Global T Cell Therapy Market
Taille du marché en milliards USD
TCAC :
% 
 USD
5.45 Billion
USD
21.28 Billion
2021
2029
USD
5.45 Billion
USD
21.28 Billion
2021
2029
| 2022 –2029 | |
| USD 5.45 Billion | |
| USD 21.28 Billion | |
|
|
|
|
Marché mondial de la thérapie par cellules thymiques (T), par modalité (recherche, commercialisation), thérapie (à base de cellules CAR T, à base de récepteurs de cellules T (TCR), à base de lymphocytes infiltrant les tumeurs (TIL)), indication (hémopathies malignes, tumeurs solides, autres), utilisateurs finaux (hôpitaux, cliniques spécialisées, soins à domicile, autres), canal de distribution (pharmacie hospitalière, pharmacie de détail, pharmacie en ligne, autres) – Tendances et prévisions de l’industrie jusqu’en 2029
Analyse et taille du marché
La thérapie par cellules T est un type d'immunothérapie utilisé pour traiter le cancer en modifiant les cellules T, qui font partie du système immunitaire. Les cellules T ont été prélevées dans un échantillon de sang d'une personne, puis génétiquement modifiées pour développer des structures spécifiques à leur surface, appelées récepteurs d'antigènes chimériques (CAR). La thérapie par cellules T est actuellement approuvée par la FDA comme traitement standard pour certains types de lymphomes non hodgkiniens agressifs, récidivants ou réfractaires, tels que le lymphome diffus à grandes cellules B, le lymphome médiastinal primaire à cellules B, le lymphome à cellules B de haut grade, le lymphome folliculaire transformé et le lymphome à cellules du manteau. Le traitement par cellules T est également approuvé pour les patients de moins de 25 ans atteints de leucémie lymphoblastique aiguë récidivante ou réfractaire.
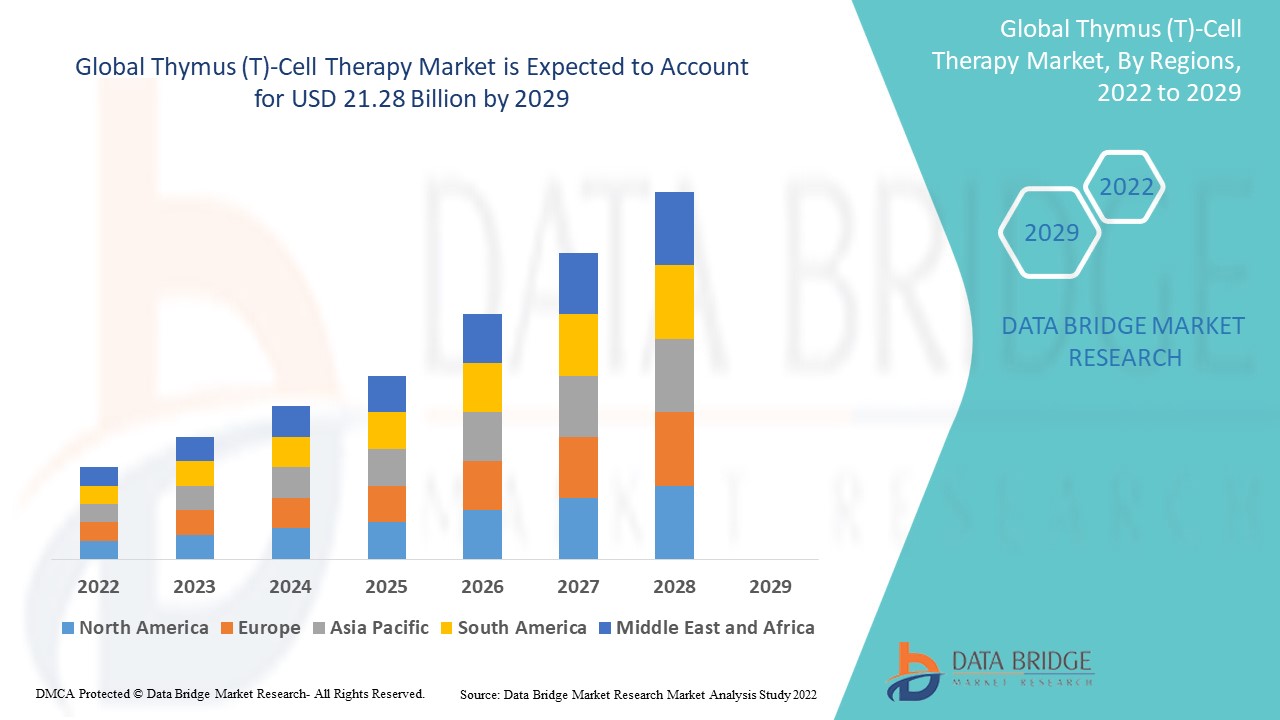
Français Data Bridge Market Research analyse que le marché de la thérapie par cellules thymiques (T) était évalué à 5,45 milliards USD en 2021 et devrait atteindre 21,28 milliards USD d'ici 2029, enregistrant un TCAC de 18,55 % au cours de la période de prévision de 2022 à 2029. Le rapport de marché organisé par l'équipe Data Bridge Market Research comprend une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
Portée du rapport et segmentation du marché
|
Rapport métrique |
Détails |
|
Période de prévision |
2022 à 2029 |
|
Année de base |
2021 |
|
Années historiques |
2020 (personnalisable de 2014 à 2019) |
|
Unités quantitatives |
Chiffre d'affaires en milliards USD, volumes en unités, prix en USD |
|
Segments couverts |
Modalité (recherche, commercialisation), thérapie (à base de cellules CAR-T, à base de récepteurs de cellules T (TCR), à base de lymphocytes infiltrant les tumeurs (TIL)), indication (hémopathies malignes, tumeurs solides, autres), utilisateurs finaux (hôpitaux, cliniques spécialisées, soins à domicile, autres), canal de distribution (pharmacie hospitalière, pharmacie de détail, pharmacie en ligne, autres) |
|
Pays couverts |
États-Unis, Canada et Mexique en Amérique du Nord, Allemagne, France, Royaume-Uni, Pays-Bas, Suisse, Belgique, Russie, Italie, Espagne, Turquie, Reste de l'Europe en Europe, Chine, Japon, Inde, Corée du Sud, Singapour, Malaisie, Australie, Thaïlande, Indonésie, Philippines, Reste de l'Asie-Pacifique (APAC) en Asie-Pacifique (APAC), Arabie saoudite, Émirats arabes unis, Afrique du Sud, Égypte, Israël, Reste du Moyen-Orient et de l'Afrique (MEA) en tant que partie du Moyen-Orient et de l'Afrique (MEA), Brésil, Argentine et Reste de l'Amérique du Sud en tant que partie de l'Amérique du Sud |
|
Acteurs du marché couverts |
Pfizer Inc. (États-Unis), GlaxoSmithKline plc (Royaume-Uni), Novartis AG (Suisse), Sanofi (France), F. Hoffmann-La Roche Ltd. (Suisse), Bristol-Myers Squibb Company (États-Unis), Merck KGaA (Allemagne), Aurora Health Care (États-Unis), bluebird bio, Inc. (États-Unis), Amgen Inc. (États-Unis), Gilead Sciences, Inc. (États-Unis), Aurora Biopharma. (États-Unis), Fate Therapeutics (États-Unis), Mustang Bio (États-Unis), Sorrento Therapeutics Inc. (États-Unis), TCR² Therapeutics (États-Unis), Bayer AG (Allemagne), Bellicum Pharmaceuticals Inc. (États-Unis), AstraZeneca (Royaume-Uni), Johnson & Johnson Private Limited (États-Unis) |
|
Opportunités de marché |
|
Définition du marché
La thérapie par lymphocytes T est une technique d'immunothérapie connue sous le nom de transfert cellulaire adoptif (ACT), dans laquelle les propres cellules immunitaires du patient sont utilisées pour traiter le cancer. Elle consiste à utiliser l'aphérèse pour extraire les lymphocytes T et les remanier en laboratoire. Ces cellules remanipulées sont ensuite reproduites et injectées dans le corps, où elles reconnaissent et tuent les cellules cancéreuses qui ont des antigènes spécifiques à leur surface. Un nombre croissant de traitements par lymphocytes T sont actuellement testés dans le cadre d'essais cliniques. L'une de ces études consiste à créer une technologie prête à l'emploi permettant de collecter des cellules immunitaires auprès de donneurs sains et de les rendre immédiatement disponibles pour utilisation.
Dynamique du marché de la thérapie par cellules thymiques (T)
Conducteurs
- Taux de prévalence élevé du cancer
L'augmentation du taux de prévalence du cancer sera un facteur majeur qui entraînera l'expansion du taux de croissance du marché. L'augmentation de la consommation de tabac, les mauvaises habitudes alimentaires et le mode de vie sédentaire des personnes contribuent tous à un risque accru de cancer. En conséquence, le marché devrait augmenter en réponse à la prévalence mondiale croissante du cancer. Selon l'American Cancer Society, 28,4 millions de nouveaux cas de cancer devraient être diagnostiqués en 2040. Ce chiffre, associé à l'augmentation du nombre de personnes diagnostiquées avec des maladies de la moelle osseuse et des tissus hématopoïétiques, est l'un des principaux facteurs qui stimulent la demande de thérapie par cellules T.
- Augmenter les investissements dans les infrastructures de santé
Un autre facteur important qui influence le taux de croissance du marché de la thérapie par cellules thymiques (T) est l’augmentation des dépenses de santé qui contribue à améliorer son infrastructure. En outre, diverses organisations gouvernementales visent à améliorer l’infrastructure des soins de santé en augmentant le financement, ce qui influencera davantage la dynamique du marché.
En outre, les initiatives croissantes des organismes publics et privés visant à sensibiliser la population et l’augmentation de la population gériatrique élargiront le marché de la thérapie par cellules thymiques (T). En outre, des politiques de remboursement favorables et des initiatives gouvernementales de plus en plus favorables entraîneront l’expansion du marché de la thérapie par cellules thymiques (T).
Opportunités
- Augmentation du nombre d'activités de recherche et développement
De plus, la croissance du marché est alimentée par une augmentation du nombre d'activités de recherche et développement. Cela offrira des opportunités bénéfiques pour la croissance du marché de la thérapie par cellules thymiques (T). La propagation généralisée de la maladie à coronavirus (COVID-19) a incité les chercheurs du monde entier à considérer la thérapie par cellules T comme une option de traitement pour les patients à haut risque. Les thérapies par cellules T ont également été récemment approuvées par la Food and Drug Administration (USFDA) des États-Unis pour traiter les enfants atteints de leucémie lymphoblastique aiguë (LAL) et les adultes atteints de lymphomes avancés. En conséquence, les producteurs devraient bénéficier de perspectives intéressantes et le marché va augmenter.
- Lancements de nouveaux produits
Au cours de la période projetée, les lancements de nouveaux produits par les acteurs de l'industrie sur le marché de la thérapie par cellules thymiques (T) en raison de la forte demande du grand public devraient favoriser de nouvelles opportunités de marché. Par exemple, la Food and Drug Administration américaine a autorisé Tecartus (brexucabtagene autoleucel), une thérapie génique cellulaire, en juillet 2020 pour le traitement des patients adultes atteints de lymphome à cellules du manteau (LCM) qui n'ont pas répondu ou ont rechuté après des traitements antérieurs. La FDA a approuvé Tecartus, une thérapie par cellules T à récepteur d'antigène chimérique (CAR), comme première thérapie génique cellulaire pour le LCM.
De plus, l’augmentation des investissements dans le développement de technologies avancées et l’augmentation du nombre de marchés émergents offriront davantage d’opportunités bénéfiques pour la croissance du marché de la thérapie par cellules thymiques (T) au cours de la période de prévision.
Contraintes/Défis
D'autre part, le coût élevé associé au traitement entravera le taux de croissance du marché de la thérapie par cellules thymiques (T) au cours de la période de prévision 2022-2029. Le manque d'infrastructures de santé dans les économies en développement et la pénurie de professionnels qualifiés mettront à mal le marché de la thérapie par cellules thymiques (T). De plus, les effets secondaires associés à la thérapie tels que les nausées, la perte de cheveux, les vomissements et autres, ainsi que le manque de sensibilisation au traitement agiront comme un frein et entraveront davantage le taux de croissance du marché au cours de la période de prévision 2022-2029.
Ce rapport sur le marché de la thérapie par cellules thymiques (T) fournit des détails sur les nouveaux développements récents, les réglementations commerciales, l'analyse des importations et des exportations, l'analyse de la production, l'optimisation de la chaîne de valeur, la part de marché, l'impact des acteurs du marché national et localisé, les opportunités d'analyse en termes de poches de revenus émergentes, les changements dans la réglementation du marché, l'analyse stratégique de la croissance du marché, la taille du marché, la croissance du marché des catégories, les niches d'application et la domination, les approbations de produits, les lancements de produits, les expansions géographiques, les innovations technologiques sur le marché. Pour obtenir plus d'informations sur le marché de la thérapie par cellules thymiques (T), contactez Data Bridge Market Research pour un briefing d'analyste, notre équipe vous aidera à prendre une décision de marché éclairée pour atteindre la croissance du marché.
Analyse épidémiologique des patients
Le marché de la thérapie par cellules thymiques (T) vous fournit également une analyse de marché détaillée pour l'analyse des patients, le pronostic et les remèdes. La prévalence, l'incidence, la mortalité et les taux d'adhésion sont quelques-unes des variables de données disponibles dans le rapport. Les analyses d'impact directes ou indirectes de l'épidémiologie sur la croissance du marché sont analysées pour créer un modèle statistique multivarié de cohorte plus robuste pour prévoir le marché pendant la période de croissance.
Impact du COVID-19 sur le marché de la thérapie par cellules thymiques (T)
Le secteur privé de la santé est l’un des domaines où la pandémie a eu un impact significatif. La pandémie de coronavirus a eu une influence significative sur le développement, la production et l’approvisionnement de médicaments, ainsi que sur les activités de différentes sociétés de soins de santé à travers le monde. Les patients atteints de cancer étant plus sensibles aux infections virales, en particulier après une chimiothérapie, une greffe de cellules souches ou une intervention chirurgicale, le marché de la thérapie par cellules T est particulièrement vulnérable aux perturbations causées par l’épidémie de coronavirus. Le COVID-19 a entraîné le retard des essais cliniques de médicaments de thérapie CAR-T. La production est également suspendue en raison du confinement international, ce qui suscite des inquiétudes au niveau de la chaîne d’approvisionnement. Cependant, maintenant que les vaccins contre le COVID-19 sont facilement disponibles, de nombreuses autorités tentent de garantir que les médicaments et les vaccins vitaux soient fournis sans interruption. En conséquence, le marché devrait se stabiliser à l’avenir.
Développement récent
- En décembre 2021, Novartis avait annoncé le lancement d'une plateforme CAR-T de nouvelle génération appelée T-Charge, qui servira de base à diverses nouvelles thérapies cellulaires CAR-T expérimentales dans le pipeline de Novartis. La capacité des cellules T à s'auto-renouveler et à se développer est préservée par la plateforme T-Charge, ce qui donne un produit avec un potentiel prolifératif accru et moins de cellules T fatiguées. T-Charge permet aux cellules CAR-T de se développer principalement à l'intérieur du corps d'un patient (in vivo), supprimant ainsi la nécessité d'une longue période de culture en dehors du corps (ex vivo).
Portée du marché mondial de la thérapie par cellules thymiques (T)
Le marché de la thérapie par cellules thymiques (T) est segmenté en fonction de la modalité, de la thérapie, de l'indication, des utilisateurs finaux et du canal de distribution. La croissance parmi ces segments vous aidera à analyser les segments de croissance faibles dans les industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour les aider à prendre des décisions stratégiques pour identifier les principales applications du marché.
Modalité
- Recherche
- Commercialisé
Thérapie
- À base de cellules CAR-T
- Basé sur le récepteur des cellules T (TCR)
- Basé sur les lymphocytes infiltrant la tumeur (TIL)
Indication
- Hémopathies malignes
- Lymphome
- Leucémie
- Myélome
- Tumeurs solides
- Mélanome
- Cerveau et système nerveux central
- Cancer du foie
- Autres
- Autres
Utilisateurs finaux
- Hôpitaux
- Cliniques spécialisées
- Soins à domicile
- Autres
Canal de distribution
- Pharmacie de l'hôpital
- Pharmacie de détail
- Pharmacie en ligne
- Autres
Analyse/perspectives régionales du marché de la thérapie par cellules thymiques (T)
The thymus (T)-cell therapy market is analysed and market size insights and trends are provided by country, modality, therapy, indication, end-users and distribution channel as referenced above.
The countries covered in the thymus (T)-cell therapy market report are U.S., Canada and Mexico in North America, Germany, France, U.K., Netherlands, Switzerland, Belgium, Russia, Italy, Spain, Turkey, Rest of Europe in Europe, China, Japan, India, South Korea, Singapore, Malaysia, Australia, Thailand, Indonesia, Philippines, Rest of Asia-Pacific (APAC) in the Asia-Pacific (APAC), Saudi Arabia, U.A.E, South Africa, Egypt, Israel, Rest of Middle East and Africa (MEA) as a part of Middle East and Africa (MEA), Brazil, Argentina and Rest of South America as part of South America
North America dominates the thymus (T)-cell therapy market in terms of market share and market revenue and will continue to flourish its dominance during the forecast period. This is due to the presence of major key players and rising healthcare expenditure will further propel the market’s growth rate in this region. Additionally, increasing research and development activities will further propel the market’s growth rate in this region.
Asia-Pacific is expected to grow during the forecast period of 2022-2029 due to rising research and development in this region. Also, development of healthcare infrastructure and growing government initiatives will further propel the market’s growth rate in this region.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impacts the current and future trends of the market. Data points like down-stream and upstream value chain analysis, technical trends and porter's five forces analysis, case studies are some of the pointers used to forecast the market scenario for individual countries. Also, the presence and availability of global brands and their challenges faced due to large or scarce competition from local and domestic brands, impact of domestic tariffs and trade routes are considered while providing forecast analysis of the country data.
Competitive Landscape and Thymus (T)-Cell Therapy Market Share Analysis
The thymus (T)-cell therapy market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in research and development, new market initiatives, global presence, production sites and facilities, production capacities, company strengths and weaknesses, product launch, product width and breadth, application dominance. The above data points provided are only related to the companies' focus related to thymus (T)-cell therapy market.
Some of the major players operating in the thymus (T)-cell therapy market are:
- Pfizer Inc. (US)
- GlaxoSmithKline plc (UK)
- Novartis AG (Switzerland)
- Sanofi (France)
- F. Hoffmann-La Roche Ltd. (Switzerland)
- Société Bristol-Myers Squibb (États-Unis)
- Merck KGaA (Allemagne)
- Aurora Health Care (États-Unis)
- bluebird bio, Inc. (États-Unis)
- Amgen Inc. (États-Unis)
- Gilead Sciences, Inc. (États-Unis)
- Aurora Biopharma. (États-Unis)
- Fate Therapeutics (États-Unis)
- Biographie de Mustang (États-Unis)
- Sorrento Therapeutics Inc. (États-Unis)
- TCR² Therapeutics (États-Unis)
- Bayer AG (Allemagne)
- Bellicum Pharmaceuticals Inc. (États-Unis)
- AstraZeneca (Royaume-Uni)
- Johnson & Johnson Private Limited (États-Unis)
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.