North America Chinese Hamster Ovary Cells Cho Market
Taille du marché en milliards USD
TCAC :
% 
 USD
158.66 Million
USD
293.69 Million
2024
2032
USD
158.66 Million
USD
293.69 Million
2024
2032
| 2025 –2032 | |
| USD 158.66 Million | |
| USD 293.69 Million | |
|
|
|
|
Cellules ovariennes de hamster chinois (CHO) en Amérique du Nord : segmentation, type (services et produits), système (système de sélection métabolique, système de sélection d’antibiotiques et autres), application (produits biologiques et recherche médicale), utilisateur final (entreprises biopharmaceutiques, entreprises de biotechnologie, organismes de développement clinique et de fabrication, organismes de recherche clinique, instituts et organismes de recherche universitaires et autres), canal de distribution (appels d’offres directs, vente au détail et autres), pays (États-Unis, Canada, Mexique) – Tendances et prévisions du secteur jusqu’en 2032
Taille du marché des cellules ovariennes de hamster chinois (CHO) en Amérique du Nord
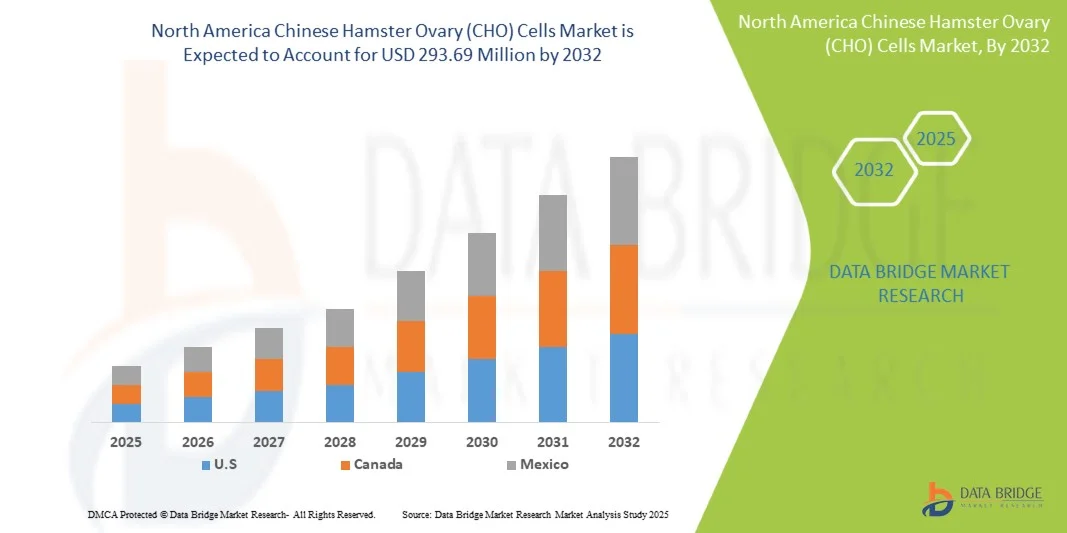
- Le marché nord-américain des cellules ovariennes de hamster chinois (CHO) était évalué à 158,66 millions de dollars en 2024 et devrait atteindre 293,69 millions de dollars d'ici 2032.
- Au cours de la période prévisionnelle allant de 2025 à 2032, le marché devrait croître à un TCAC de 8,0 %, principalement grâce à l'utilisation croissante des cellules CHO dans les études génétiques.
- Cette croissance est alimentée par des facteurs tels que la demande croissante de produits biologiques, les progrès en matière d'ingénierie des lignées cellulaires, l'augmentation des investissements dans la recherche en biotechnologie et l'expansion des applications en thérapie génique.
Analyse du marché des cellules ovariennes de hamster chinois (CHO) en Amérique du Nord
- Les cellules CHO (ovaires de hamster chinois) sont une lignée cellulaire de mammifères couramment utilisée en biotechnologie et en recherche biomédicale, notamment pour la culture cellulaire et les bioprocédés. Elles sont devenues des outils essentiels dans ces domaines. Les cellules CHO sont reconnues pour leur adhérence aux supports de culture, leur croissance rapide et leur capacité à exprimer des protéines recombinantes.
- L'une des principales applications des cellules CHO réside dans la production de protéines recombinantes, notamment d'anticorps thérapeutiques et d'enzymes. Elles peuvent être génétiquement modifiées pour exprimer des gènes d'intérêt spécifiques, et leur capacité à effectuer des modifications post-traductionnelles similaires à celles des cellules humaines garantit la qualité des produits biopharmaceutiques. Les cellules CHO sont également reconnues pour leur stabilité génétique, ce qui leur permet d'introduire des modifications génétiques de manière constante sur plusieurs générations, assurant ainsi la fiabilité et la reproductibilité des bioprocédés.
- En 2025, le segment des services devrait dominer le marché avec une part de marché de 65,50 % en raison de la demande croissante de services spécialisés liés au développement de lignées cellulaires CHO, au bioprocédé et à la fabrication à façon.
Portée du rapport et segmentation du marché des microscopes opératoires ophtalmiques
|
Attributs |
Aperçu du marché des microscopes opératoires ophtalmiques |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur du marché, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché élaborés par Data Bridge Market Research comprennent également une analyse des importations et des exportations, un aperçu de la capacité de production, une analyse de la consommation de production, une analyse des tendances des prix, un scénario de changement climatique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, les critères de sélection des fournisseurs, une analyse PESTLE, une analyse de Porter et le cadre réglementaire. |
Tendances du marché des cellules ovariennes de hamster chinois (CHO) en Amérique du Nord
« Intégration croissante de l’intelligence artificielle (IA) dans le développement des lignées cellulaires »
- Une tendance marquante du marché nord-américain des cellules d'ovaires de hamster chinois (CHO) est l'intégration croissante de l'intelligence artificielle (IA) dans le développement des lignées cellulaires et l'optimisation des bioprocédés.
- Les algorithmes d'IA sont utilisés pour analyser de grands ensembles de données, prédire les conditions de culture optimales et identifier les clones à haut rendement plus efficacement que les méthodes traditionnelles.
- Par exemple, les modèles d'apprentissage automatique peuvent traiter des données expérimentales pour prédire les performances cellulaires, réduisant ainsi le temps et les coûts associés aux approches par essais et erreurs dans la production à base de cellules CHO.
- Cette technologie permet des délais de développement plus rapides, une meilleure qualité des produits et une meilleure évolutivité de la production biologique.
- L'utilisation de l'IA transforme la recherche sur les cellules CHO et la bioproduction, ce qui se traduit par une efficacité accrue, des coûts de développement réduits et un avantage concurrentiel pour les entreprises qui adoptent des solutions numériques avancées.
Dynamique du marché des cellules ovariennes de hamster chinois (CHO) en Amérique du Nord
Conducteur
« Utilisation croissante des cellules CHO dans les études génétiques »
- L'utilisation croissante des cellules ovariennes de hamster chinois (CHO) en recherche génétique est un moteur clair et en pleine expansion du marché nord-américain des CHO. Avec la maturation des outils génétiques (CRISPR, RMCE, RNA-seq, multi-omique unicellulaire), les cellules CHO ne sont plus de simples hôtes de production ; elles constituent désormais des plateformes expérimentales permettant d'étudier les relations génotype-phénotype, de tester des stratégies d'édition génique et de concevoir rationnellement des caractéristiques de l'hôte (croissance, productivité, glycosylation, tolérance au stress).
- Ce changement élargit la demande sur trois vecteurs de marché liés : (1) en amont — lignées cellulaires CHO sous licence/modifiées et services d'édition de gènes ; (2) développement de procédés — outils d'analyse, d'omique, de jumeau numérique et d'IA nécessaires pour traduire les connaissances génétiques en clones stables à haut rendement ; et (3) fabrication — capacité CDMO et plateformes à usage unique/continues optimisées pour les hôtes génétiquement améliorés.
- En bref, l'activité d'étude génétique alimente un écosystème en expansion (logiciels, analyses, banques de cellules, réactifs, production CDMO), augmentant à la fois l'étendue et la profondeur des offres commerciales CHO et augmentant ainsi la taille du marché, la facturation et l'importance stratégique des capacités de fabrication dérivées des CHO.
- Par exemple, en janvier 2025, un article du NIH indiquait qu'un ensemble de données de criblage par inactivation CRISPR non virale à l'échelle du génome pour les cellules CHO-K1 et les cellules recombinantes dérivées avait été publié (Sci Data), fournissant une ressource complète pour identifier les gènes qui affectent la capacité de la cellule et la production de protéines - preuve que le criblage génomique fonctionnel dans les cellules CHO est maintenant mature, documenté publiquement et informe directement les programmes d'ingénierie cellulaire.
- En avril 2025, un article publié dans NIH indiquait qu'une méta-analyse des transcriptomes CHO (« Fantastic genes… ») avait été acceptée et publiée sur PMC (accès libre) après révision début 2025 ; l'article intègre des données RNA-seq et épigénétiques sur des centaines d'échantillons CHO et souligne la nécessité d'une ingénierie génétique ciblée pour contrôler les programmes d'expression — il s'agit d'une preuve directe que les études génomiques/épigénomiques des CHO définissent des cibles d'ingénierie pour l'amélioration des lignées cellulaires industrielles.
- L'utilisation croissante des cellules CHO dans les études génétiques renforce non seulement leur position dominante en tant que système d'expression de référence pour les produits biologiques, mais élargit également leur rôle en tant que plateformes expérimentales pour la génomique fonctionnelle, les tests réglementaires et l'ingénierie cellulaire.
Opportunité
« Développement continu des technologies de culture cellulaire »
- Les progrès constants des technologies de culture cellulaire créent un ensemble d'opportunités uniques pour le marché nord-américain des cellules d'ovaires de hamster chinois (CHO).
- Les améliorations apportées à la conception des bioréacteurs et aux procédés de perfusion, associées à une ingénierie précise des lignées cellulaires, à des outils d'intégration spécifiques au site et à une optimisation des procédés basée sur l'apprentissage automatique, augmentent constamment la productivité, la stabilité et la qualité des produits CHO tout en réduisant les coûts par lot.
- Par exemple, en juin 2021, l'Organisation mondiale de la santé a indiqué qu'elle soutenait un consortium sud-africain visant à établir le premier centre de transfert de technologie pour les vaccins à ARNm contre la COVID afin de développer les capacités de production régionales et de partager le savoir-faire en matière de production de produits biologiques avancés.
- En avril 2022, Medicines Patent Pool a rapporté que l'Organisation mondiale de la santé et le MPP avaient annoncé que 15 fabricants recevraient une formation du programme de transfert de technologie de l'ARNm de l'OMS, démontrant un effort public pluriannuel continu pour diffuser les capacités de fabrication avancées de vaccins et de produits biologiques.
- Les exemples sélectionnés montrent collectivement que le développement continu des technologies de culture cellulaire ne se limite plus aux laboratoires isolés ou aux feuilles de route des fournisseurs ; il est activement accéléré par des efforts publics coordonnés, des mises à jour réglementaires et des innovations techniques évaluées par les pairs.
Retenue/Défi
« Le coût élevé de la production à base de cellules CHO constitue un frein au marché »
- Les coûts d'investissement et d'exploitation élevés associés à la production de produits biologiques à base de cellules CHO constituent un frein majeur au marché nord-américain des cellules CHO.
- Des investissements initiaux importants dans la capacité des bioréacteurs, les salles blanches, les systèmes à usage unique ou l'infrastructure en acier inoxydable, et les équipements de purification en aval spécialisés — combinés à des matières premières coûteuses (milieux, résines de chromatographie), à une main-d'œuvre qualifiée, à une conformité réglementaire complexe et à de longs délais de validation — augmentent le coût total de possession pour les fabricants et les CDMO.
- Ces pressions sur les coûts augmentent les délais de mise sur le marché, compriment les marges, dissuadent les petits développeurs de fabriquer en interne et peuvent ralentir l'adoption dans les régions à faibles revenus ; collectivement, elles limitent la demande de nouvelles lignées cellulaires, de consommables et de contrats de service liés aux plateformes CHO.
- Par exemple, en juillet 2025, le Bureau du secrétaire adjoint à la planification et à l'évaluation du département américain de la Santé et des Services sociaux (HHS-ASPE) a publié une note d'information soulignant que les dépenses consacrées aux produits biologiques ont considérablement augmenté et que la complexité et le profil de coût des produits biologiques (développement et fabrication) sont des facteurs importants de l'augmentation des dépenses pharmaceutiques, ce qui met en évidence la contrainte économique que représentent les coûts de fabrication des produits biologiques/CHO.
- En mars 2024, la Commission européenne a publié des documents de politique et de recherche (analyses du Centre commun de recherche / DG R&I) soulignant que les biotechnologies et la bioproduction nécessitent des équipements hautement spécialisés et une main-d'œuvre multidisciplinaire qualifiée – des facteurs qui augmentent les coûts unitaires de fabrication et constituent des obstacles à l'augmentation de la production dans toute l'UE.
- Bien que la production de protéines thérapeutiques et d'anticorps monoclonaux par culture cellulaire CHO soit la méthode de référence, son développement reste fortement limité par des coûts élevés. Les infrastructures coûteuses, les matières premières onéreuses, la main-d'œuvre spécialisée requise et les procédures de conformité rigoureuses alourdissent les dépenses de production, limitant la participation des petites entreprises et entravant l'accès aux marchés émergents.
Portée du marché des cellules ovariennes de hamster chinois (CHO) en Amérique du Nord
Le marché est segmenté en fonction du type, du système, de l'application, de l'utilisateur final et du canal de distribution.
- Par type
Le marché est segmenté en fonction du type de prestations (services, produits et autres). En 2025, le segment des services devrait dominer le marché avec 66,50 % de parts de marché et afficher la croissance la plus rapide (TCAC de 8,3 %). Cette croissance s'explique par le recours accru aux organismes de recherche sous contrat (CRO) et aux organismes de développement et de fabrication sous contrat (CDMO) pour le développement de lignées cellulaires spécialisées, l'optimisation des procédés et la bioproduction à grande échelle. Par ailleurs, la complexité croissante de la production de produits biologiques, les coûts élevés liés aux installations internes et la demande croissante de solutions personnalisées incitent les entreprises pharmaceutiques et biotechnologiques à externaliser davantage de services. L'ensemble de ces facteurs contribue à l'expansion du segment des services, renforçant ainsi sa part de marché et son potentiel de croissance au cours de la période prévisionnelle.
- Par système
Selon le système utilisé, le marché est segmenté en systèmes de sélection métabolique, systèmes de sélection antibiotique et autres. En 2025, le segment des systèmes de sélection métabolique devrait dominer le marché avec 58,17 % et afficher la croissance la plus rapide (TCAC de 8,3 %). Cette croissance est principalement due à l'adoption croissante des systèmes de sélection métabolique pour une production de protéines recombinantes stable et à haut rendement. Ces systèmes éliminent en effet le besoin de marqueurs de résistance aux antibiotiques, réduisent les contraintes réglementaires et améliorent l'efficacité des cultures. Par ailleurs, la préférence grandissante pour des techniques de développement de lignées cellulaires plus sûres, économiques et adaptables à grande échelle dans la fabrication de produits biologiques et biosimilaires contribue également à l'expansion de ce segment.
- Sur demande
En fonction de l'application, le marché est segmenté en produits biologiques et recherche médicale. En 2025, le segment des produits biologiques devrait dominer le marché avec 71,48 % des parts de marché et afficher la croissance la plus rapide, avec un TCAC de 8,2 %. Cette croissance est principalement due à la demande croissante de protéines thérapeutiques, d'anticorps monoclonaux et de vaccins, conjuguée à la prévalence accrue des maladies chroniques et auto-immunes. Par ailleurs, les progrès des technologies de bioprocédés, l'augmentation des investissements en R&D biopharmaceutique et l'adoption croissante des cellules CHO (ovaires de hamster chinois) pour une production à grande échelle et de haute qualité contribuent à consolider la position dominante et à accélérer la croissance du segment des produits biologiques sur le marché.
- Par l'utilisateur final
On the basis of end user, the market is segmented into Biopharmaceutical Companies, Biotechnology Companies, Clinical Development and Manufacturing Organizations, Clinical Research Organizations, Academic Institutes and Research Organizations, and Others, In 2025, the Biopharmaceutical Companies segment is expected to dominate the market with 43.07%, This dominance is driven by the increasing focus of biopharmaceutical companies on developing biologics, monoclonal antibodies, and gene therapies, which heavily rely on advanced cell lines like CHO cells for high-yield and high-quality protein production. Additionally, rising R&D investments, the expansion of biologics pipelines, and the need for scalable and cost-effective production processes further reinforce the leading position of biopharmaceutical companies in the market.
- By Distribution Channel
On the basis of Distribution Channel, the market is segmented into Direct Tenders, Retail Sales, and Others, In 2025, the Direct Tenders segment is expected to dominate the market with 55.46%, and Retail Sales expected to be fastest growing segment with 7.8% CAGR. The dominance of direct tenders is driven by bulk procurement by large biopharmaceutical and research organizations seeking cost efficiency, reliable supply, and long-term contracts. Meanwhile, the rapid growth of retail sales is fueled by increasing accessibility of research products to smaller laboratories and academic institutes, rising demand for ready-to-use kits, and the expansion of e-commerce platforms for scientific supplies, making products more readily available across diverse end users.
North America Chinese Hamster Ovary (CHO) Cells Market Regional Analysis
“North America is the Dominant Region in the Chinese Hamster Ovary (CHO) Cells”
- U.S. holds the largest share in the North America CHO cells market, driven by a well-established biopharmaceutical industry, advanced healthcare infrastructure, and the strong presence of leading pharmaceutical and biotechnology companies.
- The United States, in particular, contributes significantly to regional dominance due to high R&D spending, increasing demand for biologics, and extensive use of CHO cells in the production of therapeutic proteins such as monoclonal antibodies.
- Government support for biologics development, coupled with robust regulatory frameworks and favorable reimbursement policies, further accelerates market growth. Moreover, strategic collaborations, technological advancements in cell line development, and an increase in FDA approvals for CHO cell-derived products continue to boost market expansion across the region.
U.S. CHO cells Market Insight
- The U.S. CHO cells market accounted for the largest market revenue share in the North American CHO cells market in 2025, attributed to the country’s well-established biopharmaceutical industry, significant R&D investments, presence of major biotechnology and pharmaceutical companies, advanced biologics manufacturing infrastructure, and strong government support for innovation in cell line development and biologics production.
Canada CHO cells Market Insight
- The Canada CHO cells market is expected to register a significant CAGR in North America from 2025 to 2032, driven by growing investments in biopharmaceutical research, increasing adoption of advanced cell line technologies, expansion of biologics and biosimilar production facilities, and supportive government initiatives promoting biotechnology and life sciences innovation.
North America Chinese Hamster Ovary (CHO) Cells Market Share
The market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, North America presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to market.
The Major Market Leaders Operating in the Market Are:
- Thermo Fisher Scientific Inc. (U.S.)
- AcceGen (U.S.)
- RayBiotech Life, Inc. (U.S.)
- Cytion (Germany)
- BPS Bioscience, Inc. (U.S.)
- GenTarget Inc. (U.S.)
- Merck KGaA (Germany)
- Promega Corporation (U.S.)
- Abeomics (U.S.)
- Applied Biological Materials Inc. (Canada)
- ATCC (U.S.)
- Sartorius AG (Germany)
- Lonza (Switzerland)
- Revvity Discovery Limited (U.S.)
- Cytiva (U.S.)
- GTP Bioways (France)
- Curia North America, Inc. (U.S.)
- Abbott (U.S.)
Latest Developments in North America Chinese Hamster Ovary (CHO) Cells
- In February 2022, Sartorius acquired business from Novasep, added a complementary offering to its chromatography portfolio. The acquired portfolio includes chromatography systems primarily suited for small biomolecules such as oligonucleotides, peptides, and insulin, and innovative systems for the continuous production of biopharmaceuticals.
- In July 2023, Lonza launched TheraPRO CHO Media System, a new cell culture medium that simplifies processes and optimizes productivity and protein quality when using GS-CHO cell lines. The start-up supports pharmaceutical and biotech companies producing therapeutic proteins to further improve product quality. The TheraPRO CHO Media System provides efficient performance, achieving high concentrations of viable cells and protein titers above 5 g/L over a 15-day culture period. This represents more than double the protein titer that can be produced with commercially available solutions. This launch has helped the company to expand its product portfolio in the market.
- In October 2022, Thermo Fisher Scientific Inc. collaborated with ProBioGen to develop better platform the Gibco Freedom ExpiCHO-S Cell Line Development Kit. This kit allows users to generate cell lines suitable for clinical development without their own original cells, vectors, or previous experience in the field. ProBioGen significantly contributed to the performance of the Freedom ExpiCHO-S kit by leveraging its strong expertise in cell line and process development. The new series utilizes Thermo Fisher's ExpiCHO-S cell line expanding the company's product portfolio for the CHO cell line development series.
- In July 2023, Merck announced that it is expanding its facility in Lenexa, Kansas, U.S., adding 9,100 square meters of laboratory space and production capacity for the production of cell culture media. This expansion makes Lenexa the company's largest dry powder cell culture facility and a center of excellence in North America. The investment in the region reflects the company's strategy to expand and diversify its supply chain to meet current and future demand for cell culture platforms.
- In November 2022, ATCC, the world's leading regulatory and standards organization for biological materials, announced a new line of CAR-T Target luciferase reporter cell lines to support immuno-oncology (IO) discovery and the development of new immunotherapies. These models have a high endogenous expression of relevant chimeric antigen receptor (CAR) T target antigens such as HER2, CD19, and CD20. These new IO tools consist of both hematologic cancers and solid tumor cell lines expressing a luciferase reporter. This helped the company to expand its product portfolio.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTAL ANALYSIS
4.2 PORTER'S FIVE FORCES ANALYSIS
4.3 PATENT ANALYSIS – NORTH AMERICA CHO CELLS MARKET
4.3.1 PATENT QUALITY AND STRENGTH
4.3.2 PATENT FAMILIES
4.3.3 LICENSING AND COLLABORATIONS
4.3.4 COMPETITIVE LANDSCAPE
4.3.5 IP STRATEGY AND MANAGEMENT
4.3.6 OTHER OBSERVATIONS
4.4 INDUSTRY INSIGHTS
4.4.1 MICRO AND MACRO ECONOMIC FACTORS
4.4.2 PENETRATION AND GROWTH PROSPECT MAPPING
4.4.3 KEY PRICING STRATEGIES
4.5 INNOVATION TRACKER AND STRATEGIC ANALYSIS
4.5.1 MAJOR DEALS AND STRATEGIC ALLIANCES ANALYSIS
4.5.1.1 JOINT VENTURES
4.5.1.2 MERGERS AND ACQUISITIONS
4.5.1.3 LICENSING AND PARTNERSHIP
4.5.1.4 TECHNOLOGY COLLABORATIONS
4.5.1.5 STRATEGIC DIVESTMENTS
4.5.2 NUMBER OF PRODUCTS IN DEVELOPMENT
4.5.3 STAGE OF DEVELOPMENT
4.5.4 TIMELINES AND MILESTONES
4.5.5 INNOVATION STRATEGIES AND METHODOLOGIES
4.5.6 RISK ASSESSMENT AND MITIGATION
4.5.7 FUTURE OUTLOOK
4.6 OPPORTUNITY MAP
4.7 PRICING ANALYSIS – NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET
4.8 RAW MATERIAL COVERAGE
4.9 VALUE CHAIN ANALYSIS
4.1 CONSUMER BUYING BEHAVIOUR
4.11 TECHNOLOGICAL ADVANCEMENTS
5 TARIFFS & IMPACT ON THE MARKET
5.1 CURRENT TARIFF RATE
5.2 OUTLOOK — LOCAL PRODUCTION VS. IMPORT RELIANCE
5.3 VENDOR SELECTION CRITERIA DYNAMICS
5.4 IMPACT ON SUPPLY CHAIN
5.4.1 RAW MATERIAL PROCUREMENT
5.4.2 MANUFACTURING AND PRODUCTION
5.4.3 LOGISTICS AND DISTRIBUTION
5.4.4 PRICE PITCHING AND MARKET POSITION
5.5 INDUSTRY PARTICIPANTS
5.5.1 SUPPLY CHAIN OPTIMIZATION
5.5.2 JOINT VENTURE AND LOCAL PARTNERSHIPS
5.6 IMPACT ON PRICES
5.7 REGULATORY INCLINATION
5.7.1 GEOPOLITICAL SITUATION
5.7.2 TRADE PARTNERSHIPS BETWEEN COUNTRIES
5.7.2.1 FREE TRADE AGREEMENTS
5.7.2.2 ALLIANCES ESTABLISHMENTS
5.7.3 STATUS ACCREDITATION (INCLUDING MFN)
5.7.4 DOMESTIC COURSE OF CORRECTION
5.7.4.1 INCENTIVE SCHEMES TO BOOST PRODUCTION OUTPUTS
5.7.4.2 ESTABLISHMENT OF SPECIAL ECONOMIC ZONES / INDUSTRIAL PARKS
6 REGULATORY FRAMEWORK – NORTH AMERICA CHO CELLS MARKET
6.1 NORTH AMERICA
6.2 EUROPE
6.3 ASIA-PACIFIC
6.4 SOUTH AMERICA
6.5 MIDDLE EAST & AFRICA
7 MARKET OVERVIEW
7.1 DRIVER
7.1.1 RISING USE OF CHO CELLS IN THE GENETIC STUDY
7.1.2 GROWING DEMAND FOR BIOPHARMACEUTICALS
7.1.3 RISING INVESTMENTS IN BIOTECHNOLOGY R&D
7.1.4 RISING DEMAND FOR MONOCLONAL ANTIBODIES
7.2 RESTRAINT
7.2.1 HIGH COST OF CHO CELL–BASED PRODUCTION AS A MARKET RESTRAINT
7.2.2 STRICT REGULATORY REQUIREMENTS FOR CHO CELL-BASED PRODUCTION
7.3 OPPORTUNITY
7.3.1 CONTINUOUS DEVELOPMENT OF CELL-CULTURE TECHNOLOGIES
7.3.2 RISING NUMBER OF APPLICATIONS OF CHO CELLS
7.3.3 ADVANCES IN CELL-LINE ENGINEERING & SYNTHETIC BIOLOGY
7.4 CHALLENGES
7.4.1 TIME-CONSUMING AND INCONSISTENCY IN CHO CELL LINE DEVELOPMENT PROCESS
7.4.2 CONTAMINATION RISK OF CHO CELL CULTURES
8 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE
8.1 OVERVIEW
8.2 SERVICES
8.3 PRODUCT
8.3.1 CHO-K1
8.3.1.1 CHO-K1 ATCC
8.3.1.2 CHO-K1 ECACC
8.3.1.3 Others
8.3.2 CHO-DG44
8.3.3 CHO-S
8.3.4 CHO-DXB11
8.3.5 OTHERS
9 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM
9.1 OVERVIEW
9.2 METABOLIC SELECTION SYSTEM
9.3 ANTIBIOTIC SELECTION SYSTEM
9.4 OTHERS
10 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION
10.1 OVERVIEW
10.2 BIOLOGICS
10.2.1 MONOCLONAL ANTIBODIES
10.2.2 FC-FUSION PROTIEN
10.2.3 ENZYMES
10.2.4 HORMONES
10.2.5 CYTOKINES
10.2.6 CLOTTING FACTORS
10.2.7 OTHERS
10.3 MEDICAL RESEARCH
11 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER
11.1 OVERVIEW
11.2 BIOPHARMACEUTICAL COMPANIES
11.2.1 MEDIUM
11.2.2 SMALL
11.3 CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS
11.3.1 MEDIUM
11.3.2 SMALL
11.4 BIOTECHNOLOGY COMPANIES
11.4.1 MEDIUM
11.4.2 SMALL
11.5 ACADEMIC INSTITUTES AND RESEARCH ORGANIZATIONS
11.6 CLINICAL RESEARCH ORGANIZATIONS
11.6.1 MEDIUM
11.6.2 SMALL
11.7 OTHERS
12 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDERS
12.3 RETAIL SALES
12.4 OTHERS
13 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 SARTORIUS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 LONZA
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENT
16.3 THERMO FISHER SCIENTIFIC INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 CYTIVA
16.4.1 COMPANY SNAPSHOT
16.4.2 COMPANY SHARE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENT
16.5 MERCK KGAA
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 ABEOMICS
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENT
16.7 ACCEGEN
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENT
16.8 APPLIED BIOLOGICAL MATERIALS INC. (ABM)
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 ATCC
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENT
16.1 BPS BIOSCIENCE, INC.
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 CYTION
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENT
16.12 CURIA GLOBAL, INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 SERVICE PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 GENTARGET INC.
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENT
16.14 GTP BIOWAYS
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENT
16.15 PROMEGA CORPORATION
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENT
16.16 RAYBIOTECH LIFE, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENT
16.17 REVVITY DISCOVERY LIMITED.
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 2 NORTH AMERICA SERVICES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 3 NORTH AMERICA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 4 NORTH AMERICA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 5 NORTH AMERICA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 6 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 7 NORTH AMERICA METABOLIC SELECTION SYSTEM IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 8 NORTH AMERICA ANTIBIOTIC SELECTION SYSTEM IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 9 NORTH AMERICA OTHERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 10 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 11 NORTH AMERICA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 12 NORTH AMERICA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 13 NORTH AMERICA MEDICAL RESEARCH IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 14 NORTH AMERICA CHINESE HAMSTER OVARY CELLS CHO MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 15 NORTH AMERICA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 16 NORTH AMERICA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 17 NORTH AMERICA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 18 NORTH AMERICA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 19 NORTH AMERICA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 20 NORTH AMERICA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 21 NORTH AMERICA ACADEMIC INSTITUTES AND RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 22 NORTH AMERICA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 23 NORTH AMERICA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 24 NORTH AMERICA OTHERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 25 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 26 NORTH AMERICA DIRECT TENDERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 27 NORTH AMERICA RETAIL SALES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 28 NORTH AMERICA OTHERS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY REGION, 2018-2032 (USD THOUSAND)
TABLE 29 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY COUNTRY, 2018-2032 (USD THOUSAND)
TABLE 30 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 31 NORTH AMERICA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 32 NORTH AMERICA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 33 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 34 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 35 NORTH AMERICA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 36 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 37 NORTH AMERICA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 38 NORTH AMERICA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 39 NORTH AMERICA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 40 NORTH AMERICA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 41 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 42 U.S. CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 43 U.S. PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 44 U.S. CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 45 U.S. CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 46 U.S. CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 47 U.S. BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 48 U.S. CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 49 U.S. BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 50 U.S. CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 51 U.S. BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 52 U.S. CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 53 U.S. CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 54 CANADA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 55 CANADA PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 56 CANADA CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 57 CANADA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 58 CANADA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 59 CANADA BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 60 CANADA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 61 CANADA BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 62 CANADA CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 63 CANADA BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 64 CANADA CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 65 CANADA CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
TABLE 66 MEXICO CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 67 MEXICO PRODUCT IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 68 MEXICO CHO-K1 IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 69 MEXICO CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY SYSTEM, 2018-2032 (USD THOUSAND)
TABLE 70 MEXICO CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY APPLICATION, 2018-2032 (USD THOUSAND)
TABLE 71 MEXICO BIOLOGICS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 72 MEXICO CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY END USER, 2018-2032 (USD THOUSAND)
TABLE 73 MEXICO BIOPHARMACEUTICAL COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 74 MEXICO CLINICAL DEVELOPMENT AND MANUFACTURING ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 75 MEXICO BIOTECHNOLOGY COMPANIES IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 76 MEXICO CLINICAL RESEARCH ORGANIZATIONS IN CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY TYPE, 2018-2032 (USD THOUSAND)
TABLE 77 MEXICO CHINESE HAMSTER OVARY (CHO) CELLS MARKET, BY DISTRIBUTION CHANNEL, 2018-2032 (USD THOUSAND)
Liste des figures
FIGURE 1 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EXECUTIVE SUMMARY: NORTH AMERICA CHINESE HAMSTER OVARY CELLS CHO MARKET
FIGURE 11 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: SEGMENTATION
FIGURE 12 STRATEGIC DECISIONS
FIGURE 13 TWO SEGMENTS COMPRISE THE NORTH AMERICA CHINESE HAMSTER OVARY CELLS CHO MARKET, BY TYPE
FIGURE 14 RISING DEMAND FOR BIOLOGICS AND THERAPEUTIC PROTEINS EXPECTED TO DRIVE THE NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET GROWTH IN THE FORECAST PERIOD OF 2025 TO 2032
FIGURE 15 TYPE SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET IN THE FORECAST PERIOD OF 2025 & 2032
FIGURE 16 NUMBER OF PATENTS PER COUNTRY OR REGION
FIGURE 17 NUMBER OF PATENTS PER APPLICANTS
FIGURE 18 NUMBER OF PATENTS PER YEAR.
FIGURE 19 DROC ANALYSIS
FIGURE 20 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, 2024
FIGURE 21 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, 2025 TO 2032 (USD THOUSAND)
FIGURE 22 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, CAGR (2025- 2032)
FIGURE 23 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, 2024
FIGURE 25 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, 2025 TO 2032 (USD THOUSAND)
FIGURE 26 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, CAGR (2025- 2032)
FIGURE 27 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY SYSTEM, LIFELINE CURVE
FIGURE 28 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, 2024
FIGURE 29 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, 2025 TO 2032 (USD THOUSAND)
FIGURE 30 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, CAGR (2025- 2032)
FIGURE 31 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 32 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, 2024
FIGURE 33 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, 2025 TO 2032 (USD THOUSAND)
FIGURE 34 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, CAGR (2025- 2032)
FIGURE 35 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY END USER, LIFELINE CURVE
FIGURE 36 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, 2024
FIGURE 37 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, 2025 TO 2032 (USD THOUSAND)
FIGURE 38 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2025- 2032)
FIGURE 39 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 40 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: SNAPSHOT (2022)
FIGURE 41 NORTH AMERICA CHINESE HAMSTER OVARY (CHO) CELLS MARKET: COMPANY SHARE 2024 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.