Marché de l'échantillonnage aseptique en Amérique du Nord, par produit (échantillonnage aseptique manuel et système/instruments d'échantillonnage automatisé), type (échantillonnage aseptique manuel et échantillonnage aseptique automatisé), technique (technique d'échantillonnage hors ligne, technique d'échantillonnage en ligne et échantillonnage aseptique en ligne), application (processus en amont et processus en aval), utilisateur final (fabricants de biotechnologie et de produits pharmaceutiques, organisation de fabrication sous contrat, organisation de recherche sous contrat, départements universitaires et de R&D, et autres), canal de distribution (appel d'offres direct, distributeur tiers et autres) Tendances de l'industrie et prévisions jusqu'en 2030.
Analyse et perspectives du marché de l'échantillonnage aseptique en Amérique du Nord
Le marché des échantillons aseptiques fournit des produits et des services pour la collecte, le transport et le stockage d'échantillons stériles dans diverses industries, notamment les produits pharmaceutiques, la biotechnologie et l'alimentation et les boissons. L'échantillonnage aseptique fait référence à l'utilisation de techniques et de méthodes qui empêchent l'entrée de micro-organismes et d'autres contaminants pendant le processus d'échantillonnage et garantissent l'intégrité et la précision de l'échantillon.
Le marché de l'échantillonnage aseptique est influencé par la demande croissante de produits biologiques, de vaccins et d'autres médicaments complexes, les investissements dans les activités de R&D et les exigences de conformité réglementaire. Les produits biologiques et autres médicaments complexes nécessitent un contrôle strict de la stérilité et de la pureté des produits, ce qui augmente la demande de produits et de services d'échantillonnage aseptique. Les investissements dans la R&D pour développer des traitements avancés tels que la thérapie génique, la thérapie par cellules souches et la médecine personnalisée contribuent également à la croissance du marché. Les exigences croissantes de conformité réglementaire en matière de contrôle de la qualité et de sécurité augmentent également la demande de produits et de services d'échantillonnage aseptique.
Cependant, le marché est confronté à des défis tels que le coût élevé des produits et services d’échantillonnage aseptique, le manque de personnel qualifié et les exigences réglementaires complexes.
Le marché de l'échantillonnage aseptique a connu des avancées technologiques importantes ces dernières années visant à améliorer l'efficacité et la précision du processus d'échantillonnage. Les fabricants du marché nord-américain de l'échantillonnage aseptique se sont concentrés sur la collaboration avec des marques populaires pour attirer une plus grande base de consommateurs et proposer des instruments d'échantillonnage nouveaux et innovants.
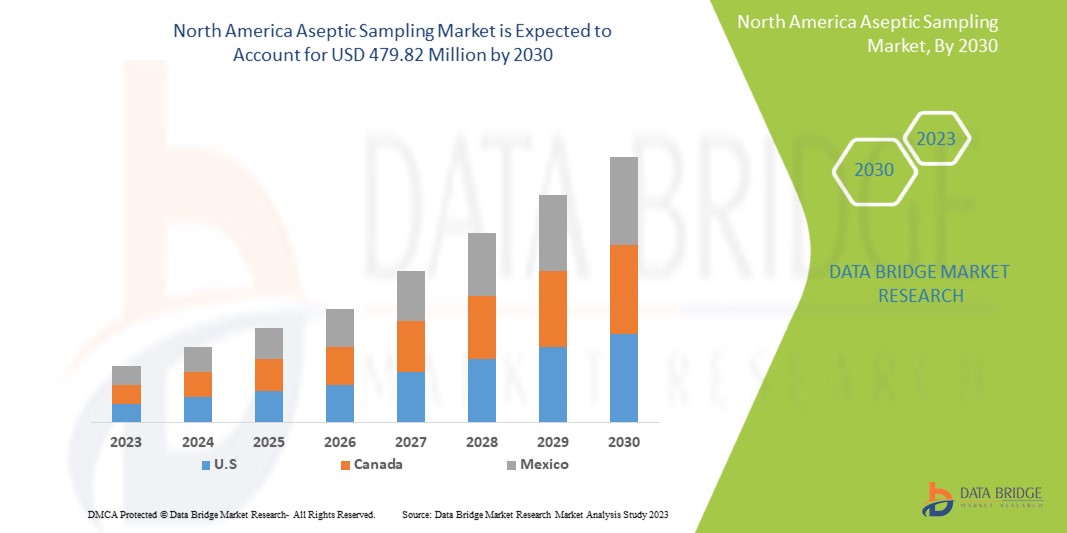
Data Bridge Market Research analyse que le marché de l'échantillonnage aseptique en Amérique du Nord devrait atteindre 479,82 millions USD d'ici 2030, à un TCAC de 12,8 % au cours de la période de prévision.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable de 2015 à 2020) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD |
|
Segments couverts |
Produit (échantillonnage aseptique manuel et système/instruments d'échantillonnage automatisé), type (échantillonnage aseptique manuel et échantillonnage aseptique automatisé), technique (technique d'échantillonnage hors ligne, technique d'échantillonnage en ligne et échantillonnage aseptique en ligne), application (processus en amont et processus en aval), utilisateur final (fabricants de produits biotechnologiques et pharmaceutiques, organisation de fabrication sous contrat, organisation de recherche sous contrat, départements universitaires et de R&D, et autres), canal de distribution (appel d'offres direct, distributeur tiers et autres) |
|
Pays couverts |
États-Unis, Canada et Mexique |
|
Acteurs du marché couverts |
Sartorius AG, KEOFITT A/S, KIESELMANN GmbH, THERMO FISHER SCIENTIFIC INC., GEMU Group, Flownamics, Merck KGaA, Advanced Microdevices Pvt. Ltd. Mdi, SAINT-GOBAIN,GEA Group Aktiengesellschaft, Avantor, Inc., ALFA LAVAL, WL Gore & Associates, Inc., QualiTru Sampling Systems, Aerre Inox Srl, Shanghai LePure Biotech Co., Ltd., JONENG VALVES CO., LIMITED, Burkle GmbH et Dietrich Engineering Consultants, entre autres |
Définition du marché de l'échantillonnage aseptique en Amérique du Nord
Le marché de l'échantillonnage aseptique désigne le marché des produits et services utilisés pour collecter et traiter des échantillons stériles à des fins de test, d'analyse et de contrôle qualité dans diverses industries, notamment les industries pharmaceutique, biotechnologique et agroalimentaire. L'échantillonnage aseptique implique des techniques et des procédures qui empêchent l'entrée de micro-organismes et d'autres contaminants pendant le processus d'échantillonnage et garantissent l'intégrité et la précision de l'échantillon.
L'échantillonnage aseptique est essentiel pour maintenir la qualité et la sécurité des produits, en particulier dans les industries pharmaceutiques et biotechnologiques, où la stérilité et la pureté des produits sont essentielles. Le processus utilise divers produits et équipements pour collecter des échantillons, tels que des sacs de collecte stériles, des bouteilles, des échantillonneurs, des pelles et d'autres fournitures.
Dynamique du marché de l'échantillonnage aseptique en Amérique du Nord
Cette section traite de la compréhension des moteurs, des avantages, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
- Réglementations et directives strictes
Des lois et des directives strictes ont été un facteur majeur de l'expansion constante du marché des échantillons aseptiques. L'échantillonnage aseptique implique la collecte d'échantillons dans un environnement stérile pour éviter la contamination et protéger l'intégrité de l'échantillon. Des règles et des directives sont en place pour garantir que ces articles respectent des exigences strictes de qualité et de sécurité. Les secteurs pharmaceutique et biotechnologique ont des normes très strictes qui doivent être respectées pour garantir la sécurité et l'efficacité de leurs produits. L'échantillonnage aseptique est donc devenu une procédure cruciale dans diverses entreprises pour éviter la contamination et garantir la qualité de leur production.
L'échantillonnage aseptique est une procédure cruciale dans les secteurs pharmaceutique et biotechnologique pour garantir la sécurité et l'efficacité des médicaments et des produits biologiques. Les organismes de réglementation comme l'Agence européenne des médicaments (EMA) et la Food and Drug Administration (FDA) des États-Unis ont établi des règles strictes pour le traitement aseptique afin d'éviter toute contamination tout au long du processus de fabrication. Ces directives exigent l'utilisation de techniques d'échantillonnage aseptiques. Ces réglementations exigent l'utilisation de techniques d'échantillonnage aseptiques pour garantir que les échantillons sont exempts de contamination microbiologique, d'endotoxines et d'autres contaminants qui pourraient affecter la qualité du produit final.
La demande de produits et services d'échantillonnage aseptique a considérablement augmenté ces dernières années. Les entreprises du marché de l'échantillonnage aseptique investissent dans la R&D pour développer de nouveaux produits et services qui répondent à des exigences et directives réglementaires strictes.
Retenue
- Manque de normalisation
L'échantillonnage aseptique dans diverses industries, notamment les produits pharmaceutiques, les biotechnologies , l'alimentation et les boissons, implique la collecte d'échantillons dans un environnement stérile pour éviter toute contamination. Les méthodes d'échantillonnage aseptique utilisées dans diverses industries et applications ne sont cependant pas universelles, ce qui peut entraîner des résultats incohérents et limiter la comparabilité des données. L'absence de normes et de standards clairs de la part des organismes de réglementation entraîne un manque de normalisation dans l'échantillonnage aseptique. Bien que les organismes de réglementation proposent des recommandations générales sur l'échantillonnage aseptique, il n'existe aucune normalisation pour les procédures d'échantillonnage, les tailles d'échantillon, les outils ou les techniques de validation. Par conséquent, il peut être difficile de produire des résultats cohérents dans diverses applications et industries et entraîner une confusion commerciale.
Des données inexactes ou incohérentes peuvent conduire à des conclusions erronées et à de mauvaises décisions dans le développement de produits, le contrôle qualité et la conformité réglementaire. Cela peut avoir de graves conséquences sur la santé et la sécurité publiques, ainsi que sur la santé financière des entreprises.
Ainsi, le manque de normalisation devrait freiner la croissance du marché de l’échantillonnage aseptique en Amérique du Nord.
Opportunité
Croissance des progrès technologiques
Le développement de nouvelles technologies dans le domaine de l'échantillonnage aseptique est l'un des facteurs les plus importants contribuant à l'expansion du marché de l'échantillonnage aseptique en Amérique du Nord. Les entreprises peuvent désormais améliorer la précision, l'efficacité et la fiabilité de leurs opérations grâce au développement d'équipements et de techniques d'échantillonnage avancés. Ces développements ouvrent de nouvelles opportunités de croissance pour le marché et, par conséquent, les entreprises font des investissements importants dans la recherche et le développement pour maintenir leur position de leader du marché.
Le développement de systèmes automatisés est l'une des avancées technologiques les plus importantes qui ont été réalisées dans le domaine de l'échantillonnage aseptique. Ces systèmes utilisent la robotique et l'intelligence artificielle pour améliorer la précision des procédures d'échantillonnage tout en augmentant leur vitesse. Ils améliorent la cohérence des résultats tout en réduisant simultanément le risque de contamination, ce qui conduit à une augmentation de l'efficacité globale des opérations. Les systèmes automatisés sont particulièrement utiles dans les opérations à grande échelle avec un débit d'échantillons élevé en raison de leur rapidité et de leur précision.
Les progrès des techniques d’échantillonnage stimulent également la croissance du marché nord-américain de l’échantillonnage aseptique. Par exemple, le développement de techniques d’échantillonnage en boucle fermée permet aux entreprises de prélever des échantillons représentatifs sans exposer le produit à l’environnement. Cette technique réduit le risque de contamination et améliore la précision des résultats. Ainsi, la croissance des avancées technologiques constitue une opportunité pour la croissance du marché nord-américain de l’échantillonnage aseptique.
Défi
Manque de main d'oeuvre qualifiée
Des équipements spécialisés et du personnel formé sont essentiels pour exploiter, entretenir et dépanner les systèmes d'échantillonnage aseptique. Cependant, une pénurie de personnel formé et qualifié pour exploiter ces systèmes pourrait freiner l'expansion du marché.
Le marché de l'échantillonnage aseptique est très avancé sur le plan technique et les employeurs recherchent des candidats ayant de l'expérience en microbiologie, en ingénierie et en automatisation. D'un autre côté, il se peut qu'il n'y ait pas suffisamment de travailleurs qualifiés dans ces régions pour soutenir la mise en œuvre de systèmes d'échantillonnage aseptique. Cela pourrait entraîner des délais de mise en œuvre du système plus longs, des coûts de formation plus élevés et une baisse de productivité en raison du manque d'expérience du personnel.
De plus, le taux de rotation élevé du personnel qualifié peut rendre le problème du manque de main-d'œuvre qualifiée encore plus difficile à résoudre. Lorsque des employés compétents quittent leur poste, ils emportent avec eux les connaissances et l'expérience nécessaires pour exploiter efficacement les systèmes d'échantillonnage aseptique. Par conséquent, cela peut réduire l'efficacité, la productivité, les temps d'arrêt et les coûts de maintenance.
En conclusion, le manque de main-d’œuvre qualifiée constitue un défi important pour l’expansion du marché nord-américain de l’échantillonnage aseptique.
Développements récents
- En juin 2022, Merck kGaA a annoncé une collaboration avec Agilent Technologies pour combler le manque de technologies d'analyse de processus pour le traitement en aval. Cette collaboration contribuera à une nouvelle augmentation des revenus.
- En novembre 2021, Sartorius AG, l'un des principaux partenaires internationaux de la recherche en sciences de la vie et de l'industrie biopharmaceutique, a annoncé avoir reçu le prix du « Meilleur fournisseur de bioprocédés » lors des Europe Bioprocessing Excellence Awards 2021. Ce prix a aidé l'entreprise à faire reconnaître son travail.
Portée du marché de l'échantillonnage aseptique en Amérique du Nord
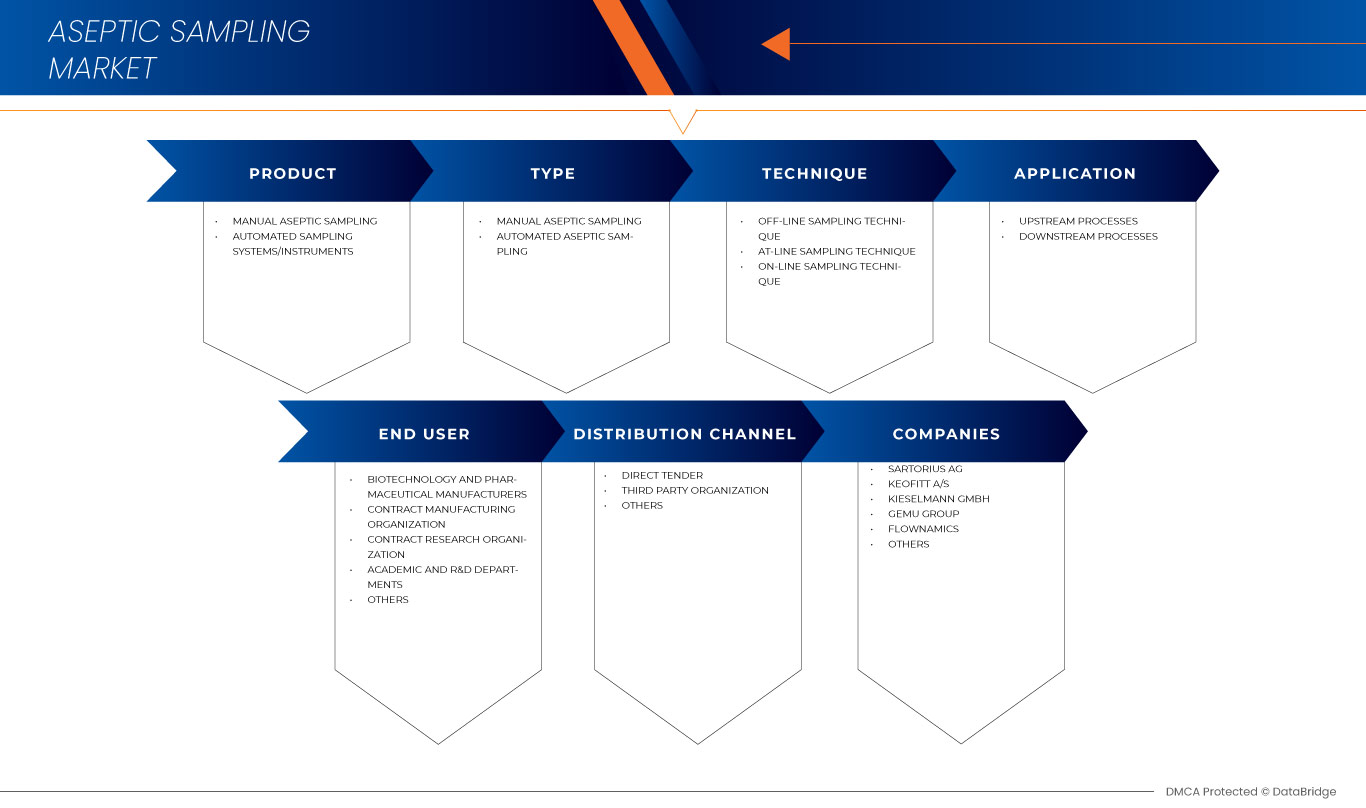
Le marché nord-américain de l'échantillonnage aseptique est divisé en six segments importants : produit, type, technique, application, utilisateur final et canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
Produit
- Échantillonnage aseptique manuel
- Système/instruments d'échantillonnage automatisé
Sur la base du produit, le marché de l’échantillonnage aseptique en Amérique du Nord est segmenté en échantillonnage aseptique manuel et en systèmes/instruments d’échantillonnage automatisés.
Taper
- Échantillonnage aseptique manuel
- Échantillonnage aseptique automatisé
Sur la base du type, le marché de l’échantillonnage aseptique en Amérique du Nord est segmenté en échantillonnage aseptique manuel et échantillonnage aseptique automatisé.
Technique
- Technique d'échantillonnage hors ligne
- Technique d'échantillonnage en ligne
- Technique d'échantillonnage en ligne
Sur la base de la technique, le marché de l'échantillonnage aseptique en Amérique du Nord est segmenté en technique d'échantillonnage hors ligne, technique d'échantillonnage en ligne et technique d'échantillonnage en ligne.
Application
- Processus en amont
- Processus en aval
Sur la base de l'application, le marché nord-américain de l'échantillonnage aseptique est segmenté en processus en amont et en processus en aval
Utilisateur final
- Fabricants de produits biotechnologiques et pharmaceutiques
- Organisation de fabrication sous contrat
- Organisation de recherche sous contrat
- Départements académiques et de recherche et développement
- Autres
Sur la base de l'utilisateur final, le marché de l'échantillonnage aseptique en Amérique du Nord est segmenté en fabricants de biotechnologie et de produits pharmaceutiques, organisations de fabrication sous contrat, organisations de recherche sous contrat, départements universitaires et de R&D, et autres.
Canal de distribution
- Appel d'offres direct
- Distributeur tiers
- Autres
Sur la base du canal de distribution, le marché de l'échantillonnage aseptique en Amérique du Nord est segmenté en appel d'offres direct, distributeur tiers et autres.
Analyse/perspectives régionales du marché de l'échantillonnage aseptique en Amérique du Nord
Le marché de l’échantillonnage aseptique en Amérique du Nord est classé en six segments et zones géographiques notables.
Le marché de l’échantillonnage aseptique en Amérique du Nord est segmenté en six segments notables : produit, type, technique, application, utilisateur final et canal de distribution.
Les pays couverts dans ce rapport de marché sont les États-Unis, le Canada et le Mexique.
Les États-Unis dominent la région Amérique du Nord en raison de la forte présence d’acteurs clés et de la demande croissante d’échantillonnage aseptique sur les marchés émergents et en expansion.
La section du rapport consacrée aux pays fournit également des facteurs individuels ayant un impact sur le marché et des changements de réglementation nationale qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie des pays, les actes réglementaires et les tarifs douaniers d'import-export sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario du marché pour les différents pays. En outre, la présence et la disponibilité des marques nord-américaines et leurs défis en raison de la concurrence importante ou rare des marques locales et nationales et de l'impact des canaux de vente sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché de l'échantillonnage aseptique en Amérique du Nord
Le paysage concurrentiel du marché de l'échantillonnage aseptique en Amérique du Nord fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements en R&D, les nouvelles initiatives du marché, les sites et installations de production, les forces et les faiblesses de l'entreprise, le lancement du produit, les approbations de produits, la largeur et la portée du produit, la domination des applications, la courbe de survie du type de produit. Les points de données ci-dessus ne concernent que l'accent mis par l'entreprise sur le marché de l'échantillonnage aseptique en Amérique du Nord.
Français Certains des principaux acteurs opérant sur le marché de l'échantillonnage aseptique sont Sartorius AG, KEOFITT A/S, KIESELMANN GmbH, THERMO FISHER SCIENTIFIC INC., GEMU Group, Flownamics, Merck KGaA, Advanced Microdevices Pvt. Ltd. Mdi, SAINT-GOBAIN,GEA Group Aktiengesellschaft, Avantor, Inc., ALFA LAVAL, WL Gore & Associates, Inc., QualiTru Sampling Systems, Aerre Inox Srl, Shanghai LePure Biotech Co., Ltd., JONENG VALVES CO., LIMITED, Burkle GmbH et Dietrich Engineering Consultants, entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA ASEPTIC SAMPLING MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 STRATEGIC INITIATIVES
5 INDUSTRY INSIGHTS
6 REGULATORY FRAMEWORK
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 STRINGENT REGULATIONS AND GUIDELINES
7.1.2 RISING DEMAND FOR ADVANCED PHARMACEUTICAL AND BIOTECHNOLOGY PRODUCTS
7.1.3 INCREASING FOCUS ON QUALITY ASSURANCE AND GROWING AWARENESS OF ASEPTIC PRACTICES
7.1.4 GROWING R&D ACTIVITIES AND NEW APPROVALS AND LAUNCHES OF ASEPTIC SAMPLING PRODUCTS
7.2 RESTRAINTS
7.2.1 LACK OF STANDARDISATION
7.2.2 ALTERNATIVE SAMPLING TECHNIQUES
7.3 OPPORTUNITIES
7.3.1 GROWTH IN TECHNOLOGICAL ADVANCEMENTS
7.3.2 INCREASING ADOPTION OF SINGLE-USE ASEPTIC SAMPLING SYSTEMS
7.3.3 INCREASING DEMAND FOR STERILE PHARMACEUTICAL FORMULATIONS
7.4 CHALLENGES
7.4.1 HIGH INSTALLATION AND OPERATIONAL COSTS
7.4.2 LACK OF SKILLED WORKFORCE
8 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 MANUAL ASEPTIC SAMPLING
8.3 AUTOMATED SAMPLING INSTRUMENTS/SYSTEMS
9 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY TYPE
9.1 OVERVIEW
9.2 MANUAL ASEPTIC SAMPLING
9.3 AUTOMATED ASEPTIC SAMPLING
10 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY TECHNIQUE
10.1 OVERVIEW
10.2 OFF-LINE SAMPLING TECHNIQUE
10.3 AT-LINE SAMPLING TECHNIQUE
10.4 ON-LINE SAMPLING TECHNIQUE
11 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 UPSTREAM PROCESSES
11.3 DOWNSTREAM PROCESSES
12 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY END USER
12.1 OVERVIEW
12.2 BIOTECHNOLOGY AND PHARMACEUTICAL MANUFACTURERS
12.3 CONTRACT MANUFACTURING ORGANIZATION
12.4 CONTRACT RESEARCH ORGANIZATION
12.5 ACADEMIC AND R&D DEPARTMENTS
12.6 OTHERS
13 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTOR
13.4 OTHERS
14 NORTH AMERICA ASEPTIC SAMPLING MARKET, BY REGION
14.1 NORTH AMERICA
14.1.1 U.S.
14.1.2 CANADA
14.1.3 MEXICO
15 NORTH AMERICA ASEPTIC SAMPLING MARKET_: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
16 COMPANY PROFILE
16.1 SARTORIUS AG
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 KIESELMANN GMBH
16.2.1 COMPANY SNAPSHOT
16.2.2 COMPANY SHARE ANALYSIS
16.2.3 PRODUCT PORTFOLIO
16.2.4 RECENT DEVELOPMENTS
16.3 SAINT-GOBAIN
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENTS
16.4 MERCK KGAA
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 ALFA LAVAL
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 ADVANCED MICRODEVICES PVT. LTD. MDI
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 AVANTOR, INC.
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 COMPANY SHARE ANALYSIS
16.7.4 PRODUCT PORTFOLIO
16.7.5 RECENT DEVELOPMENT
16.8 AERRE INOX S.R.L.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENTS
16.9 BURKLE GMBH
16.9.1 COMPANY SNAPSHOT
16.9.2 PRODUCT PORTFOLIO
16.9.3 RECENT DEVELOPMENTS
16.1 DIETRICH ENGINEERING CONSULTANTS
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 FLOWNAMICS
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 GEMU GROUP
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENT
16.13 GEA GROUP AKTIENGESELLSCHAFT (2022)
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 COMPANY SHARE ANALYSIS
16.13.4 PRODUCT PORTFOLIO
16.13.5 RECENT DEVELOPMENTS
16.14 JONENG VALVES CO., LIMITED
16.14.1 COMPANY SNAPSHOT
16.14.2 PRODUCT PORTFOLIO
16.14.3 RECENT DEVELOPMENTS
16.15 KEOFITT A/S
16.15.1 COMPANY SNAPSHOT
16.15.2 PRODUCT PORTFOLIO
16.15.3 RECENT DEVELOPMENTS
16.16 QUALITRU SAMPLING SYSTEMS
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 SHANGHAI LEPURE BIOTECH CO., LTD.
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENT
16.18 THERMO FISHER SCIENTIFIC INC.
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 COMPANY SHARE ANALYSIS
16.18.4 PRODUCT PORTFOLIO
16.18.5 RECENT DEVELOPMENT
16.19 W.L. GORE & ASSOCIATES, INC.
16.19.1 COMPANY SNAPSHOT
16.19.2 COMPANY SHARE ANALYSIS
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des figures
FIGURE 1 NORTH AMERICA ASEPTIC SAMPLING MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA ASEPTIC SAMPLING MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ASEPTIC SAMPLING MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA ASEPTIC SAMPLING MARKET : NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ASEPTIC SAMPLING MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ASEPTIC SAMPLING MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ASEPTIC SAMPLING MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ASEPTIC SAMPLING MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA ASEPTIC SAMPLING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ASEPTIC SAMPLING MARKET: SEGMENTATION
FIGURE 11 STRINGENT REGULATIONS AND GUIDELINES IN VARIOUS INDUSTRIES FOR QUALITY PRODUCTS ARE EXPECTED TO DRIVE THE NORTH AMERICA ASEPTIC SAMPLING MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 MANUAL ASEPTIC SAMPLING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ASEPTIC SAMPLING MARKET IN THE FORECAST PERIOD OF 20213 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ASEPTIC SAMPLING MARKET
FIGURE 14 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY PRODUCT, 2022
FIGURE 15 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 17 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 18 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TYPE, 2022
FIGURE 19 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TECHNIQUE, 2022
FIGURE 23 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TECHNIQUE, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TECHNIQUE, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY TECHNIQUE, LIFELINE CURVE
FIGURE 26 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY APPLICATION, 2022
FIGURE 27 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY APPLICATION, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY APPLICATION, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 30 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY END USER, 2022
FIGURE 31 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY END USER, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 35 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 NORTH AMERICA ASEPTIC SAMPLING MARKET: SNAPSHOT (2022)
FIGURE 39 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY COUNTRY (2022)
FIGURE 40 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 NORTH AMERICA ASEPTIC SAMPLING MARKET: BY PRODUCT (2023-2030)
FIGURE 43 NORTH AMERICA ASEPTIC SAMPLING MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.