Marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique, par type (anxiété, troubles de l’humeur, dépression, troubles bipolaires, troubles psychotiques et troubles de l’alimentation ), type de test (séquençage du génome entier et tests basés sur des matrices chromosomiques), type de patient (enfant, adulte et gériatrique), type de gène (CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT et autres), produits (instruments, consommables et logiciels et services), utilisateur final (hôpitaux et cliniques, laboratoires de diagnostic, instituts universitaires et de recherche, et autres), canal de distribution (appel d’offres direct, distribution par des tiers, pharmacie hospitalière et autres) – Tendances et prévisions de l’industrie jusqu’en 2029
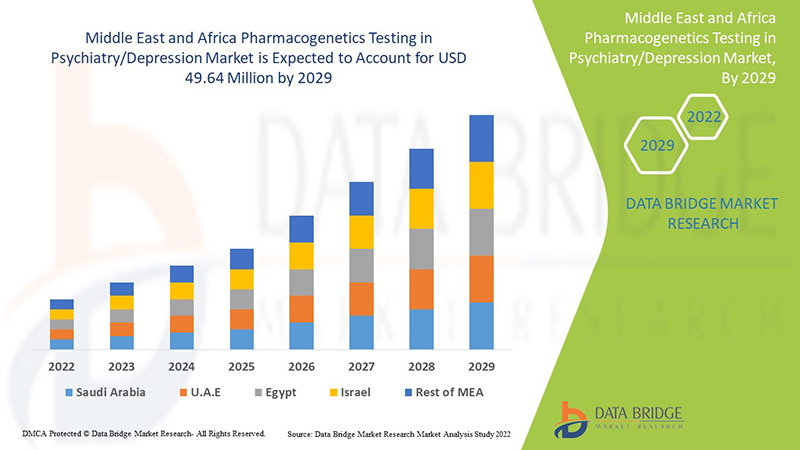
Analyse et perspectives du marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique
Les tests pharmacogénétiques aident les professionnels de la santé en leur fournissant des informations sur la façon dont une personne métabolise un médicament. Ces informations peuvent aider les médecins et autres professionnels à éviter de prescrire des antidépresseurs qui pourraient produire des effets indésirables. La pharmacogénomique s'est révélée prometteuse pour prédire la réponse et la tolérance aux antidépresseurs dans le traitement du trouble dépressif majeur (TDM). La pharmacogénomique peut améliorer les résultats cliniques en guidant le choix et le dosage des antidépresseurs. Le secteur des biotechnologies en pleine croissance et l'augmentation des dépenses de santé ont accéléré la demande de tests pharmacogénétiques en psychiatrie/dépression.
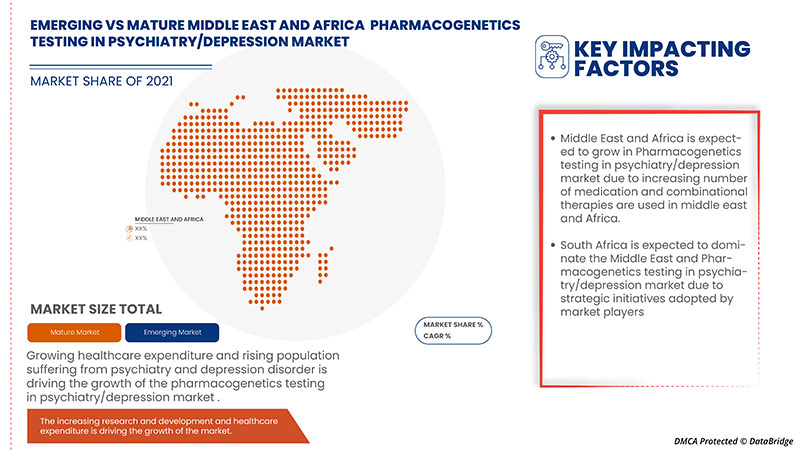
La prévalence croissante du cancer, les nouvelles technologies dans le traitement de la dépression et/ou d'autres troubles psychiatriques augmentent l'adoption des tests pharmacogénétiques dans les dispositifs et procédures de psychiatrie/dépression, et la préférence croissante pour les procédures non chirurgicales sont les principaux facteurs qui ont propulsé la demande du marché au cours de la période de prévision. Cependant, le coût élevé associé aux tests, la réglementation stricte et le manque de sensibilisation pourraient freiner la croissance du marché des tests pharmacogénétiques en psychiatrie/dépression au cours de la période de prévision.
Data Bridge Market Research analyse que le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique devrait atteindre la valeur de 49,64 millions USD d'ici 2029, à un TCAC de 7,1 % au cours de la période de prévision. L'anxiété représente le segment de type le plus important du marché en raison du taux croissant de dépression parmi les populations du Moyen-Orient et d'Afrique. Ce rapport de marché couvre également en profondeur l'analyse des prix, l'analyse des brevets et les avancées technologiques.
|
Rapport métrique |
Détails |
|
Période de prévision |
2022 à 2029 |
|
Année de base |
2021 |
|
Années historiques |
2020 |
|
Unités quantitatives |
Chiffre d'affaires en millions USD, volumes en unités, prix en USD |
|
Segments couverts |
Le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique, par application (nouveaux candidats médicaments, tests et approbations précliniques d'optimisation et de réorientation des médicaments, surveillance des médicaments, recherche de nouvelles cibles et voies associées aux maladies, compréhension des mécanismes des maladies, agrégation et synthèse des informations, formation et qualification d'hypothèses, conception de nouveaux médicaments, recherche de cibles médicamenteuses d'un ancien médicament, et autres), technologie (apprentissage automatique, apprentissage profond, traitement du langage naturel, et autres), type de médicament (petite molécule et grande molécule), offre (logiciels et services), indication (immuno-oncologie, maladies neurodégénératives, maladies cardiovasculaires, maladies métaboliques, et autres), utilisation finale (organismes de recherche sous contrat (CRO), sociétés pharmaceutiques et biotechnologiques, centres de recherche et instituts universitaires, et autres) |
|
Pays couverts |
Émirats arabes unis, Israël, Afrique du Sud, Égypte, Koweït et reste du Moyen-Orient et de l'Afrique |
|
Acteurs du marché couverts |
Genelex (une partie de la société Invitae), Genewiz (une partie d'Azenta Life Sciences), MD Labs, BiogeneiQ, Inc., ONEOME, LLC, Myriad Genetics, Inc., GenXys, Castle Biosciences, Inc., PacBio, QIAGEN, Thermo Fisher Scientific Inc., AB-Biotics, SA, Coriell Life Sciences, Eurofins Scientific, Illumina, Inc., Dynamic DNA Laboratories, STADAPHARM GmbH, Color, cnsdose, Genomind, Inc., Healthspek, myDNA Life Australia Pty Ltd., HudsonAlpha, Sonic Healthcare Limited, entre autres. |
Définition du marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique
Les tests pharmacogénomiques sont récemment devenus évolutifs et disponibles pour guider le trouble dépressif majeur (TDM). Les cliniciens reconnaissent de plus en plus les tests pharmacogénomiques (PGx) comme un outil essentiel pour guider les décisions en matière de médicaments pour les maladies psychiatriques. La mise en œuvre à grande échelle des tests PGx stimule le marché au cours de la période de prévision.
Les termes médecine personnalisée, médecine stratifiée et médecine de précision sont proches de la pharmacogénétique, mais ce sont des termes plus larges qui couvrent également d'autres facteurs non génétiques. Néanmoins, la pharmacogénétique est une composante importante de ces domaines. La pharmacogénétique s'intéresse principalement à la variation de l'ADN germinal humain, mais des avancées importantes ont également été réalisées récemment dans la compréhension des troubles de l'humeur et des maladies mentales.
Les tests pharmacogénétiques étudient l'interaction entre un médicament et la réponse génique d'une personne et recherchent la variation génétique responsable de l'influence de l'effet du médicament. Ce test est de plus en plus demandé car de nombreux chercheurs et scientifiques ont identifié l'interaction unique entre les médicaments et les gènes individuels et fournissent des informations précieuses qui peuvent ensuite être utilisées pour développer des médicaments personnalisés.
Dynamique du marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique
Cette section traite de la compréhension des moteurs, des avantages, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
- AUGMENTATION DU NOMBRE DE PATIENTS SOUFFRANT DE TROUBLES PSYCHIATRIQUES ET DÉPRESSIFS
La dépression est une maladie courante dans le monde entier, avec environ 3,8 % de la population touchée, dont 5,0 % chez les adultes et 5,7 % chez les adultes de plus de 60 ans. La dépression peut devenir un problème de santé grave de gravité légère à extrême, entraînant une souffrance importante chez la personne et pouvant conduire au suicide dans les cas les plus graves. Bien que plus de 45 antidépresseurs soient disponibles, la réponse sous-optimale pose un défi et est considérée comme le résultat de variations génétiques, de psychiatrie/dépression. En fonction de la gravité et du schéma des épisodes dépressifs au fil du temps, les prestataires de soins de santé peuvent proposer un diagnostic psychologique tel que l'activation comportementale, la thérapie cognitivo-comportementale, la psychothérapie interpersonnelle et/ou des médicaments antidépresseurs tels que les inhibiteurs sélectifs de la recapture de la sérotonine (ISRS) et les antidépresseurs tricycliques (ATC). Différents médicaments sont utilisés pour ce type de trouble mental.
Avec l'augmentation de la prévalence de la dépression, la demande de tests pharmacogénétiques augmente également, car ils étudient l'effet des variantes génétiques dans le but de fournir un diagnostic personnalisé. Le marché devrait croître pendant la période de foresterie.
- AUGMENTATION DE LA DEMANDE DE MÉDECINE PERSONNALISÉE ET DE PRÉCISION
Le test de pharmacogénétique aide le professionnel de la santé à choisir le meilleur médicament pour la personne, car le test recherche la variante génétique qui peut être responsable de l’influence de l’effet du médicament.
La médecine commence à devenir plus personnelle et les patients expriment peu à peu leur intérêt pour de meilleurs résultats et moins d’effets indésirables grâce à des médicaments personnalisés. La médecine personnalisée a le potentiel d’adapter la thérapie avec une marge de sécurité élevée et la meilleure réponse. Cette tendance est en grande partie due aux améliorations du séquençage du génome.
L'évolution vers des soins de santé personnalisés implique des changements dans la fabrication des médicaments. Les fabricants passent de la création de petites molécules à la combinaison de petites molécules et de thérapies géniques . Les promoteurs s'efforcent de remplacer la production inefficace de lots à grande échelle par des investissements dans de nouvelles technologies et la production de médicaments personnalisés.
Retenue
- Manque d'experts médicaux et génomiques qualifiés
La plupart des cliniciens manquent encore de confiance dans les tests pharmacogénétiques (PGx) et l'interprétation des données qui en découle, ce qui indique un manque de connaissances dans ce domaine. Cela souligne la nécessité d'améliorer les connaissances des professionnels de santé en matière d'expertise et de compréhension des tests pharmacogénétiques (PGx).
Le manque de connaissances des praticiens sur les possibilités de la pharmacogénétique et l'explication insuffisante des résultats des tests réduisent également les technologies de personnalisation pour les patients. Outre le développement de cours de formation thématiques dans les universités de médecine, il est nécessaire d'inclure les cycles de formation dans les systèmes de formation professionnelle continue et de mettre gratuitement à disposition des médecins en exercice des informations : portails Internet universitaires, webinaires, etc. Le pharmacologue clinicien joue un rôle crucial dans la mise en œuvre des tests pharmacogénétiques.
La compétence d'un pharmacologue clinicien dans le domaine de la pharmacogénétique est essentielle : c'est lui qui organise l'application du génotypage dans la pratique clinique, interprète les tests et informe les médecins sur les possibilités de la pharmacogénétique pour les patients présentant des nosologies spécifiques, c'est-à-dire qu'il agit comme le lien principal entre le monde scientifique, le système de santé et les médecins en exercice dans le processus d'introduction de la pharmacogénétique.
Opportunité
-
Progrès technologiques croissants
Les progrès de la pharmacogénomique ont ouvert un nombre croissant d’opportunités pour intégrer la médecine personnalisée dans la pratique clinique des troubles psychiatriques. La médecine personnalisée peut être définie comme une approche globale et prospective de la prévention, du diagnostic, du traitement et du suivi des maladies de manière à obtenir des décisions optimales en matière de soins de santé individuels. Plus de 100 médicaments portent désormais l’étiquetage de la Food and Drug Administration (FDA) des États-Unis relatif à des biomarqueurs pharmacogénomiques potentiellement applicables grâce aux avancées technologiques dans le domaine des soins de santé. De plus, de nouvelles méthodes avancées sont en cours de développement pour promouvoir les tests pharmacogénétiques dans les troubles de type dépressif. Ces tests utilisent des méthodes de test génétique avancées pour donner des résultats précis afin de former un schéma thérapeutique. Les améliorations de la technologie à l’appui des tests ont amélioré l’accessibilité des options de test, et le nombre croissant de ressources qui aident les cliniciens à comprendre comment utiliser ces informations lorsqu’elles sont disponibles font de cet aspect de la médecine personnalisée ou de précision une réalité. Ainsi, les prestataires doivent devenir plus conscients de la pertinence scientifique et clinique des tests pharmacogénomiques.
Les tests permettent également d'établir une relation significative entre un médicament et la constitution génétique d'un individu. Cela permet de décider quels médicaments administrer au patient pour traiter les troubles dépressifs majeurs et d'autres troubles psychiatriques.
Défi
- Réglementation gouvernementale stricte sur l'approbation de nouveaux produits et instruments
Les inquiétudes concernant l'efficacité et la sécurité des produits ont conduit la plupart des gouvernements à mettre en place des agences et des politiques de réglementation pour surveiller le développement de nouveaux produits ou tests médicaux. L'utilisation de ces produits médicaux peut être effectuée après avoir satisfait à des normes réglementaires strictes, qui garantissent que le produit est sûr, bien étudié et ne présente pas d'effets indésirables.
Les directives récentes et leur amendement fournissent des orientations adéquates aux fabricants. Les réglementations internationales telles que celles relatives aux aliments, aux médicaments et à l'administration jouent un rôle majeur dans le lancement d'un nouveau produit médical ou d'un nouveau test sur le marché. Elles peuvent donc constituer un frein majeur pour le marché. Par conséquent, une réglementation gouvernementale stricte sur les nouveaux produits et l'approbation des instruments aura probablement un impact sur le marché.
Impact post-COVID-19 sur le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique
L'épidémie de COVID-19 a eu un impact positif sur l'expansion de l'industrie des tests pharmacogénétiques. La pandémie a eu un impact négatif sur la croissance du marché de la pharmacogénomique en raison de l'arrêt temporaire des activités de recherche dans ce domaine, couplé au faible afflux de patients dans les hôpitaux et les centres de diagnostic. Depuis le second semestre 2020, avec la demande croissante de recherche sur certains médicaments et kits de test pour le COVID-19, les pratiques pharmacogénomiques sont à la mode.
Les fabricants prennent diverses décisions stratégiques pour rebondir après la COVID-19. Les acteurs mènent de multiples activités de R&D, de lancements de produits et de partenariats stratégiques pour améliorer la technologie et les résultats des tests impliqués dans le marché des tests pharmacogénétiques.
Développements récents
- En avril 2022, Blue Care Network (BCN) a lancé un programme de médecine de précision, Blue Cross Personalized Medicine, qui s'appuie sur la pharmacogénomique, ou les tests génétiques , pour personnaliser et adapter plus efficacement les traitements médicamenteux pour certains membres en fonction d'un examen de leurs médicaments prescrits pour divers diagnostics, notamment la santé comportementale, la cardiologie, les maladies cardiovasculaires et l'oncologie. OneOme LLC a aidé BCN à atteindre les objectifs de son programme de médecine de précision, à réduire le coût total des soins et à améliorer les résultats de santé des patients en réduisant les effets indésirables des médicaments. Cela a aidé l'entreprise à améliorer son portefeuille de produits.
- En février 2022, PacBio, l'un des principaux fournisseurs de plateformes de séquençage de haute qualité et de haute précision, a annoncé qu'il soutenait l'hôpital pour enfants malades (SickKids) de Toronto, au Canada, dans l'utilisation du séquençage du génome entier HiFi (HiFi WGS) pour identifier potentiellement des variantes génétiques pouvant être associées à des conditions médicales et développementales. Les échantillons examinés à l'aide du HiFi WGS ont été précédemment séquencés à l'aide de la technologie de séquençage d'ADN à lecture courte, mais il manque toujours l'identification d'une variante causant une maladie. Cela a aidé l'entreprise à améliorer l'utilisation de ses produits.
- En juillet 2022, selon une nouvelle étude nationale menée auprès de près de 2 000 vétérans par le ministère américain des Anciens Combattants (VA), les taux de rémission du trouble dépressif majeur (TDM) ont été considérablement améliorés lorsque les cliniciens ont eu accès aux résultats du test psychotrope GeneSight de Myriad Genetics, Inc. dans le cadre du plus grand essai contrôlé randomisé PGx sur la santé mentale jamais réalisé. Cela a aidé l'entreprise à montrer ses progrès en matière de tests pharmacogénétiques.
- En janvier 2021, myDNA Life Australia Pty Ltd a annoncé une fusion avec la société américaine de tests ADN grand public basée à Houston, FamilyTreeDNA, et sa société mère. Gene by Gene a révolutionné le domaine de la pharmacogénomique, faisant de la médecine véritablement personnalisée une réalité, avant de s'étendre à la nutrigénomique pour fournir des recommandations personnalisées et exploitables en matière de nutrition, de remise en forme et de soins de la peau. Cela a aidé l'entreprise à développer ses activités.
Portée du marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique
Le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique est segmenté en type, type de test, type de gène, type de patient, produit, utilisateur final et canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
- Tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique, par type
- Anxiété
- Troubles de l'humeur
- Dépression
- Troubles bipolaires
- Troubles psychotiques
- Troubles de l'alimentation
Sur la base du type, le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique est segmenté en anxiété, troubles de l'humeur, dépression, troubles bipolaires, troubles psychotiques et troubles de l'alimentation.
- Tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique, par type de test
- Séquençage du génome entier
- Tests basés sur des matrices chromosomiques
Sur la base du type de test, le marché des tests pharmacogénétiques du Moyen-Orient et de l'Afrique en psychiatrie/dépression est segmenté en tests basés sur le séquençage du génome entier et sur des matrices chromosomiques.
- Tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique, par type de gène
- CYP2C19
- CYP2C9 et VKORC1
- CYP2D6
- HLA-B
- HTR2A/C
- HLA-A
- CYP3A4
- SLC6A4
- MTHFR
- COMT
- AUTRES
Sur la base du type de gène, le marché des tests pharmacogénétiques du Moyen-Orient et de l'Afrique en psychiatrie/dépression est segmenté en CYP2C19, CYP2C9, VKORC1, CYP2D6, HLA-B, HTR2A/C, HLA-A, CYP3A4, SLC6A4, MTHFR, COMT et autres.
- Tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique, par type de patient
- Enfant
- Adulte
- Gériatrie
Sur la base du type de patient, le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique est segmenté en enfants, adultes et gériatriques.
- Tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique, par produit
- Instruments
- Consommables
- Logiciels et services
Sur la base du type de produit, le marché des tests pharmacogénétiques en psychiatrie/dépression au Moyen-Orient et en Afrique est segmenté en instruments, consommables et logiciels et services.
- Tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique, par utilisateur final
- Hôpitaux et cliniques
- Laboratoires Dignostics
- Instituts universitaires et de recherche
- Autres
On the basis of end users, Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into hospitals and clinics, diagnostics laboratories, academic and research institutes, and others.
- Middle East & Africa Pharmacogenetics Testing In Psychiatry/Depression Market, By Distribution Channel
- Direct Tender
- Third-Party Distribution
- Hospital Pharmacy
- Others

On the basis of distribution channel, the Middle East & Africa pharmacogenetics testing in psychiatry/depression market is segmented into direct tender, third party distribution hospital pharmacy, and others.
Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Regional Analysis/Insights
The Middle East & Africa pharmacogenetics testing in psychiatry/depression market is analyzed, and market size information is provided by the type, test type, gene type, patient type, product, end user, and distribution channel. The countries covered in this market report are UAE, Israel, South Africa, Egypt, Kuwait, and rest of the Middle East & Africa.
In 2022, Middle East & Africa is dominating due to the presence of key market players in the largest consumer market with high GDP. South Africa is expected to grow due to the rise in technological advancement in pharmacogenetics testing.
The country section of the report also provides individual market-impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, the presence and availability of Middle East & African brands, the challenges faced due to large or scarce competition from local and domestic brands, and the impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Middle East & Africa Pharmacogenetics Testing in Psychiatry/Depression Market Share Analysis
Middle East & Africa pharmacogenetics testing in psychiatry/depression market competitive landscape provides details by the competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Middle East & Africa pharmacogenetics testing in psychiatry/depression market.
Some of the major players operating in the Middle East & Africa pharmacogenetics testing in psychiatry/depression market are
- Genelex (Part of Invitae corporation)
- Genewiz (Part of Azenta Life Sciences)
- MD Labs
- BiogeneiQ, Inc.
- ONEOME, LLC
- Myriad Genetics, Inc.
- GenXys
- Château Biosciences, Inc.
- PacBio
- QIAGEN
- Thermo Fisher Scientific Inc.
- AB-Biotics.SA
- Coriell Sciences de la vie
- Eurofins Scientifique
- Illumina, Inc.
- Laboratoires d'ADN dynamique
- STADAPHARM GmbH
- Couleur
- Cnsdose
- Genomind, Inc.
- Parlons santé
- myDNA Life Australie Pty Ltd.
- Hudson Alpha
- Sonic Healthcare Limitée
Méthodologie de recherche : tests pharmacogénétiques sur le marché de la psychiatrie et de la dépression au Moyen-Orient et en Afrique
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. Les données du marché sont analysées et estimées à l'aide de modèles statistiques et cohérents du marché. En outre, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. La principale méthodologie de recherche utilisée par l'équipe de recherche DBMR est la triangulation des données, qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). En dehors de cela, les modèles de données comprennent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement de l'entreprise, l'analyse des parts de marché des entreprises, les normes de mesure, le Moyen-Orient et l'Afrique par rapport à la région et l'analyse des parts des fournisseurs. Veuillez demander un appel d'analyste en cas de demande de renseignements supplémentaires.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET APPLICATION COVERAGE GRID
2.8 SOURCE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
3.1 PESTEL ANALYSIS
3.2 PORTER'S FIVE FORCES MODEL
3.3 INDUSTRIAL INSIGHTS:
3.4 PIPELINE ANALYSIS FOR MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET
3.5 EPIDEMIOLOGY
4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, REGULATIONS
4.1 UNITED STATES (U.S.)
4.1.1 ROLE OF FDA
4.1.2 ROLE OF CDC AND HCFA
4.2 EUROPEAN UNION (EU)
4.3 FRANCE
4.4 AUSTRALIA
4.5 SOUTH KOREA
5 MARKET OVERVIEW
5.1 DRIVERS
5.1.1 INCREASE IN THE NUMBER OF PATIENTS SUFFERING FROM PSYCHIATRIC AND DEPRESSION DISORDER
5.1.2 RISE IN HEALTHCARE EXPENDITURE
5.1.3 RISE IN DEMAND FOR PERSONALIZED AND PRECISION MEDICINE
5.2 RESTRAINTS
5.2.1 LACK OF SKILLED MEDICAL AND GENOMIC EXPERT
5.2.2 LACK OF STRONG CLINICAL EVIDENCE
5.2.3 HIGH COST ASSOCIATED WITH THE DIAGNOSIS
5.3 OPPORTUNITIES
5.3.1 RISING ADVANCEMENTS IN TECHNOLOGY
5.3.2 INCREASING NUMBER OF KEY PLAYERS IN MARKET
5.4 CHALLENGES
5.4.1 STRICT GOVERNMENT REGULATION ON NEW PRODUCTS AND INSTRUMENTS APPROVAL
5.4.2 DEARTH OF SKILLED PERSONNEL
6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE
6.1 OVERVIEW
6.2 ANXIETY
6.3 DEPRESSION
6.4 MOOD DISORDERS
6.5 BIPOLAR DISORDERS
6.6 EATING DISORDERS
6.7 PSYCHOTIC DISORDERS
7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT
7.1 OVERVIEW
7.2 CONSUMABLES
7.2.1 WHOLE GENOME SEQUENCING KITS
7.2.2 CHROMOSOMAL ARRAY BASED KITS
7.3 INSTRUMENTS
7.4 SOFTWARE AND SERVICES
8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 WHOLE GENOME SEQUENCING
8.2.1 EXOME SEQUENCING
8.2.1.1 SNP
8.2.1.2 CNV
8.2.1.3 RARE PTV MUTATION
8.2.1.4 ULTRA PTV MUTATION
8.2.1.5 OTHERS
8.2.2 KARYOTYPE
8.2.2.1 SNP
8.2.2.2 CNV
8.2.2.3 RARE PTV MUTATION
8.2.2.4 ULTRA PTV MUTATION
8.2.2.5 OTHERS
8.2.3 LOW COVERAGE WGS
8.2.3.1 SNP
8.2.3.2 CNV
8.2.3.3 RARE PTV MUTATION
8.2.3.4 ULTRA PTV MUTATION
8.2.3.5 OTHERS
8.2.4 DEEP COVERAGE WGS
8.2.4.1 SNP
8.2.4.2 CNV
8.2.4.3 RARE PTV MUTATION
8.2.4.4 ULTRA PTV MUTATION
8.2.4.5 OTHERS
8.2.5 MICROARRAY
8.2.5.1 SNP
8.2.5.2 CNV
8.2.5.3 RARE PTV MUTATION
8.2.5.4 ULTRA PTV MUTATION
8.2.5.5 OTHERS
8.2.6 OTHERS
8.3 CHROMOSOMAL ARRAY BASED TESTS
8.3.1 MICRODELETIONS
8.3.2 MICRO DUPLICATIONS
9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE
9.1 OVERVIEW
9.2 CYP2C19
9.3 CYP2C9 AND VKORC1
9.4 CYP2D6
9.5 HLA-B
9.6 HTR2A/C
9.7 HLA-A
9.8 CYP3A4
9.9 SLC6A4
9.1 MTHFR
9.11 COMT
9.12 OTHERS
10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE
10.1 OVERVIEW
10.2 ADULT
10.3 GERIATRIC
10.4 CHILD
11 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET , BY END USER
11.1 OVERVIEW
11.2 HOSPITAL AND CLINICS
11.2.1 DRUG EFFECTIVENESS
11.2.2 SIDE EFFECTS
11.2.3 DOSAGE
11.3 DIAGNOSTICS LABORATORIES
11.3.1 DRUG EFFECTIVENESS
11.3.2 SIDE EFFECTS
11.3.3 DOSAGE
11.4 ACADEMIC AND RESEARCH INSTITUTES
11.4.1 DRUG EFFECTIVENESS
11.4.2 SIDE EFFECTS
11.4.3 DOSAGE
11.5 OTHERS
12 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 THIRD PARTY DISTRIBUTION
12.4 HOSPITAL PHARMACY
12.5 OTHERS
13 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION
13.1 MIDDLE EAST AND AFRICA
13.1.1 SOUTH AFRICA
13.1.2 SAUDI ARABIA
13.1.3 U.A.E
13.1.4 ISRAEL
13.1.5 EGYPT
13.1.6 REST OF MIDDLE EAST AND AFRICA
14 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 THERMO FISHER SCIENTIFIC INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 RECENT FINANCIALS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENT
16.2 ILLUMINA, INC.
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 MYRIAD GENETICS, INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 COMPANY SHARE ANALYSIS
16.3.3 PRODUCT PORTFOLIO
16.3.4 RECENT DEVELOPMENT
16.4 SONIC HEALTHCARE LIMITED
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 QIAGEN
16.5.1 COMPANY SNAPSHOT
16.5.2 RECENT FINANCIALS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 EUROFINS SCIENTIFIC
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AB-BIOTICS, S.A.
16.7.1 COMPANY SNAPSHOT
16.7.2 PRODUCT PORTFOLIO
16.7.3 RECENT DEVELOPMENTS
16.8 BIOGENIQ INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 PRODUCT PORTFOLIO
16.8.3 RECENT DEVELOPMENT
16.9 CASTLE BIOSCIENCE, INC.
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CNSDOSE
16.10.1 COMPANY SNAPSHOT
16.10.2 PRODUCT PORTFOLIO
16.10.3 RECENT DEVELOPMENT
16.11 COLOR HEALTH, INC.
16.11.1 COMPANY SNAPSHOT
16.11.2 PRODUCT PORTFOLIO
16.11.3 RECENT DEVELOPMENTS
16.12 CORIELL LIFE SCIENCES
16.12.1 COMPANY SNAPSHOT
16.12.2 PRODUCT PORTFOLIO
16.12.3 RECENT DEVELOPMENTS
16.13 DYNAMIC DNA LABORATORIES
16.13.1 COMPANY SNAPSHOT
16.13.2 PRODUCT PORTFOLIO
16.13.3 RECENT DEVELOPMENTS
16.14 GENELEX (SUBSIADIARY OF INVITAE CORPORATION.)
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 GENEWIZ (PART OF AZENTA LIFE SCIENCES)
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 GENOMIND, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 GENXYS
16.17.1 COMPANY SNAPSHOT
16.17.2 PRODUCT PORTFOLIO
16.17.3 RECENT DEVELOPMENTS
16.18 HEALTHSPEK
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENTS
16.19 HUDSONALPHA
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 MD LABS
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
16.21 MYDNA LIFE AUSTRALIA PTY LTD.
16.21.1 COMPANY SNAPSHOT
16.21.2 PRODUCT PORTFOLIO
16.21.3 RECENT DEVELOPMENT
16.22 ONEOME, LLC
16.22.1 COMPANY SNAPSHOT
16.22.2 PRODUCT PORTFOLIO
16.22.3 RECENT DEVELOPMENTS
16.23 PACBIO
16.23.1 COMPANY SNAPSHOT
16.23.2 REVENUE ANALYSIS
16.23.3 PRODUCT PORTFOLIO
16.23.4 RECENT DEVELOPMENTS
16.24 STADAPHARM GMBH
16.24.1 COMPANY SNAPSHOT
16.24.2 PRODUCT PORTFOLIO
16.24.3 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 2 MIDDLE EAST & AFRICA ANXIETY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA DEPRESSION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA MOOD DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA BIPOLAR DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA EATING DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA PSYCHOTIC DISORDERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 10 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 13 MIDDLE EAST & AFRICA INSTRUMENTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA SOFTWARE AND SERVICES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA CYP2C19 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA CYP2C9 AND VKORC1 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA CYP2D6 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA HLA-B IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA HTR2A/C IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HLA-A IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA CYP3A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA SLC6A4 IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA MTHFR IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA COMT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA ADULT IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA GERIATRIC IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA CHILD IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA DIAGNOSTICS LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA DIRECT TENDER IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA THIRD PARTY DISTRIBUTION IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA HOSPITAL PHARMACY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA OTHERS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 54 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 55 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 57 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 58 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 59 MIDDLE EAST AND AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 60 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 61 MIDDLE EAST AND AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 62 MIDDLE EAST AND AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 63 MIDDLE EAST AND AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 64 MIDDLE EAST AND AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 65 MIDDLE EAST AND AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 66 MIDDLE EAST AND AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 67 MIDDLE EAST AND AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 68 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 69 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 70 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 MIDDLE EAST AND AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 72 MIDDLE EAST AND AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 73 MIDDLE EAST AND AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 77 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 78 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 79 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 81 SOUTH AFRICA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 82 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 83 SOUTH AFRICA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 84 SOUTH AFRICA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 85 SOUTH AFRICA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 86 SOUTH AFRICA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 87 SOUTH AFRICA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 88 SOUTH AFRICA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 89 SOUTH AFRICA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 90 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 91 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 92 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 93 SOUTH AFRICA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 SOUTH AFRICA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 SOUTH AFRICA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 96 SOUTH AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 98 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 99 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 100 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 101 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 103 SAUDI ARABIA CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 104 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 105 SAUDI ARABIA WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 106 SAUDI ARABIA EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 107 SAUDI ARABIA KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 108 SAUDI ARABIA LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 109 SAUDI ARABIA DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 110 SAUDI ARABIA MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 111 SAUDI ARABIA CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 112 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 113 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 114 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 115 SAUDI ARABIA HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 116 SAUDI ARABIA DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 117 SAUDI ARABIA ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 118 SAUDI ARABIA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 119 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 120 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 122 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 123 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 124 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 125 UAE CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 126 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 127 UAE WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 128 UAE EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 129 UAE KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 130 UAE LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 131 UAE DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 132 UAE MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 133 UAE CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 134 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 135 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 136 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 137 UAE HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 138 UAE DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 139 UAE ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 140 UAE PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 141 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 143 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 144 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 145 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 146 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 147 ISRAEL CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 148 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 149 ISRAEL WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 150 ISRAEL EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 151 ISRAEL KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 152 ISRAEL LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 153 ISRAEL DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 154 ISRAEL MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 155 ISRAEL CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 156 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 157 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 158 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 159 ISRAEL HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 160 ISRAEL DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 161 ISRAEL ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 162 ISRAEL PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 163 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 164 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 165 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 166 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 167 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 168 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 VOLUME (UNITS)
TABLE 169 EGYPT CONSUMABLES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PRODUCT, 2020-2029 ASP (USD)
TABLE 170 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 171 EGYPT WHOLE GENOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 172 EGYPT EXOME SEQUENCING IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 173 EGYPT KARYOTYPE IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 174 EGYPT LOW COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 175 EGYPT DEEP COVERAGE WGS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 176 EGYPT MICROARRAY IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 177 EGYPT CHROMOSOMAL ARRAY BASED TESTS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TEST TYPE, 2020-2029 (USD MILLION)
TABLE 178 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY GENE TYPE, 2020-2029 (USD MILLION)
TABLE 179 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 180 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 181 EGYPT HOSPITAL AND CLINICS IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 182 EGYPT DIAGNOSTIC LABORATORIES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 183 EGYPT ACADEMIC AND RESEARCH INSTITUTES IN PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 184 EGYPT PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 185 REST OF MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET, BY TYPE, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SEGMENTATION
FIGURE 11 THE INCREASING ADOPTION OF PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION DEVICES AND PROCEDURES AND RISING PREFERENCE FOR NON-SURGICAL PROCEDURES ARE EXPECTED TO DRIVE THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SOFTWARE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET IN 2022 & 2029
FIGURE 13 PATIENT FLOW DIAGRAM
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN THE PSYCHIATRY/DEPRESSION MARKET
FIGURE 15 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2021
FIGURE 16 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 17 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 18 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE, LIFELINE CURVE
FIGURE 19 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2021
FIGURE 20 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, 2022-2029 (USD MILLION)
FIGURE 21 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 22 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 23 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2021
FIGURE 24 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, 2022-2029 (USD MILLION)
FIGURE 25 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, CAGR (2022-2029)
FIGURE 26 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 27 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2021
FIGURE 28 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, 2022-2029 (USD MILLION)
FIGURE 29 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, CAGR (2022-2029)
FIGURE 30 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY GENE TYPE, LIFELINE CURVE
FIGURE 31 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2021
FIGURE 32 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 33 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 34 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 35 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2021
FIGURE 36 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, CAGR (2022-2029)
FIGURE 38 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY END USER, LIFELINE CURVE
FIGURE 39 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: SNAPSHOT (2021)
FIGURE 44 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021)
FIGURE 45 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 MIDDLE EAST AND AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: BY TYPE (2022-2029)
FIGURE 48 MIDDLE EAST & AFRICA PHARMACOGENETICS TESTING IN PSYCHIATRY/DEPRESSION MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.













