Marché des médicaments antiviraux en Amérique du Nord, par indication (grippe, virus de l'immunodéficience humaine (VIH), virus de l'hépatite C (VHC), virus respiratoire syncytial, virus de l'herpès simplex, cytomégalovirus humain (HCMV), virus varicelle-zona (VZV), virus de l'hépatite B (VHB), infection à coronavirus et autres), type de patient (enfant, adulte et gériatrique), produits (oraux, topiques et parentéraux), type de médicament (générique et de marque), utilisateur final (hôpitaux, cliniques, soins à domicile , centres spécialisés, centres ambulatoires et autres), canal de distribution (pharmacie hospitalière, pharmacie en ligne et pharmacie de détail) - Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et perspectives du marché des médicaments antiviraux en Amérique du Nord
La sensibilisation croissante aux infections virales en Amérique du Nord a accru la demande sur le marché. L'augmentation des dépenses de santé pour de meilleurs services de santé contribue également à la croissance du marché. Les principaux acteurs du marché se concentrent sur le lancement et l'approbation de divers services pendant cette période cruciale. En outre, l'amélioration des progrès des techniques de développement de médicaments contribue également à la demande croissante de médicaments antiviraux.
Le marché nord-américain des médicaments antiviraux devrait croître au cours de l'année de prévision en raison de l'augmentation du nombre d'acteurs sur le marché et de la disponibilité de services avancés. Parallèlement à cela, les fabricants sont engagés dans l'activité de développement pour lancer de nouveaux services sur le marché. Le développement croissant dans le domaine du développement de médicaments stimule encore davantage la croissance du marché. Cependant, des difficultés telles que le manque de protocoles standardisés et le manque de professionnels qualifiés pourraient entraver la croissance du marché nord-américain des médicaments antiviraux au cours de la période de prévision.
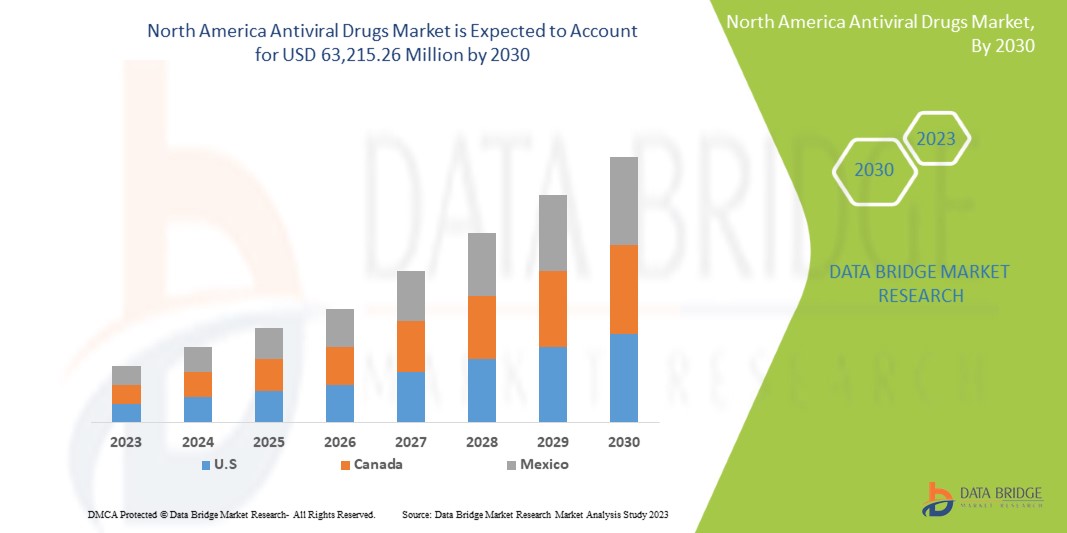
L'augmentation des dépenses de santé consacrées au développement de nouveaux médicaments devrait offrir des opportunités au marché. Cependant, l'utilisation croissante de traitements alternatifs pourrait remettre en cause la croissance du marché. Data Bridge Market Research estime que le marché nord-américain des médicaments antiviraux devrait atteindre la valeur de 63 215,26 millions USD d'ici 2030, avec un TCAC de 5,5 % au cours de la période de prévision.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable de 2015 à 2020) |
|
Unités quantitatives |
Chiffre d'affaires en millions, volumes en unités et prix en USD |
|
Segments couverts |
Indication (grippe, virus de l'immunodéficience humaine (VIH), virus de l'hépatite C (VHC), virus respiratoire syncytial, virus de l'herpès simplex, cytomégalovirus humain (HCMV), virus varicelle-zona (VZV), virus de l'hépatite B (VHB), infection à coronavirus et autres), type de patient (enfant, adulte et gériatrique), produits (oraux, topiques et parentéraux), type de médicament (générique et de marque), utilisateur final (hôpitaux, cliniques, soins à domicile , centres spécialisés, centres ambulatoires et autres), canal de distribution (pharmacie hospitalière, pharmacie en ligne et pharmacie de détail) |
|
Pays couverts |
États-Unis, Canada et Mexique |
|
Acteurs du marché couverts |
Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy's Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc. et Hetero. entre autres |
Définition du marché des médicaments antiviraux en Amérique du Nord
Les médicaments antiviraux sont des médicaments utilisés pour traiter les infections virales en inhibant la réplication des virus dans les cellules hôtes. Ces médicaments ciblent des virus ou des types de virus spécifiques et agissent soit en empêchant le virus de pénétrer dans la cellule hôte, soit en bloquant les enzymes ou protéines clés nécessaires à la réplication virale. Contrairement aux antibiotiques , qui sont utilisés pour traiter les infections bactériennes, les médicaments antiviraux sont généralement moins efficaces, car les virus ont une structure beaucoup plus simple et dépendent des cellules hôtes pour se répliquer. Cependant, ils peuvent toujours être utiles dans le traitement de certaines infections virales, telles que la grippe, l'herpès et le VIH.
Dynamique du marché des médicaments antiviraux en Amérique du Nord
Cette section traite de la compréhension des moteurs, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
- Augmentation de la prévalence des infections virales
Les infections virales ont augmenté de façon constante dans le monde au cours des dernières décennies. Un virus qui pénètre dans l’organisme, exploite ses cellules pour se répliquer et se propage provoque une infection virale. Les infections virales peuvent entraîner divers symptômes, de mineurs à majeurs, et dans certains cas, même mortels. La mondialisation est l’une des principales causes de l’augmentation des infections virales. Les gens voyagent et communiquent entre eux au-delà des frontières, ce qui rend le monde plus connecté que jamais. La transmission virale d’une région à une autre s’est accélérée en raison de cette connectivité accrue.
La prévalence croissante des infections virales est donc un problème complexe auquel contribuent de nombreux facteurs. La mondialisation, la densité de population, le changement climatique et la résistance aux antibiotiques influencent tous la propagation des virus. On s'attend donc à ce que cela stimule la croissance du marché.
- Progrès dans le développement de nouveaux médicaments antiviraux
Les traitements antiviraux sont prescrits aux patients atteints d'infections virales. La création de nouveaux médicaments antiviraux a fait d'énormes progrès au fil du temps. Ces développements ont réduit la charge de morbidité, amélioré le traitement des infections virales et sauvé des vies. De nombreux progrès sont réalisés dans le domaine des nouveaux médicaments antiviraux.
Ainsi, les progrès dans le développement de nouveaux médicaments antiviraux ont amélioré le traitement des infections virales, réduit la charge de morbidité et devraient stimuler la croissance du marché.
Retenue
- Coût élevé des médicaments antiviraux
Le coût élevé des médicaments antiviraux peut avoir des conséquences importantes pour les patients et les systèmes de santé. Les patients qui ne peuvent pas se permettre ces médicaments risquent de ne pas recevoir de traitement ou d’avoir recours à des traitements de moindre qualité, ce qui peut entraîner de moins bons résultats pour leur santé. En outre, le coût élevé des médicaments antiviraux peut grever les budgets de santé, en particulier dans les pays aux ressources limitées.
Ainsi, le coût élevé des médicaments antiviraux devrait restreindre le marché nord-américain des médicaments antiviraux.
Opportunité
-
Nouveaux systèmes d'administration de médicaments en plein essor
Les recherches sur les médicaments antiviraux ont mis l’accent sur la création de nouveaux mécanismes d’administration des médicaments. Par rapport aux techniques d’administration conventionnelles, les nouveaux systèmes d’administration présentent plusieurs avantages, tels qu’une meilleure biodisponibilité, une administration personnalisée des médicaments et moins d’effets indésirables.
Le développement de nouveaux systèmes d'administration de médicaments constitue donc un domaine de recherche important dans le domaine des médicaments antiviraux. Les systèmes d'administration de médicaments à base de nanoparticules, les hydrogels, les dendrimères, les micro-aiguilles et les peptides pénétrant dans les cellules sont des systèmes d'administration de médicaments prometteurs qui ont été étudiés pour les médicaments antiviraux. Ces systèmes d'administration offrent plusieurs avantages par rapport aux méthodes d'administration de médicaments traditionnelles et ont le potentiel d'améliorer l'efficacité et la sécurité des médicaments antiviraux et devraient créer une opportunité de croissance du marché.
Défi
- Expiration des brevets des médicaments antiviraux
Le processus d'expiration des brevets entraîne la perte du droit exclusif du développeur ou du détenteur du brevet d'origine de produire et de commercialiser un médicament spécifique. L'expiration des brevets des médicaments antiviraux peut avoir des conséquences importantes sur le secteur pharmaceutique, car elle peut entraîner la concurrence des fabricants de médicaments génériques.
Les brevets des médicaments antiviraux expirent à la fin de la période pendant laquelle leur créateur a le droit exclusif de fabriquer et de commercialiser le médicament. Après l'expiration du brevet d'un médicament, d'autres producteurs peuvent créer et commercialiser des versions génériques. Cela peut entraîner une plus grande concurrence et une baisse des prix à la consommation. Le VIH, l'hépatite B et C, l'herpès, la grippe et d'autres maladies virales sont tous traités avec des médicaments antiviraux. Les brevets des médicaments antiviraux expirent à des dates différentes selon le médicament et le pays. Les brevets de médicaments sont généralement accordés pour 20 ans à compter de la date de dépôt. D'autres producteurs sont libres de créer et de commercialiser des versions génériques de médicaments une fois leur brevet expiré. Comme le producteur n'a pas à dépenser autant en marketing, en recherche et développement et en études cliniques, les médicaments génériques sont souvent plus abordables que les médicaments de marque.
Ainsi, l’expiration des brevets des médicaments antiviraux peut avoir des conséquences importantes sur la disponibilité, l’abordabilité et l’accessibilité de ces médicaments importants et devrait constituer un défi à la croissance du marché.
Développements récents
- En janvier 2023, Merck, connue sous le nom de MSD, a annoncé la réussite de l'offre publique d'achat en numéraire, par l'intermédiaire d'une filiale, pour toutes les actions ordinaires en circulation d'Imago Biosciences, Inc. (Nasdaq : IMGO), au prix d'achat de 36,00 USD par action en numéraire, sans intérêt et sous réserve de déduction pour toute retenue fiscale requise. L'acquisition contribuera à la croissance du chiffre d'affaires.
- En avril 2021, Zydus Pharmaceuticals, Inc. a annoncé avoir reçu une autorisation d'utilisation d'urgence restreinte du Drug Controller General of India (DCGI) pour utiliser le médicament antiviral Virafin pour le traitement des infections modérées au COVID-19. Cela aidera l'entreprise à accroître sa présence en Amérique du Nord et sa réputation dans d'autres régions du monde.
Portée du marché des médicaments antiviraux en Amérique du Nord
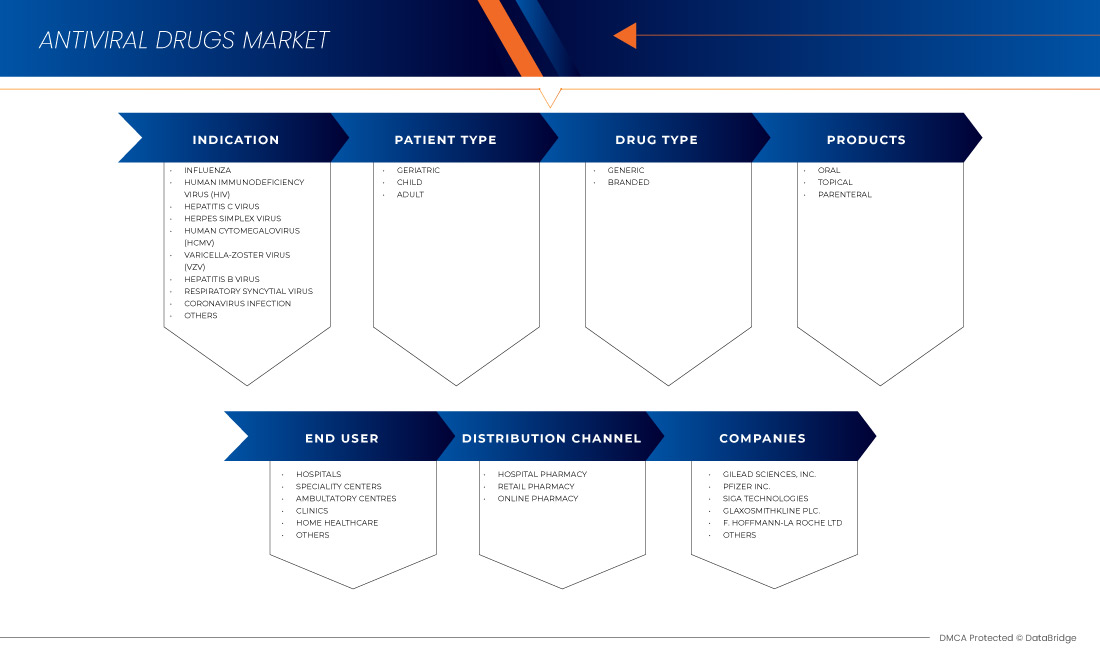
Le marché nord-américain des médicaments antiviraux est segmenté en six segments notables en fonction de l'indication, du type de patient, des produits, du type de médicament, de l'utilisateur final et du canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
Indication
- Grippe
- Virus de l'immunodéficience humaine (VIH)
- Virus de l'hépatite C
- Virus herpes simplex
- Cytomégalovirus humain (HCMV)
- Virus varicelle-zona (VZV)
- Virus de l'hépatite B
- Virus respiratoire syncytial
- Infection par corona virus
- Autres
Sur la base des indications, le marché nord-américain des médicaments antiviraux est segmenté en grippe, virus de l'immunodéficience humaine (VIH), virus de l'hépatite C (VHC), virus respiratoire syncytial, virus de l'herpès simplex, cytomégalovirus humain (HCMV), virus varicelle-zona (VZV), virus de l'hépatite B (VHB), infection à coronavirus et autres.
Type de patient
- Enfant
- Adulte
- Gériatrie
En fonction du type de patient, le marché nord-américain des médicaments antiviraux est segmenté en enfants, adultes et gériatriques.
PRODUITS
- Oral
- Topical
- Parenteral
On the basis of products, the North America antiviral drugs market is segmented into oral, topical, and parenteral.
Drug Type
- Generic
- Branded
On the basis of drug type, the North America antiviral drugs market is segmented into generic and branded.
End User
- Hospital
- Clinics
- Home Healthcare
- Speciality Centers
- Ambulatory Centers
- Others
On the basis of end user, the North America antiviral drugs market is segmented into hospitals, clinics, home healthcare, specialty centers, ambulatory centers, and others.
Distribution Channel
- Hospital Pharmacy
- Online Pharmacy
- Retail Pharmacy
On the basis of distribution channel, the North America antiviral drugs market is segmented into hospital pharmacy, online pharmacy, and retail pharmacy.
North America Antiviral Drugs Market Regional Analysis/Insights
The North America antiviral drugs market is categorized into six notable segments based on indication, patient type, products, drug type, end user, and distribution channel.
The countries covered in this market report U.S., Canada, and Mexico.
In 2023, U.S., dominates the North America region due to the strong presence of key players and due to the increasing demand from emerging markets and expansion
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of North America brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and North America Antiviral Drugs Market Share Analysis
North America antiviral drugs market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product approvals, product width and breath, application dominance, and product type lifeline curve. The above data points provided are only related to the company’s focus on the North America antiviral drugs market.
Français Certains des principaux acteurs opérant sur le marché des médicaments antiviraux en Amérique du Nord sont Gilead Sciences, Inc., F. Hoffmann-La Roche Ltd, GLAXOSMITHKLINE PLC, Abbvie, Merck & Co., Inc., Johnson & Johnson Services, Inc., Bristol-Myers Squibb Company, Cipla Inc., Aurobindo Pharma, Dr. Reddy's Laboratories Ltd., Zydus Pharmaceuticals, Inc., Mylan Pharmaceuticals ULC, Teva Pharmaceuticals USA, Inc., EMERGENT, Sun Pharmaceutical Industries Ltd., Avet Pharmaceuticals Inc., Pfizer Inc., SIGA Technologies, NAVINTA LLC., Macleods Pharmaceuticals Ltd., BioCryst Pharmaceuticals, Inc et Hetero. entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET TESTING TYPE COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS FOR NORTH AMERICA ANTIVIRAL DRUGS MARKET
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING PREVALENCE OF VIRAL INFECTIONS
8.1.2 ADVANCEMENTS IN NEW ANTIVIRAL DRUG DEVELOPMENT
8.1.3 GROWING DEMAND FOR COMBINATION THERAPIES
8.1.4 INCREASING GOVERNMENT FUNDING AND R&D ACTIVITIES
8.2 RESTRAINS
8.2.1 HIGH COST OF ANTIVIRAL DRUGS
8.2.2 EMERGENCE OF DRUG-RESISTANT STRAINS OF VIRUSES
8.3 OPPORTUNITIES
8.3.1 INCREASING COLLABORATION AND PARTNERSHIP AMONG KEY PLAYERS
8.3.2 RISING NOVEL DRUG DELIVERY SYSTEMS
8.3.3 DEVELOPMENT OF PERSONALIZED MEDICINES
8.4 CHALLENGES
8.4.1 PATENT EXPIRATION OF ANTIVIRAL DRUGS
8.4.2 DEMAND FOR ALTERNATIVE MEDICINES
9 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION
9.1 OVERVIEW
9.2 INFLUENZA
9.2.1 NEURAMINIDASE INHIBITORS
9.2.1.1 OSELTAMIVIR
9.2.1.2 ZANAMIVIR
9.2.1.3 PERAMIVIR
9.2.1.4 LANINAMIVIR
9.2.2 M2 INHIBITORS
9.2.2.1 RIMANTADINE
9.2.2.2 OTHERS
9.2.3 RNA POLYMERASE INHIBITORS
9.2.3.1 FAVIPIRAVIR
9.2.3.2 BALOXAVIR MARBOXIL
9.3 HUMAN IMMUNODEFICIENCY VIRUS (HIV)
9.3.1 REVERSE TRANSCRIPTASE INHIBITORS
9.3.1.1 NUCLEOSIDE (NRTIS)
9.3.1.1.1 LAMIVUDINE
9.3.1.1.2 ABACAVIR
9.3.1.1.3 DIDANOSINE
9.3.1.1.4 OTHERS
9.3.1.2 NONNUCLEOSIDE (NNRTIS)
9.3.1.2.1 EFAVIRENZ
9.3.1.2.2 NEVIRAPINE
9.3.1.2.3 DELAVIRDINE
9.3.1.2.4 OTHERS
9.3.1.3 INTEGRASE
9.3.1.3.1 DOLUTEGRAVIR
9.3.1.3.2 ELVITEGRAVIR
9.3.1.3.3 RALTEGRAVIR
9.3.1.3.4 BICTEGRAVIR
9.3.1.4 NUCLEOTIDE
9.3.1.4.1 TENOFOVIR
9.3.1.4.2 OTHERS
9.3.1.5 INTERFERONS
9.3.1.5.1 ALPHA
9.3.1.5.2 BETA
9.3.1.5.3 GAMMA
9.3.1.6 GP41
9.3.1.6.1 ENFUVIRTIDE
9.3.1.6.2 OTHERS
9.3.2 PROTEASE
9.3.2.1 ATAZANAVIR
9.3.2.2 DARUNAVIR
9.3.2.3 LOPINAVIR
9.3.2.4 RITONAVIR
9.3.2.5 SAQUINAVIR
9.3.2.6 INDINAVIR
9.3.2.7 NELFINAVIR
9.3.2.8 TIPRANAVIR
9.3.2.9 AMPRENAVIR
9.4 HEPATITIS C VIRUS
9.4.1 NS5B POLYMERASE
9.4.1.1 SOFOSBUVIR
9.4.1.2 DASABUVIR
9.4.2 NS3/4A PROTEASE
9.4.2.1 DANOPREVIR
9.4.2.2 GLECAPREVIR
9.4.2.3 GRAZOPREVIR
9.4.2.4 PARITAPREVIR
9.4.2.5 SIMEPREVIR
9.4.3 NS5A PHOSPHOPROTEIN
9.4.3.1 LEDIPASVIR
9.4.3.2 VELPATASVIR
9.4.3.3 OMBITASVIR
9.4.3.4 ELBASVIR
9.4.3.5 DACLATASVIR
9.4.3.6 PIBRENTASVIR
9.4.4 NEURAMINIDASE
9.4.4.1 OSELTAMIVIR
9.4.4.2 ZANAMIVIR
9.4.4.3 PERAMIVIR
9.4.4.4 LANINAMIVIR
9.4.5 RNA POLYMERASE
9.4.5.1 BALOXAVIR MARBOXIL
9.4.5.2 FAVIPIRAVIR
9.4.6 MATRIX PROTEIN 2
9.4.6.1 RIMATIDINE
9.4.6.2 FAVIPIRAVIR
9.5 HERPES SIMPLEX VIRUS
9.5.1 DNA POLYMERASE UL30
9.5.1.1 ACICLOVIR
9.5.1.2 FAMCICLOVIR
9.5.1.3 VALACICLOVIR
9.5.1.4 PENCICLOVIR TRIFLURIDINE
9.5.1.5 BRIVUDINE
9.5.1.6 FOSCARNET
9.5.1.7 IDOXURIDINE
9.5.2 ENVELOPE PROTEINS
9.5.2.1 DOCOSANOL
9.5.2.2 OTHERS
9.6 HUMAN CYTOMEGALOVIRUS (HCMV)
9.6.1 GANCICLOVIR
9.6.2 VALGANCICLOVIR
9.6.3 CIDOFOVIR
9.6.4 FOSCARNET
9.6.5 FOMIVIRSEN
9.7 VARICELLA-ZOSTER VIRUS (VZV)
9.7.1 VALACICLOVIR
9.7.2 FAMCICLOVIR
9.7.3 ACICLOVIR
9.7.4 VIDARABINE
9.7.5 BRIVUDINE
9.8 HEPATITIS B VIRUS
9.8.1 ENTECAVIR
9.8.2 TENOFOVIR
9.8.3 TELBIVUDINE
9.8.4 TENOFOVIR ALAFENAMIDE
9.8.5 OTHERS
9.9 RESPIRATORY SYNCYTIAL VIRUS
9.9.1 RNA POLYMERASE
9.9.1.1 RIBAVIRIN
9.9.1.2 OTHERS
9.9.2 FUSION GLYCOPROTEIN
9.9.2.1 PALIVIZUMAB
9.9.2.2 OTHERS
9.1 CORONAVIRUS INFECTION
9.11 OTHERS
10 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 GERIATRIC
10.2.1 MALE
10.2.2 FEMALE
10.3 CHILD
10.3.1 MALE
10.3.2 FEMALE
10.4 ADULT
10.4.1 MALE
10.4.2 FEMALE
11 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS
11.1 OVERVIEW
11.2 ORAL
11.2.1 SOLID
11.2.1.1 TABLETS
11.2.1.2 CAPSULES
11.2.1.3 OTHERS
11.2.2 SEMISOLID
11.2.2.1 GELS
11.2.2.2 EMULSIONS
11.2.2.3 ELIXIRS
11.2.2.4 OTHERS
11.2.3 LIQUID
11.2.3.1 SOLUTIONS
11.2.3.2 SYRUPS
11.2.3.3 OTHERS
11.3 TOPICAL
11.3.1 SEMI-SOLID
11.3.1.1 CREAM
11.3.1.2 OINTMENT
11.3.1.3 GELS
11.3.1.4 OTHERS
11.3.2 LIQUID
11.3.2.1 SOLUTIONS
11.3.2.2 SUSPENSIONS
11.3.3 SOLID
11.3.3.1 POWDERS
11.3.3.2 SUPPOSITORIES
11.3.3.3 ENEMA
11.3.3.4 OTHERS
11.4 PARENTERAL
11.4.1 CONVENTIONAL DRUG DELIVERY FORMUALTIONS
11.4.1.1 SOLUTIONS
11.4.1.2 RECONSTITUTED/LYOPHILIZED
11.4.1.3 SUSPENSIONS
11.4.1.4 EMULSIONS
11.4.1.5 OTHERS
11.4.2 NOVEL DRUG DELIVERY FORMULATIONS
11.4.3 COLLOIDAL DISPERSIONS
11.4.4 LONG ACTING INJECTION FORMULATION
12 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERIC
12.3 BRANDED
13 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER
13.1 OVERVIEW
13.2 HOSPITAL
13.3 SPECIALTY CENTERS
13.4 AMBULATORY CENTRES
13.5 CLINICS
13.6 HOME HEALTHCARE
13.7 OTHERS
14 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 GILEAD SCIENCES, INC. (2022)
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENT
18.2 PFIZER INC. (2022)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 SIGA TECHNOLOGIES (2022)
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 GLAXOSMITHKLINE PLC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENT
18.5 F. HOFFMANN-LA ROCHE LTD. (2022)
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENT
18.6 ABBVIE INC.
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENT
18.7 AUROBINDO PHARMA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENT
18.8 AVET PHARMACEUTICALS INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BRISTOLL MYERS SQUIBB (2022)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BIOCRYST PHARMACEUTICALS, INC. (2022)
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 CIPLA INC. (2022)
18.11.1 COMPANY SNAPSHOT
18.11.2 REVENUE ANALYSIS
18.11.3 PRODUCT PORTFOLIO
18.11.4 RECENT DEVELOPMENT
18.12 DR. REDDY’S LABORATORIES LTD. (2022)
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENTS
18.13 EMERGENT (2022)
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENT
18.14 HETERO.
18.14.1 COMPANY SNAPSHOT
18.14.2 PRODUCT PORTFOLIO
18.14.3 RECENT DEVELOPMENT
18.15 JOHNSON & JOHNSON PRIVATE LIMITED (2022)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENT
18.16 MACLEODS PHARMACEUTICALS LTD.
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MERCK & CO., INC, (2022)
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENT
18.18 MYLAN N.V (SUBSIDIARY OF VIATRIS) (2022)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 NAVINTA LLC.
18.19.1 COMPANY SNAPSHOT
18.19.2 PRODUCT PORTFOLIO
18.19.3 RECENT DEVELOPMENT
18.2 SUN PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.20.1 COMPANY SNAPSHOT
18.20.2 REVENUE ANALYSIS
18.20.3 PRODUCT PORTFOLIO
18.20.4 RECENT DEVELOPMENT
18.21 TEVA PHARMACEUTICAL INDUSTRIES LTD. (2022)
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENT
18.22 ZYDUS PHARMACEUTICALS, INC. (2022)
18.22.1 COMPANY SNAPSHOT
18.22.2 REVENUE ANALYSIS
18.22.3 PRODUCT PORTFOLIO
18.22.4 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Liste des tableaux
TABLE 1 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021- 2030 (USD MILLION)
TABLE 2 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 4 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 5 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 6 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 7 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 9 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 10 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 11 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 12 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 13 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 14 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 15 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 16 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 17 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 19 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 20 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 21 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 22 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 23 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 24 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 25 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 27 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 28 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 29 NORTH AMERICA HUMAN CYTOMEGALOVIRUS (HCMV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 31 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 33 NORTH AMERICA HEPATITIS B VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 35 NORTH AMERICA RESPIRATORY SYNCYTIAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 37 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 38 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 39 NORTH AMERICA CORONAVIRUS INFECTION IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021- 2030 (USD MILLION)
TABLE 42 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 43 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 44 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 45 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 46 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 47 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 48 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCT, 2021- 2030 (USD MILLION)
TABLE 49 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 50 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 51 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 52 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 53 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 54 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 55 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 56 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 57 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 58 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 59 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 60 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 61 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 62 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 63 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021- 2030 (USD MILLION)
TABLE 64 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021- 2030 (USD MILLION)
TABLE 65 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021- 2030 (USD MILLION)
TABLE 66 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 67 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 68 NORTH AMERICA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 69 NORTH AMERICA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 70 NORTH AMERICA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 71 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 72 NORTH AMERICA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 73 NORTH AMERICA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 74 NORTH AMERICA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 75 NORTH AMERICA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 76 NORTH AMERICA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 77 NORTH AMERICA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 78 NORTH AMERICA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 79 NORTH AMERICA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 80 NORTH AMERICA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 81 NORTH AMERICA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 82 NORTH AMERICA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 83 NORTH AMERICA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 84 NORTH AMERICA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 85 NORTH AMERICA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 86 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 87 NORTH AMERICA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 88 NORTH AMERICA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 89 NORTH AMERICA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 90 NORTH AMERICA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 91 NORTH AMERICA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 92 NORTH AMERICA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 93 NORTH AMERICA DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 94 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 95 NORTH AMERICA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 96 NORTH AMERICA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 97 NORTH AMERICA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 98 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 99 NORTH AMERICA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 100 NORTH AMERICA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 101 NORTH AMERICA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 102 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 103 NORTH AMERICA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 104 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 105 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 106 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 107 NORTH AMERICA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 108 NORTH AMERICA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 109 NORTH AMERICA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 110 NORTH AMERICA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 111 NORTH AMERICA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 112 NORTH AMERICA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 113 NORTH AMERICA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 114 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 115 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 116 NORTH AMERICA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 117 U.S. ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 118 U.S. INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 119 U.S. NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 120 U.S. M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 121 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 122 U.S. HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 123 U.S. REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 124 U.S. NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 125 U.S. NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 126 U.S. INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 127 U.S. NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 128 U.S. INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 129 U.S. GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 130 U.S. PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 131 U.S. HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 132 U.S. NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 133 U.S. NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 134 U.S. NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 135 U.S. NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 136 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 137 U.S. MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 138 U.S. HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 139 U.S. DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 140 U.S. ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 141 U.S. HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 142 U.S. VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 143 U.S. DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 144 U.S. RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 145 U.S. RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 146 U.S. FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 147 U.S. ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 148 U.S. GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 149 U.S. CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 150 U.S. ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 151 U.S. ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 152 U.S. ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 153 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 154 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 155 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 156 U.S. TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 157 U.S. SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 158 U.S. LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 159 U.S. SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 160 U.S. PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 161 U.S.CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 162 U.S. NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 163 U.S. ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 164 U.S. ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 U.S. ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 CANADA ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 167 CANADA INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 168 CANADA NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 169 CANADA M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 170 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 171 CANADA HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 172 CANADA REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 173 CANADA NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 174 CANADA NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 175 CANADA INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 176 CANADA NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 177 CANADA INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 178 CANADA GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 179 CANADA PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 180 CANADA HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 181 CANADA NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 182 CANADA NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 183 CANADA NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 184 CANADA NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 185 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 186 CANADA MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 187 CANADA HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 188 CANADA DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 189 CANADA ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 190 CANADA HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 191 CANADA VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 192 CANADA RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 193 CANADA RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 194 CANADA FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 195 CANADA ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 196 CANADA GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 197 CANADA CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 198 CANADA ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 199 CANADA ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 200 CANADA ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 201 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 202 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 203 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 204 CANADA TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 205 CANADA SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 206 CANADA LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 207 CANADA SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 208 CANADA PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 209 CANADA CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 210 CANADA NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 211 CANADA ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 212 CANADA ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 213 CANADA ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 214 MEXICO ANTIVIRAL DRUGS MARKET, BY INDICATION, 2021-2030 (USD MILLION)
TABLE 215 MEXICO INFLUENZA IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 216 MEXICO NEURAMINIDASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 217 MEXICO M2 INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 218 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 219 MEXICO HUMAN IMMUNODEFICIENCY VIRUS (HIV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 220 MEXICO REVERSE TRANSCRIPTASE INHIBITORS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 221 MEXICO NUCLEOSIDE (NRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 222 MEXICO NONNUCLEOSIDE (NNRTIS) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 223 MEXICO INTEGRASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 224 MEXICO NUCLEOTIDE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 225 MEXICO INTERFERONS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 226 MEXICO GP41 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 227 MEXICO PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 228 MEXICO HEPATITIS C VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 229 MEXICO NS5B POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 230 MEXICO NS3/4A PROTEASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 231 MEXICO NS5A PHOSPHOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 232 MEXICO NEURAMINIDASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 233 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 234 MEXICO MATRIX PROTEIN 2 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 235 MEXICO HERPES SIMPLEX VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 236 MEXICO DNA POLYMERASE UL30 IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 237 MEXICO ENVELOPE PROTEINS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 238 MEXICO HUMAN CYTOMEGALOVIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 239 MEXICO VARICELLA-ZOSTER VIRUS (VZV) IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 240 MEXICO DNA POLYMERASE ADEFOVIR IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 241 MEXICO RESPIRATORY SYNCYTICAL VIRUS IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 242 MEXICO RNA POLYMERASE IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 243 MEXICO FUSION GLYCOPROTEIN IN ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 244 MEXICO ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 245 MEXICO GERIATRIC IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 246 MEXICO CHILD IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 247 MEXICO ADULT IN ANTIVIRAL DRUGS MARKET, BY PATIENT TYPE, 2021-2030 (USD MILLION)
TABLE 248 MEXICO ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 249 MEXICO ORAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 250 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 251 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 252 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 253 MEXICO TOPICAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 254 MEXICO SEMI-SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 255 MEXICO LIQUID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 256 MEXICO SOLID IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 257 MEXICO PARENTERAL IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 258 MEXICO CONVENTIONAL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 259 MEXICO NOVEL DRUG DELIVERY FORMULATIONS IN ANTIVIRAL DRUGS MARKET, BY PRODUCTS, 2021-2030 (USD MILLION)
TABLE 260 MEXICO ANTIVIRAL DRUGS MARKET, BY DRUG TYPE, 2021-2030 (USD MILLION)
TABLE 261 MEXICO ANTIVIRAL DRUGS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 262 MEXICO ANTIVIRAL DRUGS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 NORTH AMERICA ANTIVIRAL DRUGS: SEGMENTATION
FIGURE 2 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA ANTIVIRAL DRUGS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA ANTIVIRAL DRUGS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA ANTIVIRAL DRUGS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA ANTIVIRAL DRUGS MARKET: MARKET TESTING TYPE COVERAGE GRID
FIGURE 9 NORTH AMERICA ANTIVIRAL DRUGS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE OF VIRAL INFECTIONS IS EXPECTED TO DRIVE THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN THE FORECAST PERIOD
FIGURE 12 THE INFLUENZA SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA ANTIVIRAL DRUGS MARKET
FIGURE 14 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2022
FIGURE 15 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , 2023-2030 (USD MILLION)
FIGURE 16 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , CAGR (2023-2030)
FIGURE 17 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION , LIFELINE CURVE
FIGURE 18 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2022
FIGURE 19 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, 2023-2030 (USD MILLION)
FIGURE 20 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, CAGR (2023-2030)
FIGURE 21 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 22 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2022
FIGURE 23 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 24 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 25 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 26 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2022
FIGURE 27 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, 2023-2030 (USD MILLION)
FIGURE 28 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, CAGR (2023-2030)
FIGURE 29 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 30 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2022
FIGURE 31 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 32 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 33 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY END USER, LIFELINE CURVE
FIGURE 34 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 35 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 36 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 37 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 38 NORTH AMERICA ANTIVIRAL DRUGS MARKET: SNAPSHOT (2022)
FIGURE 39 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022)
FIGURE 40 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 NORTH AMERICA ANTIVIRAL DRUGS MARKET: BY INDICATION (2023-2030)
FIGURE 43 NORTH AMERICA ANTIVIRAL DRUGS MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.