North America Epilepsy Monitoring Devices Market
Taille du marché en milliards USD
TCAC :
% 
 USD
470.56 Million
USD
738.76 Million
2024
2032
USD
470.56 Million
USD
738.76 Million
2024
2032
| 2025 –2032 | |
| USD 470.56 Million | |
| USD 738.76 Million | |
|
|
|
|
Segmentation du marché nord-américain des dispositifs de surveillance de l'épilepsie, par type de produit (dispositifs portables, dispositifs intelligents et dispositifs conventionnels), type (crises focales et généralisées), type de patient (pédiatrie, gériatrie et adultes), utilisateur final (hôpitaux, services de soins à domicile, centres de neurologie, centres de diagnostic, centres et cliniques de chirurgie ambulatoire, etc.), canal de distribution (vente au détail, vente en ligne, appels d'offres directs, etc.) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché des dispositifs de surveillance de l'épilepsie en Amérique du Nord
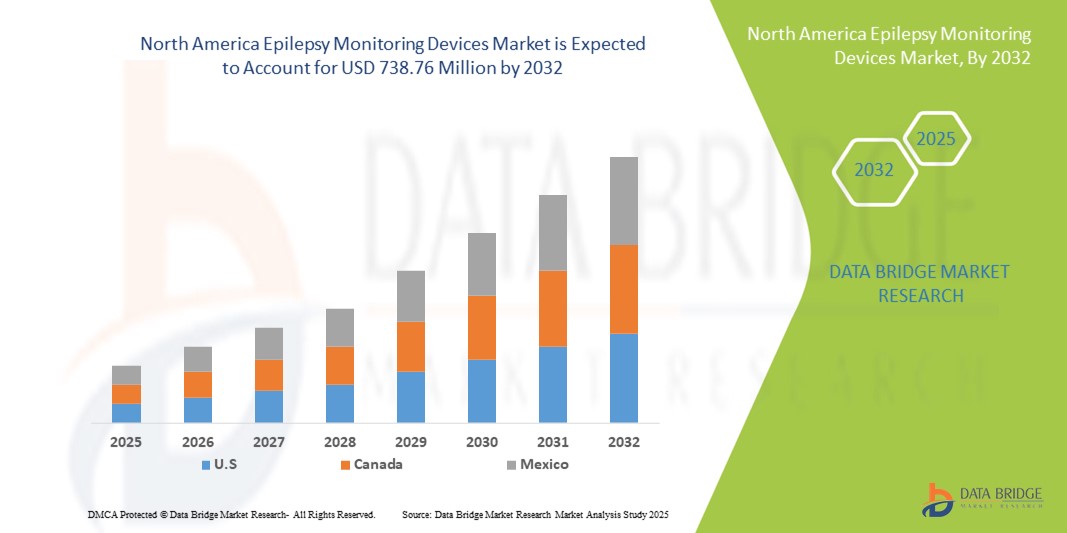
- La taille du marché des dispositifs de surveillance de l'épilepsie en Amérique du Nord était évaluée à 470,56 millions USD en 2024 et devrait atteindre 738,76 millions USD d'ici 2032 , à un TCAC de 5,8 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par la prévalence croissante de l’épilepsie, la sensibilisation croissante aux troubles neurologiques et les progrès des technologies de diagnostic et de surveillance, qui améliorent la gestion et les soins des patients.
- De plus, la demande croissante de solutions de surveillance précises, en temps réel et non invasives, tant en milieu hospitalier qu'à domicile, positionne les dispositifs de surveillance de l'épilepsie comme des outils essentiels en neurologie. Ces facteurs convergents accélèrent l'adoption de systèmes de surveillance avancés, stimulant ainsi significativement la croissance du marché.
Analyse du marché nord-américain des dispositifs de surveillance de l'épilepsie
- Les dispositifs de surveillance de l'épilepsie, y compris les appareils portables , les appareils intelligents et les appareils conventionnels, sont de plus en plus essentiels dans les hôpitaux et les établissements de soins à domicile en raison de leur capacité à fournir une surveillance neurologique précise et en temps réel, à soutenir le diagnostic et à améliorer la gestion des patients.
- La demande croissante d’appareils de surveillance de l’épilepsie est principalement alimentée par la prévalence croissante de l’épilepsie, la sensibilisation croissante aux troubles neurologiques et le besoin de solutions de surveillance continue et non invasive qui améliorent la sécurité des patients et les résultats cliniques.
- Les États-Unis ont dominé le marché nord-américain des dispositifs de surveillance de l'épilepsie avec la plus grande part de revenus de 73,3 % en 2024, grâce à une infrastructure de soins de santé avancée, une forte adoption des technologies de santé numériques et une forte présence d'acteurs clés de l'industrie, avec une croissance significative de l'adoption des dispositifs dans les hôpitaux, les centres de neurologie et les établissements de soins à domicile, soutenue par des innovations dans les systèmes de surveillance sans fil, portables et basés sur l'IA.
- Le Canada devrait être le pays qui connaîtra la croissance la plus rapide sur le marché des dispositifs de surveillance de l’épilepsie au cours de la période de prévision en raison de l’augmentation des investissements dans les soins de santé, de la sensibilisation croissante aux troubles neurologiques et de la demande croissante de solutions de surveillance à domicile.
- Le segment des appareils intelligents a dominé le marché nord-américain des dispositifs de surveillance de l'épilepsie avec une part de marché de 48,8 % en 2024, grâce à leurs fonctionnalités avancées, leurs capacités de surveillance à distance et leur intégration avec les plateformes de santé numériques pour le suivi des crises en temps réel.
Portée du rapport et segmentation du marché des dispositifs de surveillance de l'épilepsie en Amérique du Nord
|
Attributs |
Aperçu du marché des dispositifs de surveillance de l'épilepsie en Amérique du Nord |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des dispositifs de surveillance de l'épilepsie en Amérique du Nord
Progrès dans les solutions de surveillance intelligentes et portables
- Une tendance importante et croissante sur le marché nord-américain des dispositifs de surveillance de l'épilepsie est l'adoption croissante d'appareils intelligents et portables qui fournissent un suivi des crises en temps réel et une surveillance continue de l'EEG, améliorant ainsi les soins aux patients et la prise de décision clinique.
- Par exemple, les dispositifs portables de détection des crises tels qu'Embrace2 permettent aux patients de suivre les crises et d'alerter les soignants en temps réel, tandis que les casques EEG intelligents permettent une surveillance à distance dans les soins à domicile et en milieu clinique.
- L'intégration de l'IA dans ces appareils permet des fonctionnalités telles que la détection automatisée des crises, l'analyse prédictive du risque de crise et des recommandations de surveillance personnalisées, améliorant ainsi les résultats cliniques et la sécurité des patients.
- L'intégration transparente des dispositifs de surveillance de l'épilepsie avec les plateformes de santé numériques et les solutions de télémédecine permet une surveillance et une gestion centralisées, permettant aux neurologues d'accéder aux données des patients à distance et d'ajuster efficacement les plans de traitement.
- Cette tendance vers des systèmes de surveillance intelligents, connectés et centrés sur le patient transforme fondamentalement la gestion de l'épilepsie, avec des entreprises telles que NeuroSigma développant des appareils compatibles avec l'IA qui offrent des alertes en temps réel, des informations prédictives et une connectivité cloud.
- La demande d'appareils de surveillance de l'épilepsie intelligents, portables et connectés augmente rapidement dans les hôpitaux, les centres de neurologie et les établissements de soins à domicile, car les prestataires de soins de santé et les patients accordent de plus en plus d'importance à la précision, à la commodité et à la surveillance continue.
Dynamique du marché nord-américain des dispositifs de surveillance de l'épilepsie
Conducteur
Demande croissante en raison de la prévalence croissante de l'épilepsie et de l'adoption des soins de santé numériques
- La prévalence croissante de l’épilepsie, associée à l’adoption croissante des technologies de santé numériques, est un facteur clé de la demande accrue d’appareils avancés de surveillance de l’épilepsie.
- Par exemple, en mars 2024, Cadwell Laboratories a lancé un nouveau système EEG sans fil conçu pour améliorer la surveillance à domicile et améliorer le confort des patients tout en permettant l'accès à distance des neurologues.
- Alors que les prestataires de soins de santé cherchent à améliorer la précision du diagnostic et les résultats des patients, les appareils de surveillance de l'épilepsie offrent des fonctionnalités telles que le suivi continu de l'EEG, des alertes en temps réel et le partage de données basé sur le cloud, ce qui représente une mise à niveau significative par rapport aux méthodes de diagnostic conventionnelles.
- De plus, la mise en œuvre croissante des services de télésanté et des programmes de surveillance à distance des patients positionne ces appareils comme des outils essentiels dans les soins neurologiques modernes.
- La commodité des appareils portables et intelligents qui permettent un suivi en temps réel et un accès aux données à distance pour les cliniciens, combinés à des interfaces conviviales, propulse l'adoption dans les hôpitaux, les centres de neurologie et les établissements de soins à domicile.
- La tendance vers la surveillance assistée par l’IA, l’analyse prédictive et l’intégration numérique transparente soutient davantage la croissance des dispositifs de surveillance de l’épilepsie en Amérique du Nord.
Retenue/Défi
Préoccupations en matière de sécurité des données et coûts élevés des appareils
- Les préoccupations concernant la cybersécurité et la confidentialité des données des dispositifs médicaux connectés constituent un défi majeur pour l'adoption généralisée des solutions de surveillance de l'épilepsie. Les dispositifs transmettant des données sensibles sur les patients via les réseaux sont vulnérables aux failles de sécurité, ce qui suscite des appréhensions chez les professionnels de santé et les patients.
- Par exemple, les rapports sur les vulnérabilités des systèmes EEG sans fil et des dispositifs portables de suivi des crises ont rendu certaines institutions prudentes quant à l’adoption de solutions de surveillance connectées au cloud.
- Relever ces défis grâce à un cryptage robuste, des protocoles d’authentification sécurisés et le respect des directives HIPAA et de la FDA est essentiel pour instaurer la confiance entre les cliniciens et les patients.
- En outre, le coût relativement élevé des dispositifs avancés de surveillance de l’épilepsie par rapport aux systèmes conventionnels peut constituer un obstacle à l’adoption, en particulier pour les petites cliniques ou les établissements de soins à domicile à budget limité.
- Bien que les appareils de base soient devenus plus abordables, les fonctionnalités haut de gamme telles que la détection des crises assistée par l'IA, la connectivité sans fil et la surveillance continue à long terme ont souvent un prix plus élevé.
- Surmonter ces défis grâce à une cybersécurité renforcée, à la conformité réglementaire et au développement de solutions de surveillance rentables sera essentiel pour une croissance soutenue du marché en Amérique du Nord.
Portée du marché nord-américain des dispositifs de surveillance de l'épilepsie
Le marché est segmenté en fonction du type de produit, du type de crise, du type de patient, de l’utilisateur final et du canal de distribution.
- Par type de produit
Selon le type de produit, le marché des dispositifs de surveillance de l'épilepsie est segmenté en dispositifs portables, dispositifs intelligents et dispositifs conventionnels. Le segment des dispositifs intelligents a dominé le marché avec la plus grande part de chiffre d'affaires (48,8 %) en 2024, grâce à l'adoption croissante de systèmes de détection des crises d'épilepsie basés sur l'IA et de capacités de surveillance à distance. Les dispositifs intelligents permettent de suivre en temps réel l'activité neurologique des patients et d'envoyer des alertes aux soignants ou aux cliniciens en cas de crise. Leur intégration aux plateformes de santé numérique et aux solutions de télémédecine améliore la gestion des soins à distance et favorise les interventions précoces. Les hôpitaux et les centres de neurologie privilégient de plus en plus les dispositifs intelligents pour la surveillance continue des patients en raison de leur précision, de leur praticité et de leur compatibilité avec les dossiers médicaux électroniques. De plus, la préférence croissante des consommateurs pour les solutions de santé connectées dans les contextes de soins à domicile renforce encore la domination des dispositifs intelligents. Ce segment bénéficie des innovations technologiques, notamment la connectivité cloud, l'analyse prédictive et la prise en charge des applications mobiles, ce qui renforce son attractivité.
Le segment des dispositifs portables devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par la demande croissante de solutions de surveillance portables et non invasives adaptées aux soins à domicile et aux patients ambulatoires. Les dispositifs portables, tels que les bracelets ou les bandeaux, permettent une détection continue des crises sans limiter la mobilité du patient. Ils sont particulièrement intéressants pour les populations pédiatriques et gériatriques, permettant aux soignants de surveiller les patients à distance et en temps réel. Les avancées technologiques, telles que la détection assistée par l'IA et la longue durée de vie des batteries, améliorent la précision et la commodité. La sensibilisation croissante à l'épilepsie et la nécessité d'une prise en charge proactive stimulent l'adoption de ces dispositifs, tant en milieu clinique qu'à domicile. De plus, les partenariats entre fabricants de dispositifs et plateformes de santé numérique élargissent la convivialité des dispositifs portables, accélérant ainsi leur croissance.
- Par type de crise
Selon le type de crise, le marché des dispositifs de surveillance de l'épilepsie est segmenté en crises focales et crises généralisées. Le segment des crises généralisées a dominé le marché avec une part de chiffre d'affaires de 54,1 % en 2024, porté par la prévalence accrue de l'épilepsie généralisée et la demande de solutions de surveillance complètes capables de capturer l'activité cérébrale complète. Les dispositifs conçus pour les crises généralisées offrent des algorithmes de détection avancés, permettant aux professionnels de santé d'évaluer la fréquence, la gravité et les schémas des crises avec plus de précision. Les hôpitaux et les centres de neurologie privilégient les dispositifs capables de surveiller les crises généralisées pour un diagnostic précis et une prise en charge à long terme. L'utilisation croissante de l'IA et des plateformes de surveillance infonuagiques a amélioré l'efficacité des dispositifs dans la capture et l'analyse des crises généralisées. De plus, l'observance des traitements par les patients est mieux assurée grâce aux systèmes d'alerte intelligents et à la connectivité mobile. L'intégration des dispositifs de surveillance des crises généralisées aux systèmes de télésanté consolide leur domination en Amérique du Nord.
Le segment des crises focales devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par la sensibilisation croissante à la détection localisée des crises et aux approches thérapeutiques personnalisées. Les dispositifs ciblant les crises focales offrent une surveillance cérébrale spécifique à chaque région, permettant une intervention et des ajustements thérapeutiques précis. Les patients, enfants et adultes, bénéficient de plus en plus d'appareils capables de différencier les types de crises, permettant des évaluations cliniques plus précises. L'innovation continue dans les algorithmes assistés par l'IA et les outils de détection portables favorise l'adoption de solutions de surveillance des crises focales. L'évolution vers les soins neurologiques à domicile et la télésurveillance des patients stimule encore davantage la croissance de ce segment. Les cliniciens recommandent activement les dispositifs de surveillance des crises focales pour les patients nécessitant un suivi détaillé et localisé des crises afin d'améliorer l'efficacité du traitement.
- Par type de patient
Selon le type de patient, le marché des dispositifs de surveillance de l'épilepsie est segmenté en patients pédiatriques, gériatriques et adultes. Le segment des patients adultes a dominé le marché avec une part de 50,7 % en 2024, stimulé par la forte prévalence de l'épilepsie chez les adultes et l'adoption croissante des dispositifs de surveillance continue dans les hôpitaux et les services de soins à domicile. Les adultes nécessitent souvent une surveillance à long terme pour la gestion des crises, ce qui stimule la demande d'appareils intelligents et portables capables de générer des alertes en temps réel. Les hôpitaux et les centres de neurologie privilégient les solutions de surveillance destinées aux adultes pour un diagnostic précis, l'analyse des schémas épileptiques et la gestion thérapeutique. L'intégration aux plateformes de télémédecine permet la surveillance à distance, en particulier pour les adultes actifs nécessitant une perturbation minimale de leur mode de vie. La présence de fabricants d'appareils de pointe et d'une solide infrastructure de santé en Amérique du Nord renforce encore la domination du segment adulte.
Le segment des patients pédiatriques devrait connaître la croissance la plus rapide entre 2025 et 2032, stimulée par une sensibilisation croissante au diagnostic précoce de l'épilepsie et par la prévalence croissante de l'épilepsie infantile. Les dispositifs de surveillance pédiatrique sont conçus pour être non invasifs, confortables et adaptés à un port continu, ce qui encourage l'observance. Les soignants et les hôpitaux adoptent de plus en plus d'appareils portables et intelligents permettant une surveillance à distance et des alertes immédiates en cas de crise. Les innovations technologiques, telles que la détection des crises par IA et l'intégration aux applications mobiles, favorisent cette adoption rapide au sein de la population pédiatrique. La demande est particulièrement forte dans les services de soins à domicile, où les parents recherchent des outils de surveillance fiables et continus pour les enfants épileptiques. Les dispositifs pédiatriques bénéficient également des initiatives gouvernementales et des campagnes de sensibilisation favorisant le dépistage et l'intervention précoces.
- Par utilisateur final
En fonction de l'utilisateur final, le marché des dispositifs de surveillance de l'épilepsie est segmenté entre hôpitaux, services de soins à domicile, centres de neurologie, centres de diagnostic, centres et cliniques de chirurgie ambulatoire, etc. Le segment hospitalier a dominé le marché avec une part de 45,2 % en 2024, porté par la préférence pour des dispositifs de surveillance avancés capables d'enregistrer l'EEG en continu, de détecter les crises en temps réel et de s'intégrer aux systèmes informatiques hospitaliers. Les hôpitaux utilisent ces dispositifs pour améliorer la précision du diagnostic, gérer efficacement les soins aux patients et réduire la durée d'hospitalisation. L'adoption d'appareils intelligents et portables dans les hôpitaux améliore l'efficacité de la surveillance, réduit l'observation manuelle et favorise les applications de télémédecine. Les neurologues et les cliniciens s'appuient sur ces dispositifs pour recueillir des données patients complètes, permettant ainsi des décisions thérapeutiques plus éclairées. De plus, les hôpitaux nord-américains bénéficient d'une infrastructure de santé solide, de politiques de remboursement et d'une adoption accrue des technologies, ce qui renforce leur domination.
Le secteur des soins à domicile devrait connaître la croissance la plus rapide entre 2025 et 2032, alimenté par la demande croissante de soins centrés sur le patient, la télésurveillance et l'adoption d'appareils portables et intelligents. La télésurveillance permet aux patients, notamment pédiatriques et gériatriques, de préserver leur autonomie tout en garantissant la détection des crises en temps réel. L'intégration avec les applications mobiles, les plateformes cloud et les services de télésanté permet aux soignants et aux cliniciens de suivre l'état de santé des patients à distance. La praticité, la rentabilité et l'accessibilité des solutions de télésurveillance à domicile favorisent leur adoption rapide. Les avancées technologiques, telles que les appareils portables légers et les alertes assistées par IA, améliorent encore la convivialité des soins à domicile. La tendance à la gestion proactive de l'épilepsie et à la personnalisation des soins crée des opportunités significatives dans ce secteur.
- Par canal de distribution
En fonction du canal de distribution, le marché des dispositifs de surveillance de l'épilepsie est segmenté en ventes au détail, ventes en ligne, appels d'offres directs et autres. Le segment des appels d'offres directs a dominé le marché avec une part de 47,5 % en 2024, porté par les achats groupés des hôpitaux, des centres de neurologie et des prestataires de soins à domicile. Les appels d'offres directs simplifient les achats, proposent des prix compétitifs et garantissent l'authenticité des appareils. Les hôpitaux et les centres de diagnostic privilégient souvent les appels d'offres directs pour acquérir des systèmes de surveillance avancés avec des contrats de service et de maintenance. Les achats à grande échelle par appels d'offres permettent aux établissements de santé d'équiper efficacement plusieurs unités tout en respectant les normes réglementaires. La présence de grands fabricants et distributeurs en Amérique du Nord renforce la domination de ce canal.
Le segment des ventes en ligne devrait connaître la croissance la plus rapide entre 2025 et 2032, stimulé par l'adoption croissante du e-commerce, la sensibilisation croissante des patients et des soignants, et la commodité de la livraison à domicile des dispositifs de surveillance portables et intelligents. Les plateformes en ligne permettent d'accéder à une gamme plus large de produits, de comparer les fonctionnalités et d'intégrer les services de télémédecine. L'achat de dispositifs médicaux en ligne gagne du terrain, notamment pour les patients en soins à domicile et les patients pédiatriques, où les soignants recherchent un accès pratique à des solutions de surveillance avancées. Les fabricants investissent également dans les canaux de vente directe en ligne pour améliorer leur communication et offrir une assistance fluide. Le marketing numérique, les démonstrations de produits et les politiques de retour simplifiées stimulent également la croissance des ventes en ligne.
Analyse régionale du marché nord-américain des dispositifs de surveillance de l'épilepsie
- Les États-Unis ont dominé le marché des dispositifs de surveillance de l'épilepsie avec la plus grande part de revenus de 73,3 % en 2024, grâce à une infrastructure de soins de santé avancée, une forte adoption des technologies de santé numériques et une forte présence d'acteurs clés de l'industrie.
- Les patients et les prestataires de soins de santé de la région apprécient grandement la précision, les capacités de surveillance en temps réel et l'intégration des appareils avec les plateformes de santé numériques et les solutions de télémédecine.
- Cette adoption généralisée est en outre soutenue par une infrastructure de soins de santé avancée, une recherche et un développement solides dans les dispositifs neurologiques et des dépenses de santé élevées, faisant des dispositifs de surveillance de l'épilepsie des outils essentiels dans les milieux hospitaliers et de soins à domicile.
Aperçu du marché américain des dispositifs de surveillance de l'épilepsie
Le marché américain des dispositifs de surveillance de l'épilepsie a représenté la plus grande part de chiffre d'affaires en 2024 en Amérique du Nord, porté par la prévalence croissante de l'épilepsie et l'adoption croissante de solutions de surveillance neurologique avancées. Patients et professionnels de santé privilégient une surveillance précise en temps réel et la gestion à distance des crises grâce à des dispositifs intelligents et portables. La tendance croissante à la surveillance à domicile, combinée à une forte demande de détection assistée par l'IA et d'intégration de la télésanté, propulse davantage le marché. De plus, l'intégration croissante des dispositifs de surveillance de l'épilepsie aux plateformes de santé numérique et aux applications mobiles contribue significativement à l'expansion du marché.
Aperçu du marché canadien des dispositifs de surveillance de l'épilepsie
Le marché canadien des dispositifs de surveillance de l'épilepsie devrait connaître une croissance substantielle au cours de la période de prévision, principalement grâce à une sensibilisation accrue aux troubles neurologiques et à des investissements croissants dans les infrastructures de santé. La demande croissante de solutions de surveillance à domicile et d'appareils portables favorise leur adoption, notamment chez les patients pédiatriques et gériatriques. Les consommateurs et les professionnels de la santé canadiens sont attirés par la commodité, la précision et l'accès aux données en temps réel offerts par les systèmes de surveillance avancés. La région connaît une croissance importante dans les hôpitaux, les centres de neurologie et les services de soins à domicile, les appareils étant intégrés aux programmes de soins, nouveaux comme existants.
Aperçu du marché mexicain des dispositifs de surveillance de l'épilepsie
Le marché mexicain des dispositifs de surveillance de l'épilepsie devrait connaître une croissance annuelle moyenne (TCAC) remarquable au cours de la période de prévision, stimulé par la prévalence croissante de l'épilepsie et la hausse des dépenses de santé. Les préoccupations croissantes concernant la sécurité des patients et la nécessité d'une surveillance fiable des crises incitent les hôpitaux, les cliniques et les soignants à adopter des dispositifs intelligents et portables. Le développement de l'infrastructure de santé numérique au Mexique, conjugué aux initiatives gouvernementales visant à améliorer l'accès aux soins neurologiques, devrait continuer de stimuler la croissance du marché. De plus, l'intégration des dispositifs de surveillance aux applications mobiles et aux services de télésanté améliore l'accessibilité et l'observance thérapeutique des patients.
Part de marché des dispositifs de surveillance de l'épilepsie en Amérique du Nord
L'industrie nord-américaine des dispositifs de surveillance de l'épilepsie est principalement dirigée par des entreprises bien établies, notamment :
- NeuroPace, Inc. (États-Unis)
- Empatica Inc. (États-Unis)
- Medtronic (Irlande)
- Masimo (États-Unis)
- Cadwell Laboratories, Inc. (États-Unis)
- Natus Medical Incorporated (États-Unis)
- Compumedics Limited (Australie)
- Brain Sentinel, Inc. (États-Unis)
- EMOTIV (États-Unis)
- Neuroélectrique (États-Unis)
- Koninklijke Philips NV (Pays-Bas)
- GE Healthcare (États-Unis)
- NIHON KOHDEN CORPORATION (Japon)
- LivaNova PLC (Royaume-Uni)
- BioSerenity (France)
- Zeto, Inc. (États-Unis)
- Ceribell, Inc. (États-Unis)
- Seer Medical (Australie)
- SleepMed, Inc. (États-Unis)
- Advanced Brain Monitoring, Inc. (États-Unis)
Quels sont les développements récents sur le marché des dispositifs de surveillance de l’épilepsie en Amérique du Nord ?
- En avril 2025, l'application EpiWatch pour Apple Watch a reçu l'autorisation de la FDA. Elle propose des fonctionnalités telles que les rappels de prise de médicaments, l'enregistrement des crises et le suivi de la santé mentale. Développée en collaboration avec Johns Hopkins Medicine et optimisée par ResearchKit d'Apple, cette plateforme améliore le suivi à distance des personnes épileptiques.
- En avril 2025, la FDA a accordé une autorisation de mise sur le marché (De Novo) au Minder d'Epiminder, un système implantable de surveillance EEG continue. Ce dispositif est le premier du genre à être approuvé aux États-Unis pour les patients épileptiques et devrait être lancé fin 2025 dans les principaux centres de soins spécialisés.
- En avril 2025, Seer Medical, une entreprise australienne de technologie médicale spécialisée dans le suivi à domicile de l'épilepsie, a obtenu un financement de 40 millions de dollars auprès de Breakthrough Victoria. Ce financement vise à soutenir les activités de l'entreprise et à faciliter son expansion sur le marché américain, malgré des difficultés telles qu'un rappel de produit et des difficultés financières.
- En février 2025, Medtronic a reçu l'approbation de la FDA pour son système de stimulation cérébrale profonde adaptative (SCPa) BrainSense. Bien que principalement destiné à la maladie de Parkinson, l'approbation de la FDA et les annonces de Medtronic soulignent que l'engagement de l'entreprise à faire progresser la recherche sur la SCP transforme également les options thérapeutiques pour les personnes épileptiques.
- En février 2024, Empatica a annoncé le lancement d'EpiMonitor, son système de surveillance de l'épilepsie nouvelle génération, approuvé par la FDA. EpiMonitor représente une avancée significative dans le domaine des technologies portables pour l'épilepsie, offrant jusqu'à une semaine d'autonomie, des capacités de détection des crises améliorées et une application mobile intuitive fournissant des informations complètes sur la santé, notamment le suivi du sommeil et de l'activité.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.