North America Pulmonary Function Testing Market
Taille du marché en milliards USD
TCAC :
% 
 USD
1.62 Billion
USD
2.60 Billion
2024
2032
USD
1.62 Billion
USD
2.60 Billion
2024
2032
| 2025 –2032 | |
| USD 1.62 Billion | |
| USD 2.60 Billion | |
|
|
|
|
Segmentation du marché nord-américain des tests de fonction pulmonaire, par type (matériel, services et logiciels), type de patient (pédiatrique, adulte et gériatrique), application (bronchopneumopathie obstructive et bronchite restrictive), utilisateur final (hôpitaux, cliniques spécialisées, laboratoires de diagnostic, établissements de soins à domicile et autres) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des tests de la fonction pulmonaire en Amérique du Nord
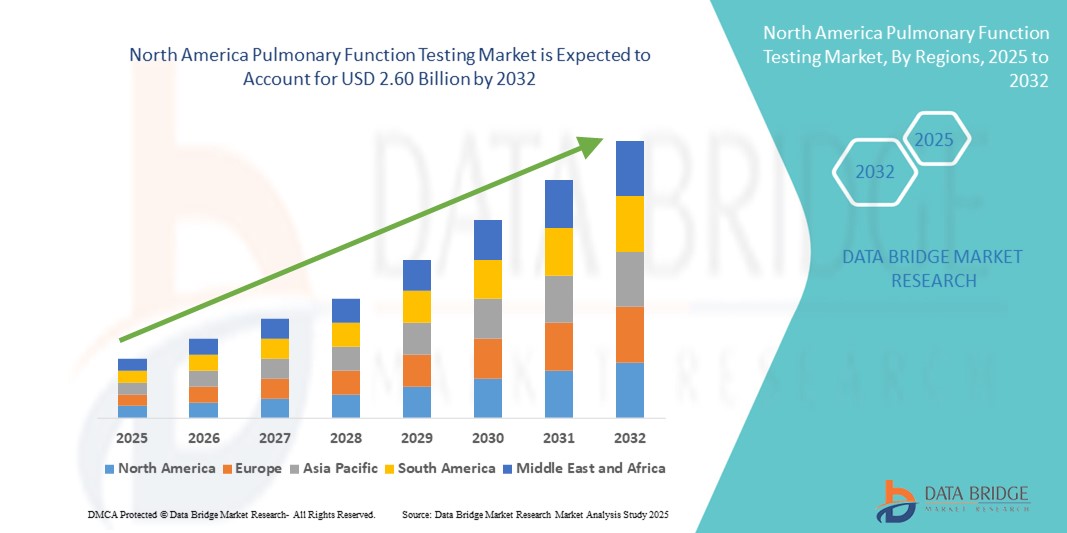
- La taille du marché des tests de la fonction pulmonaire en Amérique du Nord était évaluée à 1,62 milliard USD en 2024 et devrait atteindre 2,60 milliards USD d'ici 2032 , à un TCAC de 6,10 % au cours de la période de prévision.
- La croissance du marché est principalement tirée par l’incidence croissante des maladies respiratoires chroniques telles que la BPCO, l’asthme et la fibrose pulmonaire, ce qui stimule la demande de solutions de diagnostic précoces et précises.
- De plus, l’augmentation des dépenses de santé, la sensibilisation croissante à la santé pulmonaire et les progrès technologiques dans les dispositifs de test pulmonaire portables et conviviaux accélèrent encore l’adoption du marché, le positionnant comme un segment vital des soins respiratoires.
Analyse du marché des tests de la fonction pulmonaire en Amérique du Nord
- Les tests de fonction pulmonaire, qui permettent d'évaluer la capacité pulmonaire et l'efficacité du flux d'air, deviennent de plus en plus critiques dans le paysage des soins de santé aux États-Unis en raison de leur rôle dans la détection précoce et la gestion des maladies respiratoires chroniques en milieu hospitalier et ambulatoire.
- La prévalence croissante de l'asthme , de la BPCO et d'autres affections pulmonaires, ainsi qu'une sensibilisation accrue à la santé respiratoire et aux soins préventifs, alimentent la demande de diagnostics pulmonaires précis et non invasifs.
- Les États-Unis ont dominé le marché des tests de la fonction pulmonaire en Amérique du Nord avec la plus grande part de revenus de 39,9 % en 2024, soutenus par leur infrastructure de soins de santé avancée, l'augmentation des dépenses de santé et l'adoption rapide des technologies de diagnostic numériques et portables dans les environnements cliniques.
- Le Canada devrait être le pays connaissant la croissance la plus rapide sur le marché des tests de la fonction pulmonaire au cours de la période de prévision en raison des initiatives gouvernementales en matière de santé respiratoire et de l'augmentation des cas de troubles pulmonaires liés à l'environnement, contribuant à un déploiement plus large des systèmes PFT.
- Le segment du matériel a dominé le marché des tests de la fonction pulmonaire avec une part de marché de 47,6 % en 2024, grâce aux progrès continus des spiromètres et des pléthysmographes
Portée du rapport et segmentation du marché des tests de la fonction pulmonaire en Amérique du Nord
|
Attributs |
Analyses clés du marché des tests de fonction pulmonaire en Amérique du Nord |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des tests de la fonction pulmonaire en Amérique du Nord
Progrès technologiques dans les dispositifs pulmonaires portables et numériques
- Une tendance importante et croissante sur le marché nord-américain des tests de la fonction pulmonaire est l’adoption généralisée de systèmes de diagnostic pulmonaire portables et numériques qui permettent des tests au-delà des milieux hospitaliers et prennent en charge l’intégration des données en temps réel pour une meilleure coordination des soins.
- Par exemple, EasyOne Air de NDD Medical Technologies propose une spirométrie Bluetooth, permettant aux médecins de réaliser des tests de fonction pulmonaire à distance et d'accéder instantanément aux données des patients via des plateformes cloud. De même, les solutions PFT de Vyaire Medical s'intègrent aux dossiers médicaux électroniques pour optimiser les diagnostics et les flux de travail.
- L'intégration de la connectivité numérique permet aux professionnels de santé de suivre plus efficacement leurs patients, notamment dans le cadre des programmes de prise en charge des maladies chroniques telles que l'asthme et la BPCO. Les appareils compatibles avec les appareils mobiles et dotés d'un accès au cloud favorisent également la transition vers la télésanté et les soins à distance.
- De plus, les logiciels basés sur l’IA améliorent la précision de l’interprétation des tests et offrent des alertes intelligentes en cas de lectures anormales, réduisant ainsi les erreurs humaines et aidant les cliniciens dans la prise de décision.
- Ces innovations élargissent la portée des diagnostics pulmonaires aux milieux de soins primaires, aux environnements de soins à domicile et aux zones rurales avec un accès limité aux installations hospitalières à grande échelle.
- Cette tendance vers des solutions de diagnostic pulmonaire portables, intelligentes et connectées transforme le paysage des soins respiratoires, rendant les tests plus accessibles, plus rapides et plus adaptés aux modèles modernes de prestation de soins de santé.
Dynamique du marché des tests de la fonction pulmonaire en Amérique du Nord
Conducteur
Prévalence croissante des maladies respiratoires et accent mis sur le diagnostic précoce
- La prévalence croissante des maladies respiratoires chroniques telles que l'asthme, la BPCO et la fibrose pulmonaire aux États-Unis est un facteur clé qui alimente la demande de tests de la fonction pulmonaire dans les milieux cliniques et ambulatoires.
- Par exemple, selon le CDC, la BPCO touche plus de 16 millions d’Américains, tandis que l’asthme touche plus de 25 millions de personnes, soulignant la nécessité de tests de diagnostic de routine et précoces pour gérer la progression de la maladie.
- Les tests de la fonction pulmonaire offrent des évaluations non invasives et fiables de la santé pulmonaire, ce qui en fait des outils essentiels pour les médecins qui gèrent les affections respiratoires aiguës et à long terme.
- Les prestataires de soins de santé intègrent de plus en plus les tests de fonction pulmonaire (TFP) dans les protocoles de diagnostic standard pour améliorer les taux de détection précoce et soutenir les stratégies de traitement personnalisées.
- De plus, les initiatives nationales de santé favorisant les soins préventifs et la surveillance des maladies chroniques stimulent l’adoption de la spirométrie portable et des systèmes PFT complets dans les hôpitaux, les cliniques et les unités de test mobiles.
- La sensibilisation croissante à la santé pulmonaire et à l’importance des dépistages de routine devrait accélérer davantage la demande de diagnostics pulmonaires en Amérique du Nord.
Retenue/Défi
Coûts élevés des équipements et obstacles au remboursement en milieu ambulatoire
- Malgré le besoin croissant de tests de la fonction pulmonaire, le coût élevé des équipements de diagnostic avancés reste un obstacle important à une adoption généralisée, en particulier dans les petites cliniques et les centres de santé ruraux.
- Les systèmes PFT complets, qui incluent la capacité de diffusion et la mesure du volume pulmonaire, nécessitent souvent des investissements en capital importants et du personnel qualifié, ce qui limite leur utilisation dans les environnements aux ressources limitées.
- Par exemple, le remboursement de la spirométrie est plus courant, mais la couverture des tests avancés peut être incohérente, ce qui entraîne des difficultés financières pour les prestataires proposant des services complets de tests pulmonaires.
- En outre, la complexité des processus d’approbation des assurances et les différentes politiques de couverture peuvent dissuader les établissements de santé d’investir dans des équipements PFT.
- Bien que des appareils portables et abordables arrivent sur le marché, le coût de possession et de maintenance des systèmes avancés reste une préoccupation, en particulier pour les modèles de soins ambulatoires.
- Relever ces défis en matière de coûts et de remboursement grâce à des politiques de soutien, une couverture d’assurance élargie et l’innovation technologique sera essentiel pour garantir un accès équitable aux diagnostics pulmonaires partout en Amérique du Nord.
Portée du marché nord-américain des tests de la fonction pulmonaire
Le marché est segmenté en fonction du type, du type de patient, de l’application et de l’utilisateur final.
- Par type
En Amérique du Nord, le marché des tests de la fonction pulmonaire est segmenté en matériel, services et logiciels. Le segment du matériel a dominé le marché avec une part de marché de 47,6 % en 2024, grâce à l'utilisation généralisée d'appareils de diagnostic de base tels que les spiromètres, les pléthysmographes corporels et les systèmes de diffusion de gaz en milieu clinique. La demande en matériel de diagnostic pulmonaire avancé continue de croître en raison de l'augmentation des cas de maladies respiratoires chroniques et de l'adoption d'équipements de test portables et numériques dans les établissements de santé. Les hôpitaux et les laboratoires de diagnostic dépendent fortement de ces appareils pour les évaluations de routine et critiques de la fonction pulmonaire.
Le secteur des logiciels devrait connaître la croissance la plus rapide, soit 19,4 % entre 2025 et 2032, grâce à l'intégration croissante des systèmes de TEP aux dossiers médicaux électroniques (DME), aux plateformes de télésurveillance et aux outils d'aide au diagnostic basés sur l'IA. Ces solutions logicielles améliorent l'interprétation des tests, rationalisent les flux de travail et optimisent l'efficacité des cliniciens, contribuant ainsi à leur adoption croissante dans les grands hôpitaux comme dans les petites cliniques.
- Par type de patient
En fonction du type de patient, le marché nord-américain des tests de fonction respiratoire est segmenté en pédiatrie, en gériatrie et en soins aux adultes. Le segment adulte a dominé le marché avec une part de chiffre d'affaires de 52,6 % en 2024, en raison de la forte prévalence de l'asthme, de la bronchopneumopathie chronique obstructive (BPCO) et des affections pulmonaires professionnelles chez la population adulte. Les adultes nécessitent souvent des évaluations pulmonaires régulières pour la prise en charge de leur maladie, leur autorisation chirurgicale ou des examens de santé au travail, ce qui en fait un secteur d'activité prioritaire pour les professionnels de santé.
Le segment gériatrique devrait connaître la croissance la plus rapide, soit 20,1 % entre 2025 et 2032, en raison du vieillissement de la population nord-américaine et d'un risque accru de déclin pulmonaire lié à l'âge. La demande d'évaluations de la fonction respiratoire chez les patients âgés est stimulée par les dépistages de routine, les évaluations préopératoires et les besoins en soins chroniques liés aux affections respiratoires chroniques.
- Par application
En Amérique du Nord, le marché des tests de la fonction respiratoire est segmenté en fonction des applications : bronchopneumopathie obstructive et bronchopneumopathie restrictive. Le segment des bronchopneumopathies obstructives a dominé le marché, avec une part de chiffre d'affaires de 61,3 % en 2024, en raison de l'incidence importante de maladies telles que l'asthme et la BPCO en Amérique du Nord. Les tests de la fonction respiratoire, et notamment la spirométrie, sont des outils essentiels pour le diagnostic et le suivi de ces maladies, ce qui génère une demande constante en soins primaires et spécialisés.
Le segment des maladies pulmonaires restrictives devrait connaître une croissance régulière au cours de la période de prévision, grâce à une meilleure connaissance et à un meilleur diagnostic de pathologies telles que la fibrose pulmonaire, la sarcoïdose et les troubles neuromusculaires. L'accent étant mis sur une évaluation respiratoire complète, le besoin d'examens respiratoires complets incluant des tests de volume pulmonaire et de diffusion augmente.
- Par utilisateur final
En Amérique du Nord, le marché des tests de fonction respiratoire est segmenté en fonction de l'utilisateur final : hôpitaux, cliniques spécialisées, laboratoires de diagnostic, services de soins à domicile, etc. Le segment hospitalier a dominé le marché avec une part de chiffre d'affaires de 44,6 % en 2024, grâce à un volume important de patients, des infrastructures de pointe et la disponibilité de services de diagnostic respiratoire complets. Les hôpitaux restent les principaux centres de réalisation de tests de fonction respiratoire complexes, notamment pour les patients hospitalisés et les évaluations préopératoires.
Le secteur des soins à domicile devrait connaître la croissance la plus rapide, soit 21,5 % entre 2025 et 2032, grâce à l'adoption croissante des appareils de spirométrie portables, des solutions de télésurveillance des patients et à la transition vers des soins décentralisés. La demande croissante de prise en charge des maladies chroniques, la commodité des tests à domicile et l'amélioration de la télémédecine contribuent largement à cette tendance.
Analyse régionale du marché nord-américain des tests de la fonction pulmonaire
- Les États-Unis ont dominé le marché des tests de la fonction pulmonaire en Amérique du Nord avec la plus grande part de revenus de 39,9 % en 2024, soutenus par leur infrastructure de soins de santé avancée, l'augmentation des dépenses de santé et l'adoption rapide des technologies de diagnostic numériques et portables dans les environnements cliniques.
- Les patients et les prestataires de soins de santé aux États-Unis et au Canada adoptent de plus en plus les tests de fonction pulmonaire en raison de leur nature non invasive, de leur précision diagnostique et de leur importance dans la gestion de maladies telles que l'asthme, la BPCO et les maladies pulmonaires interstitielles.
- Cette utilisation généralisée est en outre soutenue par une infrastructure de soins de santé bien établie, des politiques de remboursement favorables et une demande croissante de solutions de test portables et à domicile, positionnant les tests de fonction pulmonaire comme un outil essentiel dans le paysage des soins respiratoires en Amérique du Nord.
Analyse du marché des tests de la fonction pulmonaire aux États-Unis et en Amérique du Nord
En 2024, le marché américain des tests de fonction pulmonaire a représenté la plus grande part de chiffre d'affaires en Amérique du Nord, avec 82 %, grâce à la charge croissante des maladies respiratoires chroniques et à la robustesse des infrastructures de santé et d'assurance du pays. Le développement des structures hospitalières, l'adoption massive des solutions de santé numériques et l'augmentation de la population gériatrique contribuent largement à l'expansion du marché. De plus, les initiatives gouvernementales, telles que le soutien des CDC à la prévention des maladies chroniques et les investissements croissants dans les diagnostics respiratoires basés sur l'IA, continuent de stimuler l'adoption des tests de fonction pulmonaire en milieu clinique et ambulatoire.
Analyse du marché canadien des tests de la fonction pulmonaire
Le marché canadien des tests de la fonction respiratoire devrait connaître une croissance soutenue tout au long de la période de prévision, grâce à l'élargissement de l'accès aux soins de santé et à la hausse du nombre de cas de maladies respiratoires. L'importance accordée par le pays aux soins préventifs, conjuguée au soutien gouvernemental important à la prise en charge des maladies chroniques, encourage le dépistage pulmonaire systématique, en particulier chez les personnes âgées. Le déploiement croissant d'unités de dépistage mobiles et l'intégration de la spirométrie numérique dans les pratiques de soins primaires améliorent encore la portée et l'efficacité du diagnostic au Canada.
Aperçu du marché mexicain des tests de la fonction pulmonaire
Le marché mexicain des tests de la fonction respiratoire devrait connaître une croissance soutenue au cours de la période de prévision, portée par une sensibilisation accrue à la santé respiratoire et des investissements croissants dans les infrastructures de santé. L'augmentation de l'incidence de l'asthme, de la BPCO et des affections pulmonaires liées à la pollution stimule la demande de solutions diagnostiques précoces et précises. Les efforts du gouvernement pour améliorer l'accès aux services de santé publics, ainsi que le développement des laboratoires de diagnostic privés, contribuent à une adoption plus large des tests de la fonction respiratoire. De plus, l'introduction progressive d'appareils de test portables et de technologies de santé numériques améliore les capacités de diagnostic dans les zones urbaines et semi-urbaines.
Part de marché des tests de la fonction pulmonaire en Amérique du Nord
L’industrie nord-américaine des tests de la fonction pulmonaire est principalement dirigée par des entreprises bien établies, notamment :
- MGC Diagnostics Corporation (États-Unis)
- Vyaire Medical, Inc. (États-Unis)
- ndd Medical Technologies, Inc. (Suisse)
- Schiller AG (Suisse)
- COSMED Srl (Italie)
- CareFusion Corporation (États-Unis)
- Medical Graphics Corporation (États-Unis)
- Morgan Scientific, Inc. (États-Unis)
- Vitalograph Ltd. (Royaume-Uni)
- Nihon Kohden Corporation (Japon)
- Diagnostics SDI (États-Unis)
- Labtech Ltd. (Hongrie)
- Medisoft SA (Belgique)
- Futuremed America Inc. (États-Unis)
- Fondation médicale Seegene (Corée du Sud)
- Enregistreurs et systèmes Medicare Pvt. Ltd. (Inde)
- Ganshorn Medizin Electronic GmbH (Allemagne)
- Jones Medical Instrument Company (États-Unis)
- ERT (États-Unis)
- Spirohome (Turquie)
Quels sont les développements récents sur le marché des tests de la fonction pulmonaire en Amérique du Nord ?
- En juin 2024, Vyaire Medical Inc., acteur majeur du diagnostic respiratoire, a lancé aux États-Unis son nouveau système Vyntus PFT, conçu pour offrir des mesures pulmonaires de haute précision et un confort accru pour le patient. Ce système s'intègre parfaitement aux dossiers médicaux électroniques (DME) et dispose d'un étalonnage avancé et de commandes tactiles. Ce lancement réaffirme l'engagement de Vyaire à améliorer la précision diagnostique et l'efficacité clinique en soins pulmonaires.
- En mai 2024, MGC Diagnostics Corporation a annoncé une collaboration avec un réseau de centres de santé communautaires du Canada pour offrir des services mobiles d'évaluation de la fonction respiratoire. Cette initiative vise à accroître l'accès aux diagnostics respiratoires dans les régions rurales et mal desservies. Grâce à ce partenariat, MGC poursuit sa mission : améliorer le dépistage précoce des maladies respiratoires chroniques tout en favorisant l'équité en santé.
- En mars 2024, NDD Medical Technologies Inc. a lancé l'EasyOne Air Next, un spiromètre sans fil approuvé par Santé Canada et la FDA. Conçu pour les tests au point de service et à distance, cet appareil prend en charge la connectivité Bluetooth et l'intégration infonuagique pour le partage de données en temps réel. Cette innovation accompagne la transition croissante vers des tests décentralisés et offre aux cliniciens des outils de diagnostic portables et précis.
- En février 2024, l'American Thoracic Society (ATS) et la Société canadienne de thoracologie ont publié conjointement des lignes directrices actualisées sur les pratiques d'évaluation de la fonction pulmonaire, mettant l'accent sur l'utilisation de systèmes assistés par l'IA pour une meilleure précision d'interprétation. Ces lignes directrices influencent les décisions d'approvisionnement des hôpitaux et des cliniques, favorisant ainsi l'adoption croissante d'appareils d'évaluation de la fonction pulmonaire assistés par l'IA en Amérique du Nord.
- En janvier 2024, Hill-Rom Holdings, Inc. a élargi son portefeuille de solutions de diagnostic en intégrant des modules de diagnostic respiratoire à sa gamme de produits Welch Allyn. Cette intégration permet aux cliniciens d'effectuer des tests de spirométrie directement depuis les stations de diagnostic existantes, améliorant ainsi le flux de travail et simplifiant les évaluations respiratoires. Cette initiative souligne la demande croissante de plateformes de diagnostic multifonctionnelles dans les établissements de santé nord-américains.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.