Middle East and Africa Glioblastoma Multiforme Treatment Market, By Type (Primary (De Novo), Secondary), Treatment (Surgery, Radiotherapy, Medications), Patient Type (AdultGeriatric, Child), Drug Type (Generics, Branded), Route of Administration (Parenteral, Oral, Others), End User (Hospitals, Clinics, Home Healthcare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) Industry Trends and Forecast to 2029.
Market Analysis and Insights
Glioblastoma multiforme (GBM) is a grade IV WHO malignant tumor with astrocytic differentiation. As one of the most common clinically diagnosed central nervous system (CNS) oncological entries, there have been a wide variety of historical reports of the description and evolution of ideas regarding these tumors. The first recorded reports of gliomas were given in British scientific reports, by Berns in 1800 and in 1804 by Abernety, with the first comprehensive histomorphological description being given in 1865 by Rudolf Virchow. In 1926 Percival Bailey and Harvey Cushing gave the base for the modern classification of gliomas. Between 1934 and 1941 the most prolific researcher in glioma research was Hans-Joachim Scherer, who postulated some of the clinico-morphological aspects of GBM. With the introduction of molecular and genetic tests the true multifomity of GBM has been established, with different genotypes bearing the same histomorphological and IHC picture, as well as some of the aspects of gliomagenesis. For a GBM to develop, a specific trigger mutation needs to occur in a GBM stem cell - primary GBM, or a slow aggregation of individual mutations, without a distinct trigger mutation - secondary GBM. Knowledge of GBM has been closely related to general medical knowledge of the CNS since these malignancies were first described more than 200 years ago. Several great leaps have been made in that time, in the footsteps of both CNS and advancements in general medical knowledge. The demand for glioblastoma multiforme treatment is increasing, for which manufacturers are involved in the new product launches, increasing pipeline products and event participation in the market. These decisions are ultimately enhancing the growth of the market.
Le rapport sur le marché du traitement du glioblastome multiforme fournit des détails sur la part de marché, les nouveaux développements, l’impact des acteurs du marché national et local, analyse les opportunités en termes de poches de revenus émergentes, les changements dans la réglementation du marché, les approbations de produits, les décisions stratégiques, les lancements de produits, les expansions géographiques et les innovations technologiques sur le marché. Pour comprendre l’analyse et le scénario du marché, contactez-nous pour un briefing d’analyste, notre équipe vous aidera à créer une solution d’impact sur les revenus pour atteindre votre objectif souhaité. Les initiatives stratégiques telles que la collaboration, l’accord et la signature d’accords de vente pour inventer et innover dans les traitements pharmacologiques sont les principaux moteurs qui ont propulsé la demande du marché au cours de la période de prévision.
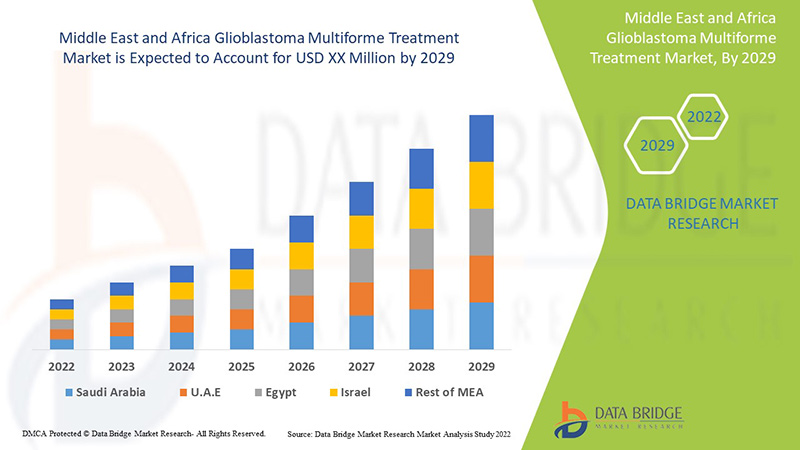
Le marché du traitement du glioblastome multiforme est favorable et vise à réduire la progression de la maladie. Data Bridge Market Research analyse que le marché du traitement du glioblastome multiforme connaîtra un TCAC de 5,9 % au cours de la période de prévision de 2022 à 2029.
|
Rapport métrique |
Détails |
|
Période de prévision |
2022 à 2029 |
|
Année de base |
2021 |
|
Années historiques |
2020 (personnalisable de 2019 à 2014) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD, prix en USD |
|
Segments couverts |
Par type (primaire (de novo), secondaire), traitement (chirurgie, radiothérapie, médicaments), type de patient (adulte, gériatrique, enfant), type de médicament (générique, de marque), voie d'administration (parentérale, orale, autres), utilisateur final (hôpitaux, cliniques, soins à domicile, autres), canal de distribution (pharmacie hospitalière, pharmacie de détail, pharmacie en ligne, autres) |
|
Pays couverts |
Afrique du Sud, Égypte, Israël, Émirats arabes unis, reste du Moyen-Orient et de l'Afrique |
|
Acteurs du marché couverts |
Français F. Hoffmann-La Roche AG, Amgen Inc., Merck & Co., Inc., Pfizer Inc., Varian Medical Systems, Inc. (une filiale de Siemens Healthcare), ZEISS International, Amneal Pharmaceuticals LLC, Elekta, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd., Eckert & Ziegler, Accord Healthcare, Angiochem, ANI Pharmaceuticals, Inc., Arbor Pharmaceuticals, LLC. (une filiale d'Azurity Pharmaceuticals, Inc.), AstraZeneca, Cantex Pharmaceuticals, Inc., CELON LABS, Diffusion Pharmaceuticals Inc., EnGeneIC, ERC.SA., Genenta science, Jazz Pharmaceuticals, Inc., Loxo Oncology (une filiale d'Eli Lilly), Novartis AG, VBL THERAPEUTICS, Viatris Inc. et Zydus Pharmaceuticals, Inc., entre autres. |
Définition du marché
Le glioblastome multiforme (GBM) est la tumeur cérébrale maligne primaire la plus courante et la plus agressive et représente 60 % des tumeurs cérébrales chez l'adulte. Les GBM peuvent survenir dans le cerveau de novo ou évoluer à partir d'un astrocytome de grade inférieur. Chez l'adulte, le GBM survient le plus souvent dans les hémisphères cérébraux, en particulier dans les lobes frontaux et temporaux du cerveau. De nombreux facteurs génétiques et environnementaux ont été étudiés dans le glioblastome multiforme, mais aucun facteur de risque responsable d'une grande proportion de GBM n'a été identifié. Ainsi, comme de nombreux autres cancers, le GBM est sporadique, bien que certaines études indiquent une prévalence élevée (17 %) d'irradiation thérapeutique antérieure chez les patients atteints de GBM. Le délai de latence entre l'irradiation et le développement du GBM varie de quelques années à plusieurs décennies. Il n'existe aucune preuve substantielle d'une association du GBM avec des facteurs liés au mode de vie comme le tabagisme, la consommation d'alcool, la consommation de drogues ou l'exposition aux composés N-nitroso. Des études ont montré que l'utilisation de téléphones portables n'augmente pas le risque de développement du GBM ; Cependant, son association avec une utilisation à long terme nécessite une confirmation supplémentaire.
Dynamique du marché du traitement du glioblastome multiforme
Conducteurs
- Prévalence croissante du glioblastome multiforme
Français Le glioblastome multiforme (GBM) est la tumeur cérébrale primaire maligne la plus fréquente, représentant 77 à 81 % de toutes les tumeurs malignes primaires du système nerveux central (SNC). L'Organisation mondiale de la santé l'a classé comme une tumeur astrocytaire et oligodendrogliale diffuse de grade IV. L'âge moyen de la présentation du GBM primaire est de 62 ans et la survie médiane est d'environ 14,6 mois. Le mauvais pronostic associé au GBM est bien documenté, tandis que les taux de survie restent décevants malgré les progrès médicaux et chirurgicaux. Selon l'étude, les études internationales révèlent un taux d'incidence annuel approximatif de 0,59 à 5 pour 100 000 personnes ; cependant, des études indiquent une augmentation de l'incidence. Miranda-Filho et al. en 2017 ont décrit des taux croissants de cancers du SNC et du cerveau dans les pays d'Amérique du Sud, d'Europe de l'Est et d'Europe du Sud, tandis que des taux décroissants n'ont été signalés qu'au Japon. Dobes et al. En 2011, deux études australiennes multicentriques ont également montré une augmentation de l'incidence des tumeurs GBM, avec une augmentation particulière des tumeurs GBM des lobes frontaux et temporaux. L'incidence accrue du glioblastome multiforme augmente la demande de détection et de diagnostic précoces grâce à l'utilisation des dernières technologies, propulsant ainsi le marché mondial du traitement du glioblastome multiforme. L'incidence croissante du glioblastome à travers le monde devrait accélérer la demande de traitement du glioblastome multiforme. Ainsi, les taux d'incidence accrus du glioblastome multiforme devraient stimuler la croissance du marché.
- Accroître la recherche et le développement (R&D)
L'augmentation des activités de recherche et développement (R&D) dans le domaine de la biotechnologie moléculaire et de la thérapie génique pour le cancer et les maladies apparentées a facilité le développement de divers médicaments biologiques. Ces médicaments contribuent à réduire les effets secondaires des méthodes de traitement existantes, créant ainsi une plus large acceptation parmi les patients. L'hétérogénéité tumorale et la variation de l'approche thérapeutique d'un patient à l'autre devraient accroître la demande d'une approche thérapeutique personnalisée pour gérer le glioblastome multiforme. L'approbation de nouveaux traitements devrait augmenter l'espérance de vie des patients atteints de glioblastome multiforme. En outre, une désignation spéciale accordée aux médicaments expérimentaux par la FDA devrait accélérer le processus d'approbation et la commercialisation de nouvelles thérapies. Une augmentation des collaborations entre les chercheurs et les acteurs du marché devrait stimuler le développement d'options thérapeutiques nouvelles et efficaces pour le glioblastome multiforme. L'approbation croissante de nouvelles thérapies et de thérapies combinées devrait stimuler le marché du traitement du glioblastome multiforme.
Opportunité
- Augmentation des approbations de médicaments
La demande croissante de traitement du glioblastome multiforme devrait entraîner davantage d'approbations réglementaires pour les médicaments associés. L'augmentation des approbations réglementaires pour les médicaments apparentés et les produits recombinants constituera une augmentation de la valeur du marché du traitement du glioblastome multiforme dans les années à venir. Dans le cadre d'une initiative de l'Organisation panaméricaine de la santé (OPS) visant à promouvoir la reconnaissance des autorités de réglementation des médicaments, le processus d'évaluation de l'ANMAT s'est terminé le 11 décembre 2009. Le secteur du traitement du glioblastome multiforme a connu de nombreuses approbations de médicaments ces dernières années, stimulées par le taux de mortalité croissant de la maladie. L'augmentation des approbations de médicaments augmentera la demande du marché du traitement du glioblastome multiforme.
Contraintes/Défis
Coût élevé du traitement du glioblastome multiforme
Les tests de diagnostic du glioblastome multiforme comprennent des produits hautement avancés sur le plan technologique. Le développement de ces produits nécessite des travaux de recherche et développement rigoureux de la part de l'acteur en développement. Ainsi, le coût du produit reste élevé, ce qui augmente proportionnellement le coût des tests.
Les outils et techniques de diagnostic utilisés pour le diagnostic du glioblastome multiforme comprennent
radiothérapie, chimiothérapie, entre autres. Les premiers stades du GBM se manifestent généralement par des symptômes minimes ou inexistants ; par conséquent, le GBM est souvent diagnostiqué à des stades avancés, ce qui entraîne un mauvais pronostic. Ainsi, le coût élevé du traitement du glioblastome multiforme à l'aide de modalités et de produits technologiques avancés constituera un facteur de restriction majeur pour la croissance du marché mondial du traitement du glioblastome multiforme.
Développements récents
- En avril 2022, Elekta et GE Healthcare ont annoncé la signature d'un accord de collaboration commerciale mondial dans le domaine de la radio-oncologie, leur permettant de proposer aux hôpitaux une offre complète d'imagerie et de traitement pour les patients cancéreux nécessitant une radiothérapie. Ce partenariat permettra aux entreprises de promouvoir conjointement des solutions adaptées aux besoins de chaque centre de cancérologie
- En juillet 2019, Amgen et Allergan plc ont annoncé que MVASI (bevacizumab-awwb), un biosimilaire d'Avastin (bevacizumab), est disponible aux États-Unis. Ce lancement renforcera les ventes du produit dans la région
Portée du marché du traitement du glioblastome multiforme
Le marché du traitement du glioblastome multiforme est divisé en sept segments notables qui sont basés sur le type, le traitement, le type de patient, le type de médicament, la voie d'administration, l'utilisateur final et le canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
Taper
- Primaire (De Novo)
- Secondaire
Sur la base du type, le marché du traitement du glioblastome multiforme est segmenté en primaire (De Novo) et secondaire.
Traitement
- Chirurgie
- Radiothérapie
- Médicaments
Sur la base du traitement, le marché du traitement du glioblastome multiforme est segmenté en chirurgie, radiothérapie et médicaments.
Type de patient
- Adulte
- Gériatrie
- Enfant
En fonction du type de patient, le marché du traitement du glioblastome multiforme est segmenté en adulte, gériatrique et enfant.
Type de médicament
- De marque
- Génériques
Sur la base du type de médicament, le marché du traitement du glioblastome multiforme est segmenté en génériques et en produits de marque.
Voie d'administration
- Oral
- Parentérale
- Autres
Sur la base de la voie d’administration, le marché du traitement du glioblastome multiforme est segmenté en voie parentérale, orale et autres.
Utilisateur final
- Hôpitaux
- Cliniques
- Soins à domicile
- Autres
Sur la base de l’utilisateur final, le marché du traitement du glioblastome multiforme est segmenté en hôpitaux, cliniques, soins à domicile et autres.
Canal de distribution
- Pharmacie de l'hôpital
- Pharmacie de détail
- Pharmacie en ligne
- Autres
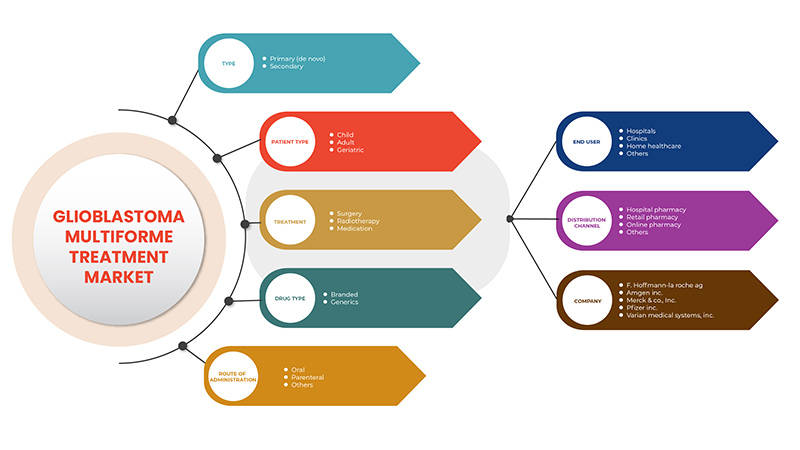
Sur la base du canal de distribution, le marché du traitement du glioblastome multiforme est segmenté en pharmacie hospitalière, pharmacie de détail et autres.
Analyse/perspectives régionales du marché du traitement du glioblastome multiforme
Le marché du traitement du glioblastome multiforme est analysé et des informations et tendances sur la taille du marché sont fournies par type, traitement, type de patient, type de médicament, voie d’administration, utilisateur final et canal de distribution comme référencé ci-dessus.
Les régions couvertes par le rapport sur le marché du traitement du glioblastome multiforme sont les Émirats arabes unis, l’Afrique du Sud, l’Égypte, Israël, le reste du Moyen-Orient et l’Afrique.
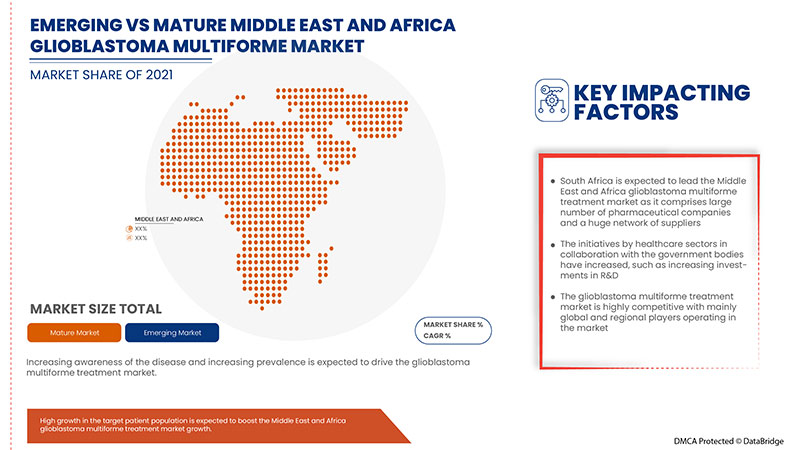
L’Afrique du Sud devrait dominer le marché en raison de la sensibilisation accrue à la maladie dans le pays.
La section pays du rapport fournit également des facteurs d'impact sur les marchés individuels et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que l'analyse de la chaîne de valeur en aval et en amont, les tendances techniques et l'analyse des cinq forces du porteur, les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques mondiales et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des tarifs nationaux et les routes commerciales sont pris en compte tout en fournissant une analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché du traitement du glioblastome multiforme
Le paysage concurrentiel du marché du traitement du glioblastome multiforme fournit des détails par concurrents. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, la présence mondiale, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises sur le marché du traitement du glioblastome multiforme.
Français Certains des principaux acteurs du marché sont F. Hoffmann-La Roche AG, Amgen Inc., Merck & Co., Inc., Pfizer Inc., Varian Medical Systems, Inc. (Une filiale de Siemens Healthcare), ZEISS International, Amneal Pharmaceuticals LLC, Elekta, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd., Eckert & Ziegler, Accord Healthcare, Angiochem, ANI Pharmaceuticals, Inc., Arbor Pharmaceuticals, LLC. (Une filiale d'Azurity Pharmaceuticals, Inc.), AstraZeneca, Cantex Pharmaceuticals, Inc., CELON LABS, Diffusion Pharmaceuticals Inc., EnGeneIC, ERC.SA., Genenta science, Jazz Pharmaceuticals, Inc., Loxo Oncology (Une filiale d'Eli Lilly), Novartis AG, VBL THERAPEUTICS, Viatris Inc. et Zydus Pharmaceuticals, Inc., entre autres.
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. Les données du marché sont analysées et estimées à l'aide de modèles statistiques et cohérents du marché. En outre, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. La principale méthodologie de recherche utilisée par l'équipe de recherche DBMR est la triangulation des données, qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). En dehors de cela, les modèles de données comprennent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement de l'entreprise, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse mondiale par rapport aux régions et la part des fournisseurs. Veuillez demander un appel d'analyste en cas de demande de renseignements supplémentaires.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS
7 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: REGULATORY SCENARIO
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GLIOBLASTOMA MULTIFORME
8.1.2 INCREASING RESEARCH AND DEVELOPMENT (R&D)
8.1.3 PRESENCE OF A STRONG PIPELINE
8.1.4 GROWING GERIATRIC POPULATION
8.2 RESTRAINTS
8.2.1 HIGH COST OF GLIOBLASTOMA MULTIFORME TREATMENT
8.2.2 ADVERSE SIDE-EFFECTS OF GLIOBLASTOMA MULTIFORME TREATMENT
8.3 OPPORTUNITIES
8.3.1 INCREASING DRUG APPROVALS
8.3.2 PARTNERSHIP AND AGREEMENT BY MAJOR PLAYERS
8.3.3 INCREASING SUPPORT OF PRIVATE AND GOVERNMENT AGENCIES FOR TREATMENT
8.4 CHALLENGES
8.4.1 LACK OF NEW TREATMENT
8.4.2 ADVERSE EFFECTS AND RISKS ASSOCIATED WITH CANCER TREATMENT DRUGS
8.4.3 LACK OF EARLY DETECTION
9 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE
9.1 OVERVIEW
9.2 PRIMARY (DE NOVO)
9.3 SECONDARY
10 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 SURGERY
10.3 RADIOTHERAPY
10.3.1 BRACHYTHERAPY
10.3.2 FRACTIONATED STEREOTACTIC RT (FSRT)
10.3.3 CONFORMAL OR INTENSITY-MODULATED RT
10.3.4 RADIOSURGERY
10.4 MEDICATIONS
10.4.1 TEMOZOLOMIDE
10.4.1.1 ORAL
10.4.1.1 INTRAVENOUS
10.4.2 NITROSOUREAS DRUGS
10.4.2.1 CARMUSTINE
10.4.2.1.1 PARENTERAL
10.4.2.1.2 IMPLANTABLE WAFERS
10.4.2.2 LOMUSTINE
10.4.2.3 NIMUSTINE
10.4.2.4 FOTEMUSTINE
10.4.3 TARGETED THERAPY
10.4.3.1 BEVACIZUMAB
10.4.3.2 OTHERS
10.4.4 ANTI-EPILEPTICS
10.4.4.1 LEVETIRACETAM
10.4.4.2 PHENYTOIN
10.4.4.3 CARBAMAZEPINE
10.4.5 CORTICOSTEROIDS
10.4.5.1 METHYLPREDNISOLONE
10.4.5.2 PREDNISONE
10.4.5.3 OTHERS
11 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE
11.1 OVERVIEW
11.2 ADULT
11.2.1 MALE
11.2.2 FEMALE
11.3 GERIATRIC
11.3.1 MALE
11.3.2 FEMALE
11.4 CHILD
12 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERICS
12.3 BRANDED
13 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 PARENTERAL
13.3 ORAL
13.3.1 CAPSULES
13.3.2 TABLETS
13.3.3 POWDERS
13.4 OTHERS
14 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITAL
14.3 CLINICS
14.4 HOME HEALTHCARE
14.5 OTHERS
15 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 HOSPITAL PHARMACY
15.3 RETAIL PHARMACY
15.4 ONLINE PHARMACY
15.5 OTHERS
16 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION
16.1 MIDDLE EAST & AFRICA
16.1.1 SOUTH AFRICA
16.1.2 U.A.E.
16.1.3 EGYPT
16.1.4 ISRAEL
16.1.5 REST OF MIDDLE EAST AND AFRICA
17 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 F.HOFFMAN-LA ROCHE
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 AMGEN INC.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.2.5.1 PRODUCT APPROVAL
19.3 MERCK & CO., INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.3.5.1 STRATETIC COLLABORATION
19.3.5.2 EVENTS
19.4 PFIZER INC.
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.4.5.1 MERGER
19.5 VARIAN MEDICAL SYSTEMS, INC. (A SUBSIDIARY OF SIEMENS HEALTHCARE)
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.5.5.1 PARTNERSHIP
19.5.5.2 ACQUISITION
19.6 ZEISS INTERNATIONAL
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.6.4.1 PRODUCT EXPANSION
19.7 AMNEAL PHARMACEUTICALS LLC
19.7.1 COMPANY SNAPSHOT
19.7.2 REVENUE ANALYSIS
19.7.3 PRODUCT PORTFOLIO
19.7.4 RECENT DEVELOPMENTS
19.7.4.1 EVENT
19.7.4.2 LAUNCH
19.7.4.3 ACQUISITION
19.8 ELEKTA
19.8.1 COMPANY SNAPSHOT
19.8.2 REVENUE ANALYSIS
19.8.3 PRODUCT PORTFOLIO
19.8.4 RECENT DEVELOPMENTS
19.8.4.1 PARTNERSHIP
19.9 SUN PHARMACEUTICAL INDUSTRIES LTD
19.9.1 COMPANY SNAPSHOT
19.9.2 REVENUE ANALYSIS
19.9.3 PRODUCT PORTFOLIO
19.9.4 RECENT DEVELOPMENT
19.9.4.1 AGREEMENT
19.1 TEVA PHARMACEUTICAL INDUSTRIES LTD
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 ECKERT & ZIEGLER
19.11.1 COMPANY SNAPSHOT
19.11.2 REVENUE ANALYSIS
19.11.3 PRODUCT PORTFOLIO
19.11.4 RECENT DEVELOPMENT
19.12 ACCORD HEALTHCARE
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 ANGIOCHEM
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENT
19.13.3.1 AGREMEENT
19.14 ANI PHARMACEUTICALS, INC.
19.14.1 COMPANY SNAPSHOT
19.14.2 REVENUE ANALYSIS
19.14.3 PRODUCT PORTFOLIO
19.14.4 RECENT DEVELOPMENTS
19.14.4.1 ACQUISITION
19.15 ARBOR PHARMACEUTICALS, LLC. A SUBSIDIARY OF AZURITY PHARMACEUTICALS, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENT
19.15.3.1 ACQUISITION
19.15.3.2 PRODUCT APPROVAL
19.16 ASTRAZENECA
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.16.4.1 AGREEMENT
19.17 CANTEX PHARMACEUTICALS, INC.
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENT
19.18 CELON LABS
19.18.1 COMPANY SNAPSHOT
19.18.2 PRODUCT PORTFOLIO
19.18.3 RECENT DEVELOPMENT
19.19 DIFFUSION PHARMACEUTICAL
19.19.1 COMPANY SNAPSHOT
19.19.2 SERVICES PORTFOLIO
19.19.3 RECENT DEVELOPMENT
19.2 ERC.SA
19.20.1 COMPANY SNAPSHOT
19.20.2 PRODUCT PORTFOLIO
19.20.3 RECENT DEVELOPMENT
19.20.3.1 PIPELINE UPDATE
19.21 ENGENEIC
19.21.1 COMPANY SNAPSHOT
19.21.2 PRODUCT PORTFOLIO
19.21.3 RECENT DEVELOPMENTS
19.21.3.1 AWARDS
19.22 GENENTA SCIENCE
19.22.1 COMPANY SNAPSHOT
19.22.2 PRODUCT PORTFOLIO
19.22.3 RECENT DEVELOPMENT
19.22.3.1 EVENT
19.23 JAZZ PHARMACEUTICALS, INC.
19.23.1 COMPANY SNAPSHOT
19.23.2 REVENUE ANALYSIS
19.23.3 PRODUCT PORTFOLIO
19.23.4 RECENT DEVELOPMENT
19.23.4.1 ACQUISITION
19.24 LOXO ONCOLOGY (A SUBSIDIARY OF ELI LILLY)
19.24.1 COMPANY SNAPSHOT
19.24.2 PRODUCT PORTFOLIO
19.24.3 RECENT DEVELOPMENT
19.25 NOVARTIS AG
19.25.1 COMPANY SNAPSHOT
19.25.2 REVENUE ANALYSIS
19.25.3 PRODUCT PORTFOLIO
19.25.4 RECENT DEVELOPMENT
19.26 VBL THERAPEUTICS
19.26.1 COMPANY SNAPSHOT
19.26.2 PRODUCT PORTFOLIO
19.26.3 RECENT DEVELOPMENT
19.26.3.1 EVENT
19.26.3.2 AWARD
19.27 VIATRIS INC
19.27.1 COMPANY SNAPSHOT
19.27.2 REVENUE ANALYSIS
19.27.3 PRODUCT PORTFOLIO
19.27.4 RECENT DEVELOPMENT
19.27.4.1 AGREEMENT
19.28 ZYDUS PHARMACEUTICALS, INC.
19.28.1 COMPANY SNAPSHOT
19.28.2 PRODUCT PORTFOLIO
19.28.3 RECENT DEVELOPMENTS
20 QUESTIONNAIRE
21 RELATED REPORTS
Liste des tableaux
TABLE 1 PIPELINE ANALYSIS FOR GLIOBLASTOMA MULTIFORME TREATMENT MARKET
TABLE 2 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 3 MIDDLE EAST & AFRICA PRIMARY (DE NOVO) IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA SECONDARY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA SURGERY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA ADULTS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA CHILD IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA GENERICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA BRANDED IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA PARENTERAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA HOSPITAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA CLINICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA HOME HEALTHCARE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITAL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA RETAIL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA ONLINE PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 MIDDLE EAST & AFRICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 42 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 43 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 44 MIDDLE EAST & AFRICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 45 MIDDLE EAST & AFRICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 46 MIDDLE EAST & AFRICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 47 MIDDLE EAST & AFRICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 48 MIDDLE EAST & AFRICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 49 MIDDLE EAST & AFRICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 50 MIDDLE EAST & AFRICA ANTI-EPILEPTIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 51 MIDDLE EAST & AFRICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 52 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 53 MIDDLE EAST & AFRICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 54 MIDDLE EAST & AFRICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 55 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 56 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 57 MIDDLE EAST & AFRICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 58 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 59 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 60 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 61 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 62 SOUTH AFRICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 63 SOUTH AFRICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 64 SOUTH AFRICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 65 SOUTH AFRICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 66 SOUTH AFRICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 67 SOUTH AFRICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 68 SOUTH AFRICA ANTI-EPILEPTIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 69 SOUTH AFRICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 70 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 71 SOUTH AFRICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 72 SOUTH AFRICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 73 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 74 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 75 SOUTH AFRICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 76 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 77 SOUTH AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 80 U.A.E. RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 81 U.A.E. MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 82 U.A.E. TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 83 U.A.E. NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 84 U.A.E. CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 85 U.A.E. TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 86 U.A.E. ANTI-EPILEPTIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 87 U.A.E. CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 88 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 89 U.A.E. ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 90 U.A.E. GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 91 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 92 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 93 U.A.E. ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 94 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 U.A.E. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 96 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 97 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 98 EGYPT RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 99 EGYPT MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 100 EGYPT TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 101 EGYPT NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 102 EGYPT CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 103 EGYPT TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 104 EGYPT ANTI-EPILEPTIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 105 EGYPT CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 106 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 107 EGYPT ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 108 EGYPT GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 109 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 110 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 111 EGYPT ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 112 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 EGYPT GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 114 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 115 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 116 ISRAEL RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 117 ISRAEL MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 118 ISRAEL TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 119 ISRAEL NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 120 ISRAEL CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 121 ISRAEL TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 122 ISRAEL ANTI-EPILEPTIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 123 ISRAEL CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 124 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 125 ISRAEL ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 126 ISRAEL GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 127 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 128 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 129 ISRAEL ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 130 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 131 ISRAEL GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 132 REST OF MIDDLE EAST AND AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET : DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MULTIVARIATE MODELLING
FIGURE 7 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 12 NORTH AMERICA IS EXPECTED TO DOMINATE AND ASIA-PACIFIC IS GROWING AT THE FASTEST PACE IN MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 13 INCREASE IN THE PREVALENCE OF GLIOBLASTOMA MULTIFORME AND INCREASE IN PIPELINE PRODUCTS ARE DRIVING THE MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 PRIMARY (DE NOVO) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN 2022 & 2029
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
FIGURE 16 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2021
FIGURE 17 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 18 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 19 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2021
FIGURE 21 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2022-2029 (USD MILLION)
FIGURE 22 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, CAGR (2022-2029)
FIGURE 23 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, LIFELINE CURVE
FIGURE 24 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2021
FIGURE 25 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 26 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 27 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 28 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2021
FIGURE 29 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2022-2029 (USD MILLION)
FIGURE 30 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, CAGR (2022-2029)
FIGURE 31 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 32 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 33 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 34 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 35 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 36 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2021
FIGURE 37 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 38 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, CAGR (2022-2029)
FIGURE 39 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 41 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 42 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 43 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SNAPSHOT (2021)
FIGURE 45 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021)
FIGURE 46 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2022 & 2029)
FIGURE 47 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021 & 2029)
FIGURE 48 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE (2022 & 2029)
FIGURE 49 MIDDLE EAST & AFRICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.