10.3 TREATMENT
10.3.1 MECHANICAL VENTILATION
10.3.1.1 HIGH-FLOW NASAL O2
10.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
10.3.1.3 CONTINOUS POSITIVE AIRWAY PRESSURE
10.3.1.4 PRONE POSITIVE VENTILATION
10.3.1.5 OTHERS
10.3.2 CORTICOSTEROIDS
10.3.2.1 METHYLPREDNISOLONE
10.3.2.2 DEXAMETHASONE
10.3.2.3 OTHERS
10.3.3 ANTIVIRAL MEDICATION
10.3.3.1 REMDESIVIR
10.3.3.2 COMBINATION DRUGS
10.3.3.3 OTHERS
10.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
10.3.5 TOCILIZUMAB
10.3.6 OTHERS
11 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
11.1 OVERVIEW
11.2 PARENTERAL
11.2.1 INTRAVENOUS
11.2.2 INTRAMUSCULAR
11.3 ORAL
11.4 OTHERS
12 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITALS
12.3 SPECIALTY CLINICS
12.4 HOME HEALTHCARE
12.5 OTHERS
13 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 HOSPITAL PHARMACY
13.4 RETAIL PHARMACY
13.5 ONLINE PHARMACY
14 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: U.S
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 GILEAD SCIENCES INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUS ANALYSIS
16.1.3 PRODUCT PORTFOLIO
16.1.4 RECENT DEVELOPMENT
16.2 TERUMO CORPORATION
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUS ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENT
16.3 GETINGE AB
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUS ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 LIVANOVA PLC
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 MEDTRONIC
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 PRODUCT PORTFOLIO
16.5.4 RECENT DEVELOPMENTS
16.6 DRÄGERWERK AG & CO. KGAA
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 F. HOFFMANN-LA ROCHE LTD
16.7.1 COMPANY SNAPSHOT
16.7.2 RECENT ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 FISHER & PAYKEL HEALTHCARE LIMITED
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 FRESENIUS SE & CO. KGAA
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUS ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 HAMILTON MEDICAL
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 NIPRO
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUS ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 PFIZER INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUS ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENT
16.13 RESMED
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 SMITHS MEDICAL
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUS ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 2 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2020-2029 (USD MILLION)
TABLE 3 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 4 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 5 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 6 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 7 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 11 U.S. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 12 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 13 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COUNTRY MARKET ANALYSIS
FIGURE 5 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2022 & 2029
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 14 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2021
FIGURE 15 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2022-2029 (USD MILLION)
FIGURE 16 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2022-2029)
FIGURE 17 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 18 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2021
FIGURE 19 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 20 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 21 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2021
FIGURE 27 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 28 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2022-2029)
FIGURE 29 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DISTRIBUTION CHANNEL, 2021
FIGURE 31 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 32 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 33 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.













