North America At-Home Testing Kits Market, By Test Type (Pregnancy Test, HIV Test Kit, Diabetes, Infectious Diseases, Glucose Tests, Ovulation Predictor Test Kit, Drug Abuse Test Kit, and Others), Type (Cassette, Strip, Midstream, Test Panel, Dip Card and Others), Age (Pediatric, Adult and Geriatric), Sample Type (Urine, Blood, Saliva and Other Sample Types), Usage (Disposable and Reusable), Distribution Channels (Retail Pharmacies, Drug Store, Supermarket/Hypermarket and Online Pharmacies)- Industry Trends and Forecast to 2029.
North America At-Home Testing Kits Market Analysis and Insights
North America at-home testing kits market is expected to grow as earlier, people used to visit hospitals often, even for basic problems, but due to the rising awareness regarding several products, this behavior has changed and has turned the trend. At-home or self-testing kits are easily available at pharmacies, and it has become extremely easy to procure them. Various medical companies are venturing into this space as they rapidly manufacture self-test kits.
This widespread availability can also be attributed to online pharmacies' medical start-ups, making availability easier by clicking a button. In addition, these self-testing kits are available without any prescription which can easily drive the at-home testing kits market. At-home testing kits allow end-users to collect their specimen at home and then either perform the tests at home or send that specimen to the lab for testing. At-home testing kits have undoubtedly eased the process of confirming the person's concern, whether it is a home pregnancy test or HIV, or any other infectious diseases test.
These at-home testing kits are easy to use and are affordable too. However, there is always a doubt about the accuracy of the results that has become a restraint for the North America at-home testing kits market. A false positive result of a test may cause anxiety and stress to the person, even if they do not have it. It is very upsetting and disturbing to the person to receive false positive or negative results. Today, many companies produce rapid diagnostic test kits for COVID-19, which can be performed at home. But there are various accuracy-related issues due to which the distribution of those at-home test kits that has been suspended from verifying their reliability.
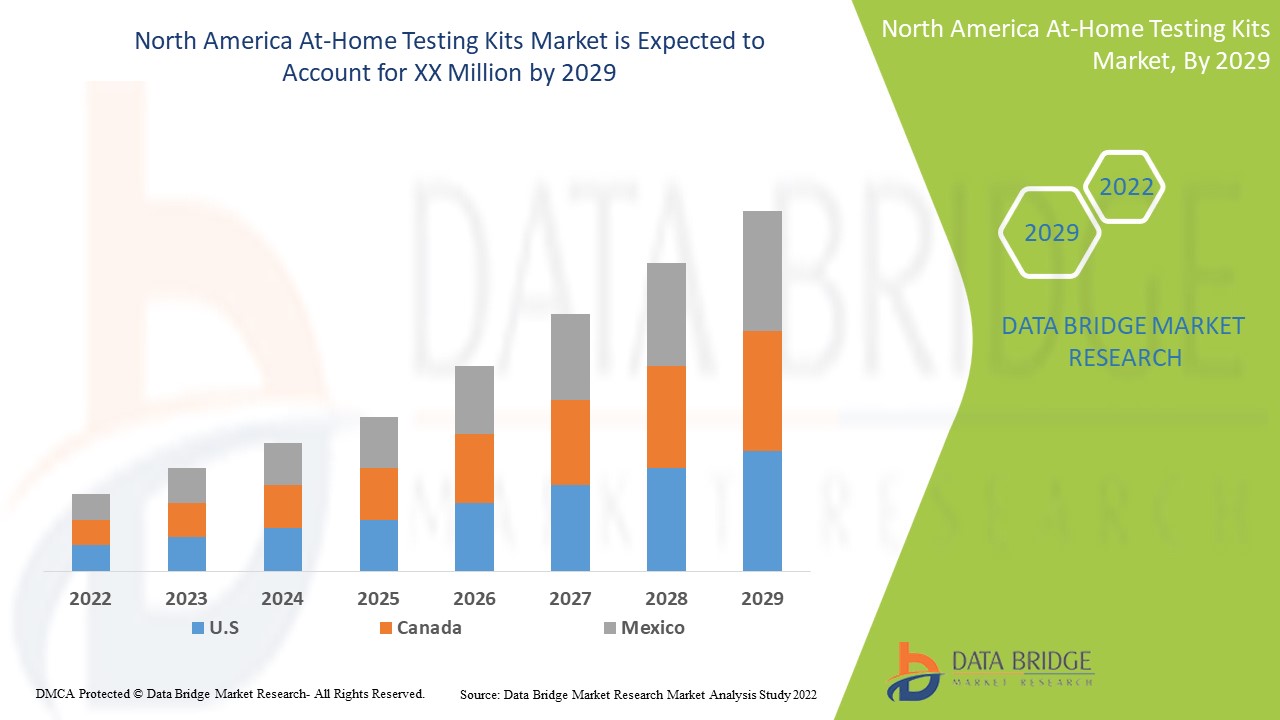
Data Bridge Market Research analyzes that the North America at-home testing kits market will grow at a CAGR of 6.3% during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019-2014) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
Par type de test (test de grossesse, kit de test du VIH, diabète, maladies infectieuses, tests de glycémie, kit de test de prédiction de l'ovulation, kit de test de toxicomanie et autres), type (cassette, bandelette, test intermédiaire, panel de test, carte à immersion et autres), âge (pédiatrique, adulte et gériatrique), type d'échantillon (urine, sang, salive et autres types d'échantillons), utilisation (jetable et réutilisable), canaux de distribution (pharmacies de détail, parapharmacies, supermarchés/hypermarchés et pharmacies en ligne) |
|
Pays couverts |
États-Unis, Canada et Mexique |
|
Acteurs du marché couverts |
Abbott, Siemens Healthcare GmbH, F. Hoffmann-La Roche Ltd, BD, Drägerwerk AG & Co. KGaA, LifeScan IP Holdings, LLC, Ascensia Diabetes Care Holdings AG, Nectar Lifesciences Ltd. (Rapikit), ACON Laboratories, Inc., Quidel Corporation, ARKRAY USA, Inc., BTNX INC., Atomo Diagnostics, Eurofins Scientific, Piramal Enterprises Ltd., Bionime Corporation, Nova Biomedical, Cardinal Health, OraSure Technologies, Biolytical Laboratories Inc., Everlywell, Inc., SA Scientific Ltd., Clearblue (une filiale de Swiss Precision Diagnostics GmbH), Biosynex, PRIMA Lab SA, MP BIOMEDICALS, Sterilab Services, Chembio Diagnostics, Inc., BioSure, Selfdiagnostics OU, entre autres |
Définition du marché des kits de test à domicile en Amérique du Nord
Les kits de test à domicile sont des instruments de test qui aident les gens à effectuer des tests à domicile et leur donnent des résultats rapides en une minute. Ils comprennent également un équipement de surveillance de la santé pour vérifier et contrôler en permanence la santé du patient diabétique. Les tests à domicile sont très pratiques à effectuer confortablement à la maison et sont disponibles à un prix très abordable. Les autotests sont généralement les versions avancées des kits de tests rapides au point de service qui ont été conçus à l'origine pour les professionnels de la santé et peuvent être effectués par une personne ordinaire. Leurs processus, leur emballage et leurs instructions ont été simplifiés afin de guider la personne tout au long des étapes de la réalisation d'un test. Divers kits de test à domicile sont disponibles, notamment des tests du VIH, des tests de grossesse, du diabète, des tests d'ovulation, des maladies infectieuses telles que le paludisme, le COVID-19 et d'autres. Pour effectuer ces tests rapides à domicile, du sang, de l'urine et du liquide buccal peuvent être prélevés comme échantillon.
Dynamique du marché des kits de test à domicile en Amérique du Nord
Conducteurs
- Adoption croissante des kits d’auto-test
Autrefois, les gens se rendaient souvent à l'hôpital, même pour des problèmes de base. Cependant, à mesure que la sensibilisation à certains produits s'est accrue, ce comportement a changé et a inversé la tendance. De nos jours, les gens préfèrent faire leurs tests de base à l'aide de kits de test à domicile avant de consulter un médecin.
Cette situation est devenue encore plus évidente en raison de la pandémie actuelle, car les gens adoptent davantage de kits de dépistage en libre-service en raison de plusieurs restrictions en vigueur. Cela s'est avéré être une aubaine déguisée pour les hôpitaux et les patients, car les hôpitaux sont déjà à court de ressources et peuvent se concentrer entièrement sur les patients atteints de COVID-19, et les patients peuvent économiser des frais importants de visites chez le médecin et de médicaments. C'est devenu très pratique pour les consommateurs car ils peuvent rapidement connaître les résultats de leurs tests à portée de main.
- Disponibilité facile des kits d'auto-test dans les pharmacies
Les kits d'auto-test ou de dépistage à domicile sont facilement disponibles dans les pharmacies et il est devenu facile de s'en procurer. Diverses sociétés médicales se lancent dans ce domaine en fabriquant rapidement des kits d'auto-test.
Cette large disponibilité peut également être attribuée aux start-ups médicales des pharmacies en ligne, qui facilitent l'accès aux médicaments en un clic. De plus, ces kits d'auto-test sont disponibles sans ordonnance.
Opportunité
- Avènement des technologies avancées
Les dernières technologies sont essentielles pour rendre les produits médicaux très avancés et fiables. Dans différents dispositifs médicaux, l'intelligence artificielle (IA) et l'apprentissage automatique (ML) sont des aspects importants du secteur de la santé avec la capacité d'améliorer la sécurité des patients et les processus administratifs grâce à l'automatisation du travail et à des performances plus rapides.
Il est certain que les instruments de diagnostic basés sur l’IA continueront de se développer par défaut. L’IA n’a fait que des progrès modestes dans le secteur de la santé, qui représente une opportunité énorme et multidimensionnelle.
Retenue/Défi
- Inexactitude des résultats des kits d'auto-test
Les kits de dépistage à domicile permettent aux utilisateurs finaux de prélever leur échantillon à domicile et d'effectuer les tests à domicile ou d'envoyer cet échantillon au laboratoire pour analyse. Les kits de dépistage à domicile ont sans aucun doute facilité le processus de confirmation de l'inquiétude de la personne, qu'il s'agisse d'un test de grossesse à domicile, d'un test de dépistage du VIH ou de tout autre test de maladies infectieuses.
Ces kits de dépistage à domicile sont faciles à utiliser et abordables. Cependant, il existe toujours un doute quant à l'exactitude des résultats. Le dépistage à domicile du COVID-19 peut être plus confortable que de se rendre à l'hôpital ou au cabinet du médecin. Il contribuera également à réduire le risque de propagation ou d'acquisition du coronavirus lors du dépistage.
Cependant, un résultat de test faussement positif peut provoquer de l'anxiété et du stress chez la personne, même si elle ne l'a pas. Il est très bouleversant et perturbant pour la personne de recevoir des résultats faussement positifs ou négatifs. Aujourd'hui, de nombreuses entreprises produisent des kits de test de diagnostic rapide pour le COVID-19 qui peuvent être effectués à domicile, mais il existe divers problèmes liés à la précision en raison desquels la distribution de ces kits de test à domicile a été suspendue pour vérifier leur fiabilité.
Impact de la pandémie de COVID-19 sur le marché nord-américain des kits de test à domicile
La COVID-19 a eu un impact considérable sur le marché des kits de test à domicile. Le marché des kits de test à domicile se développe en raison de la popularité croissante des kits de test à faire soi-même (DIY) en raison de leur commodité et de leurs résultats rapides. Les consommateurs s'inquiètent de la fiabilité des kits de test rapides à domicile, ce qui pourrait entraver l'expansion du marché des kits de test à domicile. Les kits de test rapides pour COVID-19 sont désormais nécessaires de toute urgence aux entreprises afin de réduire les décès de patients et d'augmenter les taux de guérison des patients, ainsi que d'augmenter le taux de marché des kits de test à domicile pour les patients diabétiques et les patients souffrant de problèmes cardiaques. Cela crée une opportunité de marché majeure pour les kits de test à domicile.
Développement récent
- En mars 2022, LifeScan IP Holdings, LLC a annoncé que le produit de la société, OneTouch Verio Flex, était en tête du classement Forbes Health Best Standard Glucose Meters en raison de son prix, de ses bandelettes de test abordables, de son indicateur de gamme de couleurs, de son petit échantillon de sang et de sa conception compacte. Cela a aidé l'entreprise à accroître sa présence mondiale sur le marché
Portée du marché des kits de test à domicile en Amérique du Nord
Le marché nord-américain des kits de test à domicile est classé en six segments notables en fonction du type de test, du type, de l'âge, du type d'échantillon, de l'utilisation et des canaux de distribution. La croissance parmi ces segments vous aidera à analyser les segments de croissance du marché dans les industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour les aider à prendre des décisions stratégiques pour identifier les principales applications du marché.
Type de test
- Test de grossesse
- Kit de test du VIH
- Diabète
- Maladies infectieuses
- Tests de glycémie
- Kit de test de prédiction de l'ovulation
- Kit de test de toxicomanie
- Autres
En fonction du type de test, le marché nord-américain des kits de test à domicile est segmenté en test de grossesse, kit de test du VIH, diabète, maladies infectieuses, tests de glycémie, kit de test de prédiction de l'ovulation, kit de test de toxicomanie et autres.
Taper
- Cassette
- Bande
- Au milieu du cours d'eau
- Panneau de test
- Carte à tremper
- Autres
En fonction du type, le marché nord-américain des kits de test à domicile est segmenté en cassette, bandelette, flux intermédiaire, panneau de test, carte de trempage et autres.
Âge
- Pédiatrique
- Adulte
- Gériatrie
En fonction de l’âge, le marché nord-américain des kits de test à domicile est segmenté en pédiatrie, adulte et gériatrie.
Type d'échantillon
- Urine
- Sang
- Salive
- Autres
En fonction du type d’échantillon, le marché nord-américain des kits de test à domicile est segmenté en urine, sang, salive et autres.
Usage
- Jetable
- Réutilisable
En fonction de l’utilisation, le marché nord-américain des kits de test à domicile est segmenté en kits jetables et réutilisables.
Canaux de distribution
- Pharmacies de détail
- Pharmacie
- Supermarché/Hypermarché
- Pharmacies en ligne
En fonction des canaux de distribution, le marché nord-américain des kits de test à domicile est segmenté en pharmacies de détail, parapharmacies, supermarchés/hypermarchés et pharmacies en ligne.
Analyse/perspectives régionales du marché des kits de test à domicile en Amérique du Nord
Le marché nord-américain des kits de test à domicile est analysé et des informations sur la taille du marché et les tendances sont fournies en fonction du type de test, du type, de l'âge, du type d'échantillon, de l'utilisation et des canaux de distribution.
Les régions couvertes par le rapport sur le marché des kits de test à domicile en Amérique du Nord sont les États-Unis, le Canada et le Mexique.
Les États-Unis dominent le marché nord-américain des kits de dépistage à domicile en termes de part de marché et de chiffre d'affaires et continueront de renforcer leur domination au cours de la période de prévision. Cela est dû aux dépenses de santé élevées du pays et à la sensibilisation croissante au marché nord-américain des kits de dépistage à domicile.
La section pays du rapport fournit également des facteurs d'impact sur les marchés individuels et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que l'analyse de la chaîne de valeur en aval et en amont, les tendances techniques, l'analyse des cinq forces de Porter et les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques mondiales et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des tarifs nationaux et les routes commerciales sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché des kits de test à domicile en Amérique du Nord
Le paysage concurrentiel du marché des kits de test à domicile en Amérique du Nord fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, la présence en Amérique du Nord, les sites et installations de production, les forces et les faiblesses de l'entreprise, le lancement de produits, les pipelines d'essais cliniques, l'analyse de la marque, les approbations de produits, les brevets, la largeur et l'étendue du produit, la domination des applications, la courbe de survie technologique. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises liée au marché des kits de test à domicile en Amérique du Nord.
Français Certains des principaux acteurs opérant sur le marché nord-américain des kits de test à domicile sont Abbott, ACON Laboratories, Inc., Rapikit, BD, Cardinal Health, B. Braun Melsungen AG, Piramal Enterprises Ltd., Siemens Healthcare GmbH, Quidel Corporation, Bionime Corporation, SA Scientific, ARKRAY USA, Inc., Nova Biomedical, AdvaCare Pharma, AccuBioTech Co., Ltd., Atlas Medical UK, TaiDoc Technology Corporation, Drägerwerk AG & Co. KGaA, F. Hoffmann-La Roche Ltd, Sensing Self, PTE. Ltd, Atomo Diagnostics, RUNBIO BIOTECH CO., LTD., Mylan NV (une filiale de Viatris, Inc.), MP BIOMEDICALS, VedaLab, Shanghai Chemtron Biotech Co.Ltd., et Ascensia Diabetes Care Holdings AG, entre autres acteurs nationaux.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA AT-HOME TESTING KITS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TEST TYPE
2.8 MARKET POSITION COVERAGE GRID
2.9 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.1 DBMR MARKET POSITION GRID
2.11 DISTRIBUTOR CHANNEL ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES
5 NORTH AMERICA AT- HOME TESTING KITS MARKET: REGULATIONS
5.1 REGULATION IN U.S
5.2 GUIDELINES FOR SELF-TESTING KITS
5.3 REGULATION IN EUROPE
5.4 GUIDELINES FOR TESTING KITS
5.5 REGULATION IN INDONESIA:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING ADOPTION OF SELF-TESTING KITS
6.1.2 EASY AVAILABILITY OF SELF-TESTING KITS AT PHARMACIES
6.1.3 INCREASE IN AWARENESS ABOUT THE IMPORTANCE OF HIV DIAGNOSIS
6.1.4 EASE OF USE AND LOW COSTS OF RAPID SELF-TEST KITS
6.2 RESTRAINTS
6.2.1 INACCURACY OF RESULTS BY SELF-TESTING KITS
6.2.2 STRINGENT GOVERNMENT REGULATIONS FOR MANUFACTURING AND DISTRIBUTION OF TESTING KITS
6.3 OPPORTUNITIES
6.3.1 ADVENT OF ADVANCED TECHNOLOGIES
6.3.2 EMERGING NEED FOR RAPID TESTING KITS FOR COVID-19 PANDEMIC
6.3.3 STRATEGIC INITIATIVES OF KEY PLAYERS
6.4 CHALLENGES
6.4.1 HIGH COMPETITION IN THE MEDICAL TECHNOLOGY INDUSTRY
6.4.2 REDUCTION IN RESEARCH & DEVELOPMENT BUDGETS
7 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TEST TYPE
7.1 OVERVIEW
7.2 GLUCOSE TESTS
7.3 INFECTIOUS DISEASES
7.4 PREGNANCY TEST
7.5 DRUG ABUSE TEST KITS
7.6 HIV TEST KIT
7.7 OVULATION PREDICTOR TEST KIT
7.8 OTHERS TEST TYPES
8 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TYPE
8.1 OVERVIEW
8.2 CASSETTES
8.2.1 RETAIL PHARMACIES
8.2.2 ONLINE PHARMACIES
8.2.3 DRUG STORE
8.2.4 SUPERMARKET/HYPERMARKET
8.3 STRIP
8.3.1 RETAIL PHARMACIES
8.3.2 ONLINE PHARMACIES
8.3.3 DRUG STORE
8.3.4 SUPERMARKET/HYPERMARKET
8.4 MIDSTREAM
8.4.1 RETAIL PHARMACIES
8.4.2 ONLINE PHARMACIES
8.4.3 DRUG STORE
8.4.4 SUPERMARKET/HYPERMARKET
8.5 DIP CARD
8.5.1 RETAIL PHARMACIES
8.5.2 ONLINE PHARMACIES
8.5.3 DRUG STORE
8.5.4 SUPERMARKET/HYPERMARKET
8.6 TEST PANEL
8.6.1 RETAIL PHARMACIES
8.6.2 ONLINE PHARMACIES
8.6.3 DRUG STORE
8.6.4 SUPERMARKET/HYPERMARKET
8.7 OTHERS
9 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY AGE
9.1 OVERVIEW
9.2 ADULT
9.3 GERIATRIC
9.4 PEDIATRIC
10 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY SAMPLE TYPE
10.1 OVERVIEW
10.2 BLOOD
10.3 URINE
10.4 SALIVA
10.5 OTHER SAMPLE TYPES
11 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY USAGE
11.1 OVERVIEW
11.2 DISPOSABLE
11.3 REUSABLE
12 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS
12.1 OVERVIEW
12.2 RETAIL PHARMACIES
12.3 ONLINE PHARMACIES
12.4 DRUG STORE
12.5 SUPERMARKET/HYPERMARKET
13 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY REGION
13.1 NORTH AMERICA
13.1.1 U.S.
13.1.2 CANADA
13.1.3 MEXICO
14 NORTH AMERICA AT-HOME TESTING KITS MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 ABBOTT
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUS ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 SIEMENS HEALTHCARE GMBH
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUS ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENTS
16.3 F. HOFFMANN- LA ROCHE LTD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 BD
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 PRODUCT PORTFOLIO
16.4.4 RECENT DEVELOPMENTS
16.5 DRÄGERWERK AG & CO. KGAA
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 LIFESCAN IP HOLDINGS, LLC
16.6.1 COMPANY SNAPSHOT
16.6.2 COMPANY SHARE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENTS
16.7 ASCENSIA DIABETES CARE HOLDINGS AG.
16.7.1 COMPANY SNAPSHOT
16.7.2 COMPANY SHARE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENTS
16.8 NECTAR LIFESCIENCES LTD. (RAPIKIT)
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 ACON LABORATORIES, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENTS
16.1 QUIDEL CORPORATION.
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENTS
16.11 ARKRAY USA, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 BTNX INC.
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENTS
16.13 ATOMO DIAGNOSTICS
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENTS
16.14 EUROFINS SCIENTIFIC
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 PIRAMAL ENTERPRISES LTD.
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 BIONIME CORPORATION
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENTS
16.17 NOVA BIOMEDICAL
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 CARDINAL HEALTH.
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENTS
16.19 ORASURE TECHNOLOGIES
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENTS
16.2 BIOLYTICAL LABORATORIES INC.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 EVERLYWELL, INC.
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENTS
16.22 SA SCIENTIFIC LTD.
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENTS
16.23 CLEARBLUE (A SUBSIDIARY OF SWISS PRECISION DIAGNOSTICS GMBH)
16.23.1 COMPANY SNAPSHOT
16.23.2 PRODUCT PORTFOLIO
16.23.3 RECENT DEVELOPMENTS
16.24 BIOSYNEX
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENTS
16.25 PRIMA LAB SA
16.25.1 COMPANY SNAPSHOT
16.25.2 PRODUCT PORTFOLIO
16.25.3 RECENT DEVELOPMENTS
16.26 MP BIOMEDICALS.
16.26.1 COMPANY SNAPSHOT
16.26.2 PRODUCT PORTFOLIO
16.26.3 RECENT DEVELOPMENTS
16.27 STERILAB SERVICES
16.27.1 COMPANY SNAPSHOT
16.27.2 PRODUCT PORTFOLIO
16.27.3 RECENT DEVELOPMENTS
16.28 CHEMBIO DIAGNOSTICS, INC.
16.28.1 COMPANY SNAPSHOT
16.28.2 REVENUE ANALYSIS
16.28.3 PRODUCT PORTFOLIO
16.28.4 RECENT DEVELOPMENTS
16.29 BIOSURE
16.29.1 COMPANY SNAPSHOT
16.29.2 PRODUCT PORTFOLIO
16.29.3 RECENT DEVELOPMENTS
16.3 SELFDIAGNOSTICS OU
16.30.1 COMPANY SNAPSHOT
16.30.2 PRODUCT PORTFOLIO
16.30.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA GLUCOSE TESTS IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA INFECTIOUS DISEASES IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA PREGNANCY TEST IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA DRUG ABUSE TEST KITS IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA HIV TEST KIT IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA OVULATION PREDICTOR TEST KIT IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA OTHERS TEST TYPES IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA CASSETTES IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA CASSETTES IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA STRIP IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA STRIP IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA MIDSTREAM IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA MIDSTREAM IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA DIP CARD IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA DIP CARD IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA TEST PANEL IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA TEST PANEL IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA OTHERS IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY AGE, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA ADULT IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA GERIATRIC IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA PEDIATRIC IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY SAMPLE TYPE, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA BLOOD IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA URINE IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA SALIVA IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA OTHER SAMPLE TYPES IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY USAGE, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA DISPOSABLE IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA REUSABLE IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA RETAIL PHARMACIES IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA ONLINE PHARMACIES IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA DRUG STORE IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA SUPERMARKET/HYPERMARKET IN AT-HOME TESTING KITS MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA STRIPS IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA CASSETTES IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA MIDSTREAM IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA DIP CARD IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA TEST PANEL IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY AGE, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY SAMPLE TYPE, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY USAGE, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 50 U.S. AT-HOME TESTING KITS MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 51 U.S. AT-HOME TESTING KITS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 52 U.S. STRIPS IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 53 U.S. CASSETTES IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 54 U.S. MIDSTREAM IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 55 U.S. DIP CARD IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 56 U.S. TEST PANEL IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 57 U.S. AT-HOME TESTING KITS MARKET, BY AGE, 2020-2029 (USD MILLION)
TABLE 58 U.S. AT-HOME TESTING KITS MARKET, BY SAMPLE TYPE, 2020-2029 (USD MILLION)
TABLE 59 U.S. AT-HOME TESTING KITS MARKET, BY USAGE, 2020-2029 (USD MILLION)
TABLE 60 U.S. AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 61 CANADA AT-HOME TESTING KITS MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 62 CANADA AT-HOME TESTING KITS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 63 CANADA STRIPS IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 64 CANADA CASSETTES IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 65 CANADA MIDSTREAM IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 66 CANADA DIP CARD IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 67 CANADA TEST PANEL IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 68 CANADA AT-HOME TESTING KITS MARKET, BY AGE, 2020-2029 (USD MILLION)
TABLE 69 CANADA AT-HOME TESTING KITS MARKET, BY SAMPLE TYPE, 2020-2029 (USD MILLION)
TABLE 70 CANADA AT-HOME TESTING KITS MARKET, BY USAGE, 2020-2029 (USD MILLION)
TABLE 71 CANADA AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 72 MEXICO AT-HOME TESTING KITS MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 73 MEXICO AT-HOME TESTING KITS MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 74 MEXICO STRIPS IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 75 MEXICO CASSETTES IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 76 MEXICO MIDSTREAM IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 77 MEXICO DIP CARD IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 78 MEXICO TEST PANEL IN AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
TABLE 79 MEXICO AT-HOME TESTING KITS MARKET, BY AGE, 2020-2029 (USD MILLION)
TABLE 80 MEXICO AT-HOME TESTING KITS MARKET, BY SAMPLE TYPE, 2020-2029 (USD MILLION)
TABLE 81 MEXICO AT-HOME TESTING KITS MARKET, BY USAGE, 2020-2029 (USD MILLION)
TABLE 82 MEXICO AT-HOME TESTING KITS MARKET, BY DISTRIBUTION CHANNELS, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 NORTH AMERICA AT-HOME TESTING KITS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA AT-HOME TESTING KITS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA AT-HOME TESTING KITS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA AT-HOME TESTING KITS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA AT-HOME TESTING KITS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA AT-HOME TESTING KITS MARKET: MARKET POSITION COVERAGE GRID
FIGURE 7 NORTH AMERICA AT-HOME TESTING KITS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA AT-HOME TESTING KITS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA AT-HOME TESTING KITS MARKET: DISTRIBUTOR CHANNEL ANALYSIS
FIGURE 10 NORTH AMERICA AT-HOME TESTING KITS MARKET: SEGMENTATION
FIGURE 11 GROWING ADOPTION OF SELF-TESTING KITS IS EXPECTED TO DRIVE NORTH AMERICA AT-HOME TESTING KITS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 GLUCOSE TEST IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF NORTH AMERICA AT-HOME TESTING KITS MARKET, BY TEST TYPE IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE AND ASIA-PACIFIC IS THE FASTEST GROWING REGION IN THE NORTH AMERICA AT-HOME TESTING KITS MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA AT-HOME TESTING KITS MARKET
FIGURE 15 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TEST TYPE, 2021
FIGURE 16 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 17 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 18 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 19 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TYPE, 2021
FIGURE 20 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 21 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 22 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 23 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY AGE, 2021
FIGURE 24 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY AGE, 2022-2029 (USD MILLION)
FIGURE 25 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY AGE, CAGR (2022-2029)
FIGURE 26 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY AGE, LIFELINE CURVE
FIGURE 27 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY SAMPLE TYPE, 2021
FIGURE 28 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY SAMPLE TYPE, 2022-2029 (EURO MILLION)
FIGURE 29 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY SAMPLE TYPE, CAGR (2022-2029)
FIGURE 30 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY SAMPLE TYPE, LIFELINE CURVE
FIGURE 31 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY USAGE, 2021
FIGURE 32 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY USAGE, 2022-2029 (EURO MILLION)
FIGURE 33 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY USAGE, CAGR (2022-2029)
FIGURE 34 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY USAGE, LIFELINE CURVE
FIGURE 35 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 36 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 37 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 38 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 39 NORTH AMERICA AT-HOME TESTING KITS MARKET: SNAPSHOT (2021)
FIGURE 40 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY COUNTRY (2021)
FIGURE 41 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY COUNTRY (2022 & 2029)
FIGURE 42 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY COUNTRY (2021 & 2029)
FIGURE 43 NORTH AMERICA AT-HOME TESTING KITS MARKET: BY TEST TYPE (2022-2029)
FIGURE 44 NORTH AMERICA AT-HOME TESTING KITS MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.














