North America Syndromic Multiplex Diagnostic Market - By Product and Services (Reagents & Consumables, Instruments, Software & Accessories and Services), Infection Type (Viral, Bacterial, Parasites and Fungal), Disease (Respiratory Infections, Gastroenteritis, Sexually Transmitted Infections, Sepsis, Meningitis and Others), Panels Type (Respiratory Panel, GI-Enteric Panel, Sexually Transmitted Disease Panel, Blood-Sepsis Panel, Meningitis Panel and Others), End User (Clinical Laboratories, Hospitals, Pharmaceutical & Biotechnology Companies, Research Institutes and Others) Industry Trends and Forecast to 2029.
Market Definition and Insights
Syndromic multiplex diagnostic is a type of advanced diagnostic test utilised to detect infectious diseases such as respiratory infection, infective gastroenteritis, sexually transmitted infections, sepsis, and meningitis, among other types of infectious diseases. The syndromic multiplex diagnostic also helps the clinicians or hospitals to detect the symptoms and signs of the various types of diseases. This lets the health care providers provide the right treatment for the patients and offer more precise outcomes and care that can be performed more quickly.
Syndromic multiplex testing is used to diagnose many pathogens simultaneously. In the syndromic multiplex diagnostic, various types of reagents & consumables and instruments & accessories are used, which helps maintain accuracy and provide fast diagnosis results. These multiplex tests are rapidly diagnosed with certain infections, allowing clinical management decisions to be made promptly. The tests based on multiplex technology are known as test panels. The panels used in syndromic testing are designed to diagnose multiple diseases associated with the same or similar syndrome type. These panels help evaluate the cause of the disease at the point of care. Gastrointestinal panels and respiratory panels are the types of syndromic panels.
Syndromic multiplex testing utilizes the advanced technology of multiplex PCR which provides accurate and fast diagnostic results with the help of the multiple panels used in syndromic multiplex diagnostic to provide diagnostic results within an hour. The new generations of syndromic multiplex can rapidly identify the common type of pathogens in the respiratory specimens, blood and cerebrospinal. The use of multiplex panels is associated with quicker turnaround time, reduction of other unnecessary laboratory tests, faster diagnosis and targeted treatment.
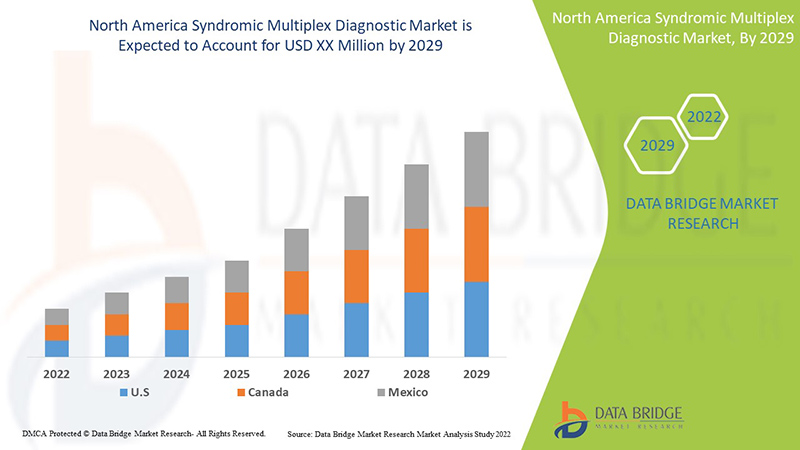
Data Bridge Market Research analyses that the North America syndromic multiplex diagnostic market will grow at a CAGR of 9.0% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Période de prévision |
2022 à 2029 |
|
Année de base |
2021 |
|
Années historiques |
2020 |
|
Unités quantitatives |
Chiffre d'affaires en millions USD, volumes en unités, prix en USD |
|
Segments couverts |
Produits et services (réactifs et consommables, instruments, logiciels et accessoires et services), type d'infection (virale, bactérienne, parasitaire et fongique), maladie (infections respiratoires, gastro-entérite, infections sexuellement transmissibles, septicémie, méningite et autres), type de panel (panel respiratoire, panel gastro-intestinal, panel de maladies sexuellement transmissibles, panel de septicémie sanguine , panel de méningite et autres), utilisateur final (laboratoires cliniques, hôpitaux, sociétés pharmaceutiques et biotechnologiques, instituts de recherche et autres) |
|
Pays couverts |
États-Unis, Canada et Mexique |
|
Acteurs du marché couverts |
BioFire Diagnostics (une filiale de bioMérieux SA), Seegene Inc. (Corée du Sud), Luminex Corporation. Une société DiaSorin (États-Unis), F. Hoffmann-La Roche Ltd (Suisse), BD (États-Unis), Bio-Rad Laboratories, Inc. (États-Unis), Cepheid (une filiale de Danaher (États-Unis)), QIAGEN (Allemagne), Abbott (États-Unis), Hologic, Inc. (États-Unis), Thermo Fisher Scientific Inc. (États-Unis), Siemens Healthcare GmbH (Allemagne), Akonni Biosystems, Inc. (États-Unis), Biocartis (Belgique), QuantuMDx Group Ltd. (Royaume-Uni), Applied BioCode, Inc. (États-Unis), Prominex Inc. (États-Unis), Nanomix, Inc. (États-Unis), Curetis (une filiale d'OpGen, Inc.) (Allemagne) |
Dynamique du marché du diagnostic multiplex syndromique
Conducteurs
- Augmentation de la prévalence des maladies infectieuses
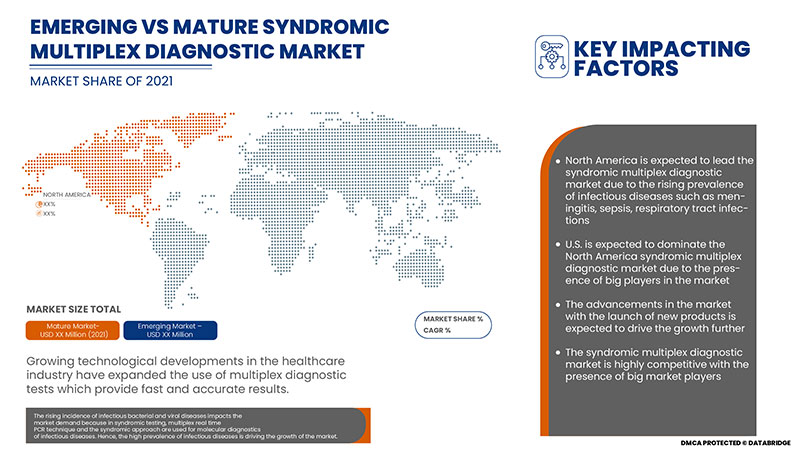
L'incidence croissante des maladies infectieuses bactériennes et virales a un impact sur la demande du marché car, dans les tests syndromiques, la technique de PCR multiplex en temps réel et l'approche syndromique sont utilisées pour le diagnostic moléculaire des maladies infectieuses.
- Augmentation de l'approbation réglementaire pour les tests de dépistage du coronavirus du syndrome respiratoire aigu
En mai 2020, biomérieux SA a reçu l'autorisation d'utilisation d'urgence (EUA) de la FDA pour le panel BIOFIRE RP2.1, qui est utilisé pour la détection de 22 agents pathogènes responsables d'infections respiratoires, dont le SARS-CoV-2 pour la maladie COVID-19.
Les autorités réglementaires, telles que la FDA ou le marquage CE, accordent une autorisation d'utilisation d'urgence (EUA) pour la commercialisation de panels SARS-CoV-2 et de tests de détection de virus associés à la maladie COVID-19, ce qui constitue un moteur de croissance du marché.
Opportunités
- Initiatives stratégiques prises par les acteurs du marché
En mars 2021, F. Hoffman-La Roche Ltd a acquis GenMark Diagnostics, un important fournisseur de diagnostics moléculaires multiplexés. Cette acquisition a permis à l'entreprise d'élargir le portefeuille de diagnostics moléculaires de Roche. Ces initiatives stratégiques prises par les acteurs du marché, notamment le lancement de produits ciblés sur certains segments, les aident à étendre leur portée mondiale et à améliorer leur portefeuille de produits, tout en constituant une opportunité pour la croissance du marché.
- Introduction de produits technologiquement avancés
Le test multiplex syndromique utilise la technologie avancée de la PCR multiplex pour détecter, isoler ou amplifier l'acide nucléique ciblé afin de fournir des résultats de diagnostic précis et rapides. La technologie avancée fournit un résultat de diagnostic dans un délai de 45 à 60 minutes. Par conséquent, le développement de produits technologiquement avancés constitue une opportunité pour la croissance du marché.
Retenue/Défi
- Coût élevé des produits de diagnostic
Les applications de tests syndromiques multiplexés utilisent la réaction en chaîne par polymérase (PCR) en temps réel, qui fournit des résultats avec des courbes d'amplification et des valeurs correctes. Les instruments utilisés dans le diagnostic syndromique multiplexé nécessitent des coûts de maintenance élevés. Le coût élevé des instruments constitue donc un défi pour le marché.
Impact post-COVID-19 sur le marché du diagnostic multiplex syndromique
La COVID-19 a eu un impact positif sur le marché. Les acteurs du marché lancent différents produits pour la détection du virus SARS-CoV. On constate une augmentation de l'approbation des produits par les organismes de réglementation après la COVID-19, ce qui entraîne une augmentation de la croissance du marché.
Développement récent
- En mars 2021, Luminex Corporation, une société du groupe Diasorin, a reçu l'autorisation d'utilisation d'urgence de la FDA et le marquage CE pour le test étendu du panel respiratoire NxTAG. Cette approbation a permis à l'entreprise d'augmenter ses revenus.
Portée du marché du diagnostic multiplex syndromique
Le marché du diagnostic multiplex syndromique est divisé en cinq segments : produits et services, type d'infection, maladie, type de panel et utilisateur final. La croissance parmi ces segments vous aidera à analyser les segments de croissance faibles dans les industries et fournira aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour les aider à prendre des décisions stratégiques pour identifier les principales applications du marché.
Produits et services
- Réactifs et consommables
- Instruments, logiciels et accessoires
- Services
Sur la base des produits et services, le marché nord-américain du diagnostic multiplex syndromique est segmenté en réactifs et consommables, instruments, logiciels et accessoires et services.
Type d'infection
- Viral
- Bactérien
- Parasites
- Fongique
En fonction du type d’infection, le marché nord-américain du diagnostic multiplex syndromique est segmenté en virus, bactéries, parasites et champignons.
Maladie
- Infection respiratoire
- Gastro-entérite
- Infections sexuellement transmissibles
- État septique
- Méningite
- Autres
En fonction de la maladie, le marché nord-américain du diagnostic multiplex syndromique est segmenté en infections respiratoires, gastro-entérite, infections sexuellement transmissibles, méningite septique et autres.
Type de panneaux
- Panel respiratoire
- Panel gastro-intestinal
- Groupe d'experts sur les maladies sexuellement transmissibles
- Panel sur la septicémie sanguine
- Panel sur la méningite
- Autres
En fonction du type de panel, le marché nord-américain du diagnostic multiplex syndromique est segmenté en panel respiratoire, panel gastro-intestinal, panel de maladies sexuellement transmissibles, panel de septicémie sanguine, panel de méningite et autres.
Utilisateur final
- Hôpitaux
- Laboratoires cliniques
- Sociétés pharmaceutiques et biotechnologiques
- Instituts de recherche
- Autres
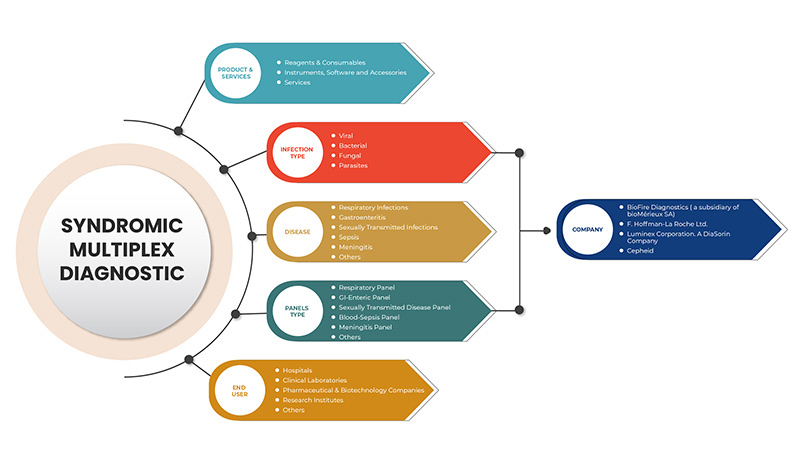
En fonction de l’utilisateur final, le marché nord-américain du diagnostic multiplex syndromique est segmenté en laboratoires cliniques, hôpitaux, sociétés pharmaceutiques et biotechnologiques, instituts de recherche et autres.
Analyse/perspectives régionales du marché du diagnostic multiplex syndromique
Le marché du diagnostic multiplex syndromique est analysé et des informations et tendances sur la taille du marché sont fournies par pays, produit et services, type d’infection, maladie, type de panels et utilisateur final comme référencé ci-dessus.
Les pays couverts par le rapport sont les États-Unis, le Canada et le Mexique.
Le marché américain du diagnostic syndromique multiplex devrait croître en raison de l’augmentation de la prévalence des maladies infectieuses et de la hausse de la demande de diagnostic précoce et précis.
La section pays du rapport fournit également des facteurs d'impact sur les marchés individuels et des changements de réglementation sur le marché national qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie des pays, l'épidémiologie des maladies et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des canaux de vente sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse des parts de marché du diagnostic multiplex syndromique
Le paysage concurrentiel du marché du diagnostic multiplex syndromique fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, la présence en Arabie saoudite, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit, la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises liée au marché du diagnostic multiplex syndromique.
Français Certains des principaux acteurs opérant sur le marché nord-américain du diagnostic multiplex syndromique sont BioFire Diagnostics (une filiale de bioMérieux SA), Seegene Inc., Luminex Corporation. A DiaSorin Company, F. Hoffmann-La Roche Ltd, BD, Bio-Rad Laboratories, Inc., Cepheid (une filiale de Danaher), QIAGEN, Abbott, Hologic, Inc., Thermo Fisher Scientific Inc., Siemens Healthcare GmbH, Akonni Biosystems, Inc., Biocartis, QuantuMDx Group Ltd., Applied BioCode, Inc., Prominex Inc., Nanomix, Inc., Curetis (une filiale d'OpGen, Inc.) entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT AND SERVICES LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
4.3 MARKET SHARE PER PANEL, FOR TOP 3 PLAYERS (2021)
5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: REGULATIONS
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 RISING PREVALENCE OF INFECTIOUS DISEASES
6.1.2 RISING ADOPTION OF MOLECULAR DIAGNOSTICS TECHNIQUES
6.1.3 INCREASING REGULATORY APPROVAL FOR SEVERE ACUTE RESPIRATORY SYNDROME CORONAVIRUS 2 (SARS-COV-2) TESTING
6.1.4 INCREASING DEMAND FOR FAST AND ACCURATE DIAGNOSTIC RESULTS
6.2 RESTRAINTS
6.2.1 HIGH COST OF DIAGNOSTIC PRODUCTS
6.2.2 EXPLICIT LIMITATION OF SYNDROMIC MULTIPLEX DIAGNOSTIC
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES TAKEN BY MARKET PLAYERS
6.3.2 RISING DIAGNOSTIC HEALTHCARE EXPENDITURE
6.3.3 INTRODUCTION OF TECHNOLOGICAL ADVANCED PRODUCTS
6.4 CHALLENGES
6.4.1 PRODUCT RECALLS
6.4.2 LACK OF SKILLED PROFESSIONALS AND BARRIERS FACED IN CONDUCTING DIAGNOSTIC TESTS
7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES
7.1 OVERVIEW
7.2 REAGENTS & CONSUMABLES
7.3 INSTRUMENTS, SOFTWARE & ACCESSORIES
7.4 SERVICES
8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE
8.1 OVERVIEW
8.2 VIRAL
8.2.1 CORONAVIRUS
8.2.2 INFLUENZA VIRUS
8.2.3 ADENOVIRUS
8.2.4 RHINOVIRUS
8.2.5 ROTAVIRUS
8.2.6 OTHERS
8.3 BACTERIAL
8.3.1 PNEUMONIAE
8.3.2 BORDETELLA PERTUSSIS
8.3.3 STAPHYLOCOCCUS
8.3.4 OTHERS
8.4 PARASITES
8.5 FUNGAL
9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE
9.1 OVERVIEW
9.2 RESPIRATORY INFECTIONS
9.3 GASTROENTERITIS
9.4 SEXUALLY TRANSMITTED INFECTIONS
9.5 SEPSIS
9.6 MENINGITIS
9.7 OTHERS
10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE
10.1 OVERVIEW
10.2 RESPIRATORY PANEL
10.3 GI-ENTERIC PANEL
10.4 SEXUALLY TRANSMITTED DISEASE PANEL
10.5 BLOOD-SEPSIS PANEL
10.6 MENINGITIS PANEL
10.7 OTHERS
11 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 CLINICAL LABORATORIES
11.4 PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES
11.5 RESEARCH INSTITUTES
11.6 OTHERS
12 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION
12.1 NORTH AMERICA
12.1.1 U.S.
12.1.2 CANADA
12.1.3 MEXICO
13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 BIOFIRE DIAGNOSTICS (A SUBSIDIARY OF BIOMÉRIEUX SA)
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENTS
15.2 F. HOFFMANN-LA ROCHE LTD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 LUMINEX CORPORATION. A DIASORIN COMPANY
15.3.1 COMPANY SNAPSHOT
15.3.2 RECENT FINANCIALS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 CEPHEID (A SUBSIDIARY OF DANAHER)
15.4.1 COMPANY SNAPSHOT
15.4.2 RECENT FINANCIALS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 QIAGEN
15.5.1 COMPANY SNAPSHOT
15.5.2 RECENT FINANCIALS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENT
15.6 ABBOTT
15.6.1 COMPANY SNAPSHOT
15.6.2 REVENUE ANALYSIS
15.6.3 PRODUCT PORTFOLIO
15.6.4 RECENT DEVELOPMENT
15.7 AKONNI BIOSYSTEMS, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 APPLIED BIOCODE, INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 BD
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 BIOCARTIS
15.10.1 COMPANY SNAPSHOT
15.10.2 REVENUE ANALYSIS
15.10.3 PRODUCT PORTFOLIO
15.10.4 RECENT DEVELOPMENT
15.11 BIO-RAD LABORATORIES, INC.
15.11.1 COMPANY SNAPSHOT
15.11.2 REVENUE ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENT
15.12 BOSCH HEALTHCARE SOLUTIONS GMBH (A SUBSIDIARY OF ROBERT BOSCH GMBH)
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENT
15.13 CURETIS (A SUBSIDIARY OF OPGEN, INC.)
15.13.1 COMPANY SNAPSHOT
15.13.2 RECENT FINANCIALS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HOLOGIC, INC.
15.14.1 COMPANY SNAPSHOT
15.14.2 RECENT FINANCIALS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENT
15.15 MIRXES PTE LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NANŌMIX, INC.
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 PROMINEX INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 PRODUCT PORTFOLIO
15.17.3 RECENT DEVELOPMENT
15.18 QUANTUMDX GROUP LTD.
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENTS
15.19 SEEGENE INC.
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUE ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 SIEMENS HEALTHCARE GMBH
15.20.1 COMPANY SNAPSHOT
15.20.2 RECENT FINANCIALS
15.20.3 PRODUCT PORTFOLIO
15.20.4 RECENT DEVELOPMENT
15.21 THERMOFISHER SCIENTIFIC INC.
15.21.1 COMPANY SNAPSHOT
15.21.2 RECENT FINANCIALS
15.21.3 PRODUCT PORTFOLIO
15.21.4 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Liste des tableaux
TABLE 1 COST OF THE PRODUCT
TABLE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT & SERVICES, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA REAGENTS & CONSUMABLES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA INSTRUMENTS, SOFTWARE & ACCESSORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA SERVICES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA PARASITES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA FUNGAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA RESPIRATORY INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA GASTROENTERITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA SEXUALLY TRANSMITTED INFECTIONS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA SEPSIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA MENINGITIS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA RESPIRATORY PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA GI-ENTERIC PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA SEXUALLY TRANSMITTED DISEASE PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA BLOOD-SEPSIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA MENINGITIS PANEL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA HOSPITALS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA CLINICAL LABORATORIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA PHARMACEUTICAL & BIOTECHNOLOGY COMPANIES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA RESEARCH INSTITUTES IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA OTHERS IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 41 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 42 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 43 U.S. VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 44 U.S. BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 45 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 46 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 47 U.S. SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 49 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 50 CANADA VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 51 CANADA BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 52 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 53 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 54 CANADA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PRODUCT AND SERVICES, 2020-2029 (USD MILLION)
TABLE 56 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 57 MEXICO VIRAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 58 MEXICO BACTERIAL IN SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY INFECTION TYPE, 2020-2029 (USD MILLION)
TABLE 59 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY DISEASE, 2020-2029 (USD MILLION)
TABLE 60 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY PANELS TYPE, 2020-2029 (USD MILLION)
TABLE 61 MEXICO SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 9 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE AND INCIDENCE OF INFECTIOUS DISEASES IS EXPECTED TO DRIVE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 REAGENTS & CONSUMABLES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET
FIGURE 15 TOTAL CASES OF COVID-19 IN NORTH AMERICA
FIGURE 16 TOTAL CASES OF COVID-19 IN EUROPE
FIGURE 17 NATIONAL HEALTH EXPENDITURE VS MEDICAL DEVICE EXPENDITURE, U.S., 2019
FIGURE 18 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2021
FIGURE 19 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, 2022-2029 (USD MILLION)
FIGURE 20 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, CAGR (2022-2029)
FIGURE 21 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT & SERVICES, LIFELINE CURVE
FIGURE 22 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2021
FIGURE 23 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, 2022-2029 (USD MILLION)
FIGURE 24 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, CAGR (2022-2029)
FIGURE 25 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY INFECTION TYPE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2021
FIGURE 27 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, 2022-2029 (USD MILLION)
FIGURE 28 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, CAGR (2022-2029)
FIGURE 29 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY DISEASE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2021
FIGURE 31 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, 2022-2029 (USD MILLION)
FIGURE 32 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, CAGR (2022-2029)
FIGURE 33 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PANELS TYPE, LIFELINE CURVE
FIGURE 34 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 35 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 36 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 37 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 39 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 40 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 41 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 42 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: BY PRODUCT AND SERVICES (2022-2029)
FIGURE 43 NORTH AMERICA SYNDROMIC MULTIPLEX DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.