Global Molecular Point Of Care Testing Using Naat Market
Taille du marché en milliards USD
TCAC :
% 
 USD
37.93 Billion
USD
86.17 Billion
2024
2032
USD
37.93 Billion
USD
86.17 Billion
2024
2032
| 2025 –2032 | |
| USD 37.93 Billion | |
| USD 86.17 Billion | |
|
|
|
|
Segmentation du marché mondial des tests moléculaires au point de soins (utilisant la technique NAAT), parProduits (instruments, consommables et réactifs), indications (dépistage des infections respiratoires, des infections sexuellement transmissibles (IST), des infections gastro-intestinales et autres), utilisateurs finaux (laboratoires, hôpitaux, cliniques, centres ambulatoires, soins à domicile, établissements d'hébergement pour personnes âgées et autres), modes de dépistage (tests sur ordonnance et tests en vente libre), circuits de distribution (pharmacies hospitalières, pharmacies de détail et pharmacies en ligne) : tendances et prévisions du secteur jusqu’en 2032
Taille du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
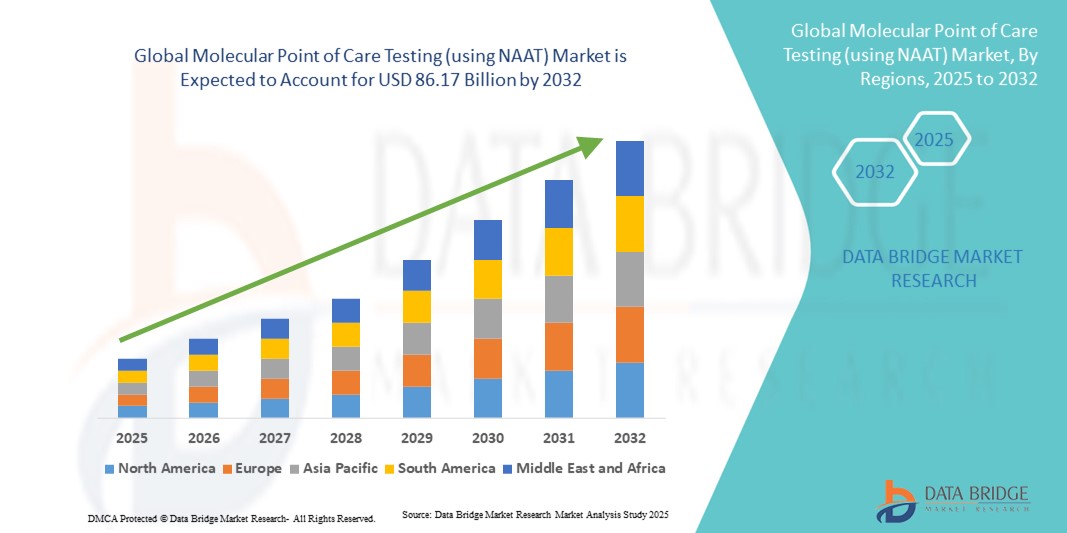
- Le marché mondial des tests moléculaires au point de soins (utilisant la technique NAAT) était évalué à 37,93 milliards de dollars américains en 2024 et devrait atteindre 86,17 milliards de dollars américains d'ici 2032 , avec un TCAC de 1,08 % au cours de la période de prévision.
- La croissance de ce marché est principalement due à la prévalence croissante des maladies infectieuses , à la demande de tests de diagnostic rapides et précis, et aux progrès des technologies de diagnostic moléculaire.
- De plus, l'évolution vers des soins de santé décentralisés et l'adoption croissante des tests multiplex contribuent à l'expansion du marché des tests moléculaires au point de soins. Ces facteurs, pris ensemble, positionnent les tests moléculaires au point de soins comme un élément essentiel du diagnostic moderne, permettant une détection rapide et précise de diverses affections.
Analyse du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
- Les tests moléculaires au point de soins (POCT) utilisant l'amplification des acides nucléiques (TAAN) permettent une détection rapide et précise des maladies infectieuses et autres affections médicales au chevet du patient ou à proximité, ce qui en fait un élément crucial du diagnostic moderne dans les hôpitaux, les cliniques et les établissements de soins décentralisés.
- L'adoption croissante des tests moléculaires au point de service (POCT) est principalement due à la prévalence croissante des maladies infectieuses, à la demande grandissante de diagnostics rapides et précis, et aux progrès technologiques réalisés dans le domaine des dispositifs de test moléculaire portables et conviviaux.
- L'Amérique du Nord a dominé le marché des tests moléculaires au point de soins (POCT) avec la plus grande part de revenus (39 %) en 2024, grâce à l'adoption précoce des technologies de diagnostic avancées, aux dépenses de santé élevées et à la forte présence d'acteurs clés du secteur. Les États-Unis ont connu une croissance substantielle grâce à l'intégration des tests NAAT dans les hôpitaux, les cliniques et les services d'urgence.
- La région Asie-Pacifique devrait connaître la croissance la plus rapide au cours de la période de prévision, portée par l'augmentation des investissements dans les infrastructures de santé, la sensibilisation croissante aux solutions de diagnostic rapide et la demande croissante de tests au point de service dans les économies émergentes.
- Le segment des tests d'infections respiratoires a dominé le marché des tests moléculaires au point de soins (POCT) avec une part de marché de 43,2 % en 2024, en raison de la forte prévalence des maladies respiratoires infectieuses et du besoin clinique d'un diagnostic rapide et précis pour orienter un traitement et des mesures de confinement opportuns.
Portée du rapport et segmentation du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
|
Attributs |
Principaux enseignements du marché des tests moléculaires au point de soins (utilisant la technique NAAT) |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
Europe
Asie-Pacifique
Moyen-Orient et Afrique
Amérique du Sud
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
En plus des informations sur les scénarios de marché tels que la valeur du marché, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché élaborés par Data Bridge Market Research comprennent également une analyse approfondie par des experts, une analyse des prix, une analyse des parts de marché des marques, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, les critères de sélection des fournisseurs, une analyse PESTLE, une analyse de Porter et le cadre réglementaire. |
Tendances du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
Progrès en matière de tests rapides, multiplexés et assistés par l'IA
- Une tendance importante et croissante sur le marché mondial des tests moléculaires au point de soins (POCT) est le développement de dispositifs NAAT multiplexés rapides et de plateformes de diagnostic basées sur l'IA, qui améliorent la rapidité, la précision et la facilité d'utilisation des tests au point de soins dans les hôpitaux, les cliniques et les établissements de soins décentralisés.
- Par exemple, le test Xpert Xpress SARS-CoV-2/Grippe/VRS intègre la détection de plusieurs agents pathogènes en une seule analyse, réduisant ainsi le délai d'obtention des résultats et rationalisant le flux de travail sur les sites des patients.
- L'intégration de l'IA dans les tests moléculaires au point de soins (POCT) permet des fonctionnalités telles que l'interprétation automatisée des résultats, l'analyse prédictive des épidémies et le contrôle qualité intelligent, améliorant ainsi la fiabilité du diagnostic et réduisant les erreurs humaines.
- Ces plateformes basées sur l'IA permettent aux professionnels de santé de gérer simultanément de multiples paramètres diagnostiques, fournissant ainsi des résultats plus rapides pour les pathologies critiques tout en réduisant la dépendance aux laboratoires centralisés.
- Cette tendance vers des solutions de test plus rapides, plus précises et interconnectées redéfinit les attentes en matière de diagnostic au point de soins. Par conséquent, des entreprises comme Abbott et Roche développent des dispositifs NAAT dotés d'intelligence artificielle, de capacités multiplex et d'interfaces conviviales pour le personnel clinique.
- La demande en dispositifs de diagnostic moléculaire au point de soins (POCT) offrant une détection multiplexe et des fonctionnalités basées sur l'IA croît rapidement, tant en milieu hospitalier qu'ambulatoire, car les prestataires de soins de santé privilégient de plus en plus des diagnostics rapides et fiables.
Dynamique du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
Conducteur
Augmentation de la demande due à la charge des maladies infectieuses et à la décentralisation des tests
- L'augmentation de la prévalence des maladies infectieuses à l'échelle mondiale, conjuguée à la décentralisation des soins de santé, est un facteur important de l'adoption accrue des tests moléculaires au point de service (POCT) utilisant la technique d'amplification des acides nucléiques (NAAT).
- Par exemple, en 2024, Cepheid a étendu les applications de sa plateforme GeneXpert aux infections sexuellement transmissibles et aux tests respiratoires, témoignant ainsi de sa volonté de répondre aux besoins croissants en matière de diagnostic au point de soins.
- Alors que les professionnels de la santé s'efforcent d'établir des diagnostics plus rapides et d'instaurer des traitements opportunistes, les tests de diagnostic rapide basés sur la technique NAAT offrent une sensibilité et une spécificité élevées, ainsi que des résultats rapides, constituant une alternative intéressante aux analyses de laboratoire centralisées traditionnelles.
- De plus, l'importance croissante accordée à la gestion des épidémies et à la préparation aux situations d'urgence fait des tests moléculaires au point de service (POCT) une composante essentielle des programmes de lutte contre les maladies infectieuses, permettant des décisions rapides en matière d'isolement et de traitement.
- La commodité des tests au chevet du patient, les exigences minimales en matière de traitement des échantillons et la possibilité de gérer plusieurs tests simultanément sont des facteurs clés qui favorisent l'adoption des tests moléculaires de diagnostic rapide (POCT) basés sur la technique NAAT dans les hôpitaux, les cliniques et sur le terrain.
Retenue/Défi
Obstacles réglementaires et limitations opérationnelles
- La conformité réglementaire et les processus d'approbation rigoureux des dispositifs de diagnostic moléculaire au point de soins constituent des défis importants pour l'expansion du marché, car les plateformes basées sur la NAAT doivent répondre à des normes rigoureuses en matière de précision, de sécurité et de fiabilité avant leur commercialisation.
- Par exemple, les retards d'approbation de la FDA ou du marquage CE peuvent ralentir le lancement de produits POCT innovants, limitant ainsi l'accès à des diagnostics avancés dans des situations où le temps est un facteur critique.
- Les limitations opérationnelles, telles que la nécessité de personnel formé, la maintenance des appareils et l'approvisionnement en consommables, peuvent restreindre l'adoption de ces dispositifs dans les contextes aux ressources limitées, malgré leurs avantages technologiques.
- De plus, le coût relativement élevé des dispositifs de diagnostic au point de soins (POCT) multiplexés ou dotés d'intelligence artificielle, comparé aux tests rapides conventionnels, peut constituer un obstacle pour les petites cliniques ou les régions à faibles revenus, limitant ainsi leur déploiement à grande échelle.
- Bien que les coûts diminuent progressivement, le surcoût perçu des plateformes moléculaires POCT sophistiquées pourrait freiner leur adoption dans les établissements de santé aux budgets limités.
- Le dépassement de ces défis grâce à des procédures réglementaires simplifiées, à la formation du personnel et au développement de dispositifs rentables sera essentiel à la croissance durable du marché.
Portée du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché est segmenté en fonction du produit, de l'indication, de l'utilisateur final, du mode de test et du canal de distribution.
- Sous-produit
Le marché des tests moléculaires au point de soins (POCT) est segmenté, selon le type de produit, en instruments et en consommables/réactifs. Le segment des instruments a dominé le marché en 2024, représentant 55,3 % des revenus, grâce au rôle crucial des dispositifs NAAT automatisés pour obtenir des résultats rapides et précis. Les établissements de santé privilégient l'adoption de ces instruments en raison de leur débit élevé, de leur fiabilité et de leur intégration aux systèmes d'information de laboratoire . Investir dans des instruments modernes permet aux hôpitaux et aux laboratoires d'optimiser leurs flux de travail, d'améliorer leur efficacité et de réduire les délais d'obtention des résultats pour le diagnostic des maladies infectieuses. Ce segment bénéficie des innovations technologiques, telles que les instruments portables et compacts adaptés aux tests décentralisés. Les instruments sont souvent associés à des plateformes logicielles pour faciliter la génération automatisée de rapports et le contrôle qualité. L'assistance technique et les services de maintenance continus proposés par les fabricants favorisent également leur adoption.
Le segment des consommables et réactifs devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide, soit 12,5 %, entre 2025 et 2032. Cette croissance est alimentée par le besoin constant en cartouches, kits de test et réactifs utilisés dans les tests de diagnostic rapide (POCT) basés sur la technique NAAT. Les consommables sont essentiels aux opérations de test quotidiennes dans les hôpitaux, les cliniques et les laboratoires, générant ainsi des revenus continus pour les fournisseurs. La demande est stimulée par une sensibilisation accrue au dépistage des maladies infectieuses et par l'augmentation de la fréquence des tests. Les analyses multiplexées, qui nécessitent des réactifs spécialisés, accélèrent encore cette croissance. Les innovations technologiques améliorant la stabilité et la facilité d'utilisation des réactifs favorisent leur adoption. Ce segment bénéficie également des améliorations apportées à la chaîne d'approvisionnement, qui réduisent les délais et les coûts de livraison.
- Par indication
En fonction des indications, le marché est segmenté en tests de dépistage des infections respiratoires, des infections sexuellement transmissibles (IST), des infections gastro-intestinales et autres. Le segment des tests de dépistage des infections respiratoires dominait le marché avec une part de 43,2 % en 2024, en raison de la forte prévalence d'agents pathogènes tels que le SARS-CoV-2, la grippe et le VRS. La détection rapide et précise des infections respiratoires est essentielle dans les hôpitaux, les cliniques et les services d'urgence pour une prise en charge et un confinement précoces. Les tests de diagnostic rapide (TDR) basés sur la technique NAAT offrent une sensibilité et une spécificité élevées, ce qui en fait la méthode de choix pour les infections respiratoires. Leur adoption est également favorisée par les programmes gouvernementaux de surveillance et de contrôle des épidémies. Les hôpitaux et les laboratoires intègrent ces tests dans leurs protocoles de soins d'urgence pour une prise de décision rapide. Les innovations continues en matière de panels multiplexés permettent d'accroître le débit et l'efficacité.
Le segment des tests de dépistage des IST devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide, soit 13,1 %, entre 2025 et 2032, sous l'effet d'une sensibilisation accrue, des programmes de dépistage et de l'augmentation de l'incidence des infections sexuellement transmissibles. Les tests de diagnostic rapide (TDR) basés sur la technique NAAT permettent une détection rapide, confidentielle et précise des IST, et sont parfaitement adaptés aux cliniques et à l'autotest. Les tests multiplexés, qui détectent plusieurs IST en un seul test, accélèrent leur adoption. Le développement de l'autotest et des initiatives de santé sexuelle stimule la demande. L'intégration aux plateformes de télémédecine améliore la communication des résultats et le conseil. Enfin, la multiplication des partenariats entre les entreprises de diagnostic et les professionnels de santé contribue également à la croissance du marché.
- Par l'utilisateur final
Selon l'utilisateur final, le marché des tests moléculaires au point de service (POCT) se segmente en laboratoires, hôpitaux, cliniques, centres ambulatoires, soins à domicile, établissements d'hébergement pour personnes âgées et autres. Le segment des hôpitaux a dominé le marché en 2024, représentant la plus grande part de revenus (48,6 %), grâce à un volume élevé de patients et au besoin de diagnostics rapides. Les hôpitaux investissent dans des appareils NAAT de pointe pour optimiser leurs flux de travail, gérer les épidémies et renforcer les services d'urgence. L'intégration aux systèmes d'information hospitaliers améliore l'efficacité opérationnelle. Ce segment bénéficie d'innovations technologiques continues et d'incitations gouvernementales. Les hôpitaux ont souvent besoin d'instruments capables de réaliser plusieurs types de tests. Des contrats à long terme avec les fournisseurs garantissent un approvisionnement constant en instruments et consommables.
Le segment des soins à domicile devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide, soit 14,2 %, entre 2025 et 2032, porté par la demande croissante de solutions de dépistage à domicile, la commodité et la confidentialité. Les kits NAAT pour soins à domicile permettent aux patients de se faire dépister sans se rendre en clinique ou à l'hôpital. L'autotest des infections respiratoires et des IST favorise leur adoption. L'intégration d'applications pour smartphones facilite l'interprétation des résultats et la transmission des informations en télémédecine. Les campagnes de sensibilisation et les partenariats avec les prestataires de télémédecine élargissent la portée de ces tests. L'acceptation croissante des tests à domicile par les autorités sanitaires soutient cette croissance durable.
- Par mode de test
Selon le mode de dépistage, le marché se divise en tests sur ordonnance et tests en vente libre. En 2024, le segment des tests sur ordonnance dominait le marché avec une part de 62,3 %, sous l'impulsion des exigences réglementaires et du contrôle des professionnels de santé. Ces tests offrent précision, fiabilité et interprétation clinique, notamment dans les hôpitaux et les cliniques. Leur intégration aux processus de soins garantit la sécurité des patients et l'assurance qualité. Ils sont particulièrement utilisés pour les maladies infectieuses à haut risque nécessitant l'avis d'experts. Les dispositifs sont souvent connectés aux réseaux hospitaliers pour le suivi et la communication des résultats. Les fabricants s'efforcent d'améliorer l'ergonomie tout en respectant les normes réglementaires.
Le segment des tests en vente libre devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide, soit 13,5 %, entre 2025 et 2032, porté par la demande croissante d'autotests pour les infections respiratoires, les IST et les infections gastro-intestinales. La simplicité d'utilisation des kits et l'interprétation des résultats via smartphone améliorent l'accessibilité. Les consommateurs privilégient les solutions en vente libre pour leur praticité, leur confidentialité et la réduction des consultations médicales. Les campagnes de sensibilisation et l'intégration de la télémédecine favorisent leur adoption. Les points de vente et les pharmacies en ligne contribuent à accroître la disponibilité des tests. Ce segment bénéficie de kits de test simplifiés et pré-emballés, conçus pour un usage domestique.
- Par canal de distribution
Selon le canal de distribution, le marché se segmente en pharmacies hospitalières, pharmacies de détail et pharmacies en ligne. Le segment des pharmacies hospitalières dominait le marché en 2024 avec une part de 57,4 %, grâce aux achats directs pour les analyses réalisées en hospitalisation et en ambulatoire. Les hôpitaux s'appuient sur les pharmacies pour un approvisionnement constant en dispositifs NAAT, consommables et réactifs. Les accords d'achat groupé permettent de réduire les coûts et d'assurer un réapprovisionnement rapide. L'intégration aux systèmes de la chaîne d'approvisionnement hospitalière minimise les ruptures de stock. Les hôpitaux bénéficient souvent d'un soutien technique et de formations via leurs contrats avec les pharmacies. Les partenariats avec les fabricants renforcent la disponibilité des produits et le service après-vente.
Le segment des pharmacies en ligne devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide, à 15,1 %, entre 2025 et 2032, grâce à la facilité de commande, la livraison à domicile des kits NAAT et l'essor de la télémédecine. Les plateformes en ligne permettent l'achat discret de tests, qu'ils soient sur ordonnance ou en vente libre. Les campagnes de marketing numérique contribuent à sensibiliser les consommateurs. L'intégration avec les applications de télésanté facilite la transmission des résultats. Les consommateurs privilégient de plus en plus l'accès en ligne pour sa praticité et la confidentialité qu'il offre. La croissance du commerce électronique et les progrès logistiques soutiennent le développement rapide de ce canal de distribution.
Analyse régionale du marché des tests moléculaires au point de soins (utilisant la technique NAAT)
- L'Amérique du Nord a dominé le marché des tests moléculaires au point de soins (POCT) avec la plus grande part de revenus (39 %) en 2024, grâce à l'adoption précoce des technologies de diagnostic avancées, aux dépenses de santé élevées et à la forte présence d'acteurs clés du secteur. Les États-Unis ont connu une croissance substantielle grâce à l'intégration des tests NAAT dans les hôpitaux, les cliniques et les services d'urgence.
- Dans la région, les établissements de santé privilégient les tests rapides, précis et fiables, ce qui rend les dispositifs de diagnostic rapide basés sur la technique NAAT indispensables aux hôpitaux, aux cliniques et aux services d'urgence. Aux États-Unis, en particulier, on observe une forte croissance grâce à l'intégration de ces dispositifs dans les hôpitaux, les cliniques ambulatoires et les services de soins à domicile, soutenue par les innovations des entreprises de diagnostic établies et des jeunes pousses.
- Cette adoption généralisée est également favorisée par une infrastructure de santé solide, des dépenses de santé élevées et une population technologiquement avancée.
Analyse du marché américain des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché américain des tests moléculaires de diagnostic au point de service (POCT) a généré 79 % des revenus en Amérique du Nord en 2024, porté par l'adoption rapide des technologies de diagnostic avancées et le besoin croissant de détection précoce des maladies infectieuses. Les professionnels de santé privilégient de plus en plus les tests d'amplification des acides nucléiques (TAAN) pour le diagnostic des infections respiratoires , des IST et des infections gastro-intestinales, en raison de leur grande précision et de leur rapidité d'exécution. La tendance croissante à la décentralisation des soins et aux tests à domicile stimule davantage ce marché. Par ailleurs, l'intégration des dispositifs POCT moléculaires aux systèmes d'information hospitaliers et aux plateformes de télémédecine contribue significativement à son expansion. La forte présence d'entreprises clés du diagnostic et les innovations technologiques constantes soutiennent également cette croissance durable.
Analyse du marché européen des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché européen des tests moléculaires au point de soins (POCT) devrait connaître une croissance annuelle composée (TCAC) importante tout au long de la période de prévision, principalement sous l'effet de la demande croissante de diagnostics rapides et de l'augmentation de l'incidence des maladies infectieuses. Les initiatives gouvernementales promouvant les tests au point de soins et la numérisation des soins de santé encouragent leur adoption dans les hôpitaux, les cliniques et les laboratoires. Cette croissance est également soutenue par l'urbanisation et la prévalence croissante des infections multirésistantes. Les établissements de santé européens adoptent de plus en plus les tests d'amplification des acides nucléiques (TAAN) pour une prise en charge rapide des maladies et un contrôle efficace des épidémies. Les établissements de santé, tant résidentiels que commerciaux, intègrent des dispositifs POCT pour améliorer la prise en charge des patients. Le marché connaît une croissance significative dans des pays comme l'Allemagne, la France et l'Italie.
Analyse du marché britannique des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché britannique des tests moléculaires de diagnostic au point de soins (POCT) devrait connaître une croissance annuelle composée (TCAC) remarquable au cours de la période de prévision, portée par la demande croissante de diagnostics rapides et de solutions de tests décentralisées. La sensibilisation accrue des professionnels de santé aux avantages des tests d'amplification des acides nucléiques (TAAN), tels que leur haute sensibilité et spécificité, encourage leur adoption dans les hôpitaux et les cliniques. Les initiatives gouvernementales en matière de dépistage précoce et de prise en charge des maladies infectieuses soutiennent également l'expansion du marché. La solide infrastructure de santé et les systèmes de santé numérique performants du pays facilitent l'intégration des dispositifs et la communication des résultats. L'adoption croissante dans les secteurs de la santé publique et privée stimule la croissance du marché. Les initiatives de télémédecine et de tests à domicile devraient encore accélérer l'adoption au Royaume-Uni.
Analyse du marché allemand des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché allemand des tests moléculaires de diagnostic au point de service (POCT) devrait connaître une croissance annuelle composée (TCAC) considérable au cours de la période de prévision, portée par la prévalence croissante des maladies infectieuses et la demande de diagnostics rapides. L'infrastructure de santé avancée de l'Allemagne, son fort accent mis sur l'innovation technologique et la sensibilisation élevée des professionnels de santé favorisent l'adoption des POCT basés sur les tests d'amplification des acides nucléiques (TAAN). L'intégration des dispositifs POCT aux systèmes d'information hospitaliers et aux réseaux de laboratoires améliore l'efficacité opérationnelle. On observe une préférence croissante pour les tests décentralisés dans les cliniques externes et les centres de soins ambulatoires. L'adoption de ces tests progresse tant dans les établissements de soins résidentiels que dans les centres de diagnostic commerciaux. La recherche et le développement continus, ainsi que la fabrication locale d'instruments et de consommables POCT, contribuent à la croissance du marché.
Analyse du marché des tests moléculaires au point de soins (utilisant la technique NAAT) en Asie-Pacifique
Le marché des tests moléculaires de diagnostic au point de service (POCT) en Asie-Pacifique devrait connaître la croissance annuelle composée la plus rapide, à 23,5 %, entre 2025 et 2032. Cette croissance est portée par l'augmentation de l'incidence des maladies infectieuses, la hausse des investissements dans le secteur de la santé et le développement des infrastructures de dépistage dans des pays comme la Chine, le Japon et l'Inde. L'intérêt croissant de la région pour les solutions de dépistage décentralisées et à domicile stimule l'adoption de ces tests. Les programmes gouvernementaux promouvant la santé numérique et la surveillance des maladies infectieuses favorisent l'expansion du marché. La présence de fabricants locaux garantit l'accessibilité des dispositifs et consommables NAAT. L'urbanisation croissante, l'adoption des technologies et la sensibilisation accrue au diagnostic précoce contribuent également à cette croissance. La demande dans les hôpitaux, les cliniques et les services de soins à domicile est en constante augmentation.
Analyse du marché japonais des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché japonais des tests moléculaires de diagnostic au point de soins (POCT) est en plein essor grâce à des normes de santé élevées, à l'adoption de technologies de diagnostic avancées et à une sensibilisation accrue aux tests rapides. Le pays met l'accent sur la détection précise et rapide des maladies infectieuses, ce qui favorise l'adoption de ces tests dans les hôpitaux, les cliniques et les services de soins à domicile. L'intégration des dispositifs NAAT aux réseaux hospitaliers et aux plateformes de télémédecine améliore l'efficacité. Le vieillissement de la population accroît encore la demande de solutions de test conviviales et accessibles. Les innovations constantes dans les dispositifs NAAT multiplexés et portables soutiennent le marché. La préférence croissante pour la médecine préventive et le diagnostic numérique accélère l'adoption de ces tests dans les secteurs de la santé, tant résidentiels que commerciaux.
Analyse du marché indien des tests moléculaires au point de soins (utilisant la technique NAAT)
Le marché indien des tests moléculaires de diagnostic au point de service (POCT) a représenté la plus grande part de revenus de la région Asie-Pacifique en 2024, grâce à la forte prévalence des maladies infectieuses, à l'expansion de la classe moyenne et à une sensibilisation accrue aux questions de santé. L'urbanisation rapide et les initiatives gouvernementales en faveur de la santé connectée et du diagnostic numérique sont des moteurs de croissance essentiels. Les hôpitaux, les cliniques et les services de soins à domicile adoptent de plus en plus les dispositifs POCT basés sur la technique NAAT pour un diagnostic et un traitement plus rapides. La disponibilité de dispositifs abordables, fabriqués localement, améliore l'accessibilité dans les zones urbaines et périurbaines. La demande croissante de solutions de tests multiplexés accélère encore l'adoption de ces tests. Des programmes de santé publique solides et des partenariats avec des entreprises de diagnostic continuent de stimuler la croissance du marché.
Part de marché des tests moléculaires au point de soins (utilisant la technique NAAT)
Le secteur des tests moléculaires au point de soins (utilisant la technique NAAT) est principalement dominé par des entreprises bien établies, notamment :
- Thermo Fisher Scientific Inc. (États-Unis)
- Hologic, Inc. (États-Unis)
- BD (États-Unis)
- F. Hoffmann-La Roche Ltd (Suisse)
- Abbott (États-Unis)
- QIAGEN (Pays-Bas)
- BIOMÉRIEUX (France)
- Danaher (États-Unis)
- Illumina, Inc. (États-Unis)
- Sysmex Corporation (Japon)
- Siemens Healthineers AG (Allemagne)
- Seegene Inc. (Corée du Sud)
- Guardant Health, Inc. (États-Unis)
- Labcorp (États-Unis)
- Exact Sciences Corporation (États-Unis)
- 10x Genomics, Inc. (États-Unis)
- DNA Genotek Inc. (Canada)
- PathoNostics (Pays-Bas)
- Molbio Diagnostics Limited (Inde)
Quels sont les développements récents sur le marché mondial des tests moléculaires au point de soins (utilisant la technique NAAT) ?
- En juillet 2025, BD (Becton, Dickinson and Company) a reçu l'autorisation 510(k) de la FDA pour son système BD Veritor™ pour le SARS-CoV-2, un test numérique conçu pour détecter les antigènes du COVID-19 chez les personnes symptomatiques en environ 15 minutes dans des lieux de soins tels que les cabinets médicaux, les centres de soins d'urgence et les cliniques de détail.
- En octobre 2024, l'Organisation mondiale de la Santé (OMS) a approuvé le premier test de diagnostic de la variole du singe (mpox) pour une utilisation d'urgence. Cette approbation vise à améliorer l'accès mondial à des diagnostics rapides et précis de la mpox, notamment dans les contextes aux ressources limitées. Le test utilise la technologie d'amplification des acides nucléiques, permettant un diagnostic au point de soins avec une sensibilité et une spécificité élevées.
- En avril 2024, Roche Diagnostics a lancé le système cobas® 5800, une plateforme d'automatisation de tests moléculaires de nouvelle génération conçue pour améliorer la productivité et réduire les erreurs en laboratoire. Ce système propose des analyses standardisées et des solutions évolutives, ce qui le rend adapté à différents volumes et compositions d'échantillons. En renforçant l'automatisation du diagnostic moléculaire, le système cobas® 5800 vise à simplifier les flux de travail et à garantir des résultats cohérents dans tous les environnements de test.
- En mars 2023, QuidelOrtho Corporation a annoncé avoir obtenu l'autorisation de mise sur le marché (De Novo) de la FDA (Food and Drug Administration) américaine, lui permettant ainsi de commercialiser son nouveau test antigénique rapide Sofia® 2 SARS Antigen+ FIA. Ce test est le premier test antigénique rapide de détection de la COVID-19 à recevoir l'autorisation de mise sur le marché de la FDA.
- En mars 2023, le test LumiraDx SARS-CoV-2 Ag a été autorisé pour une utilisation au chevet du patient dans les établissements de soins fonctionnant sous un certificat d'exemption, de conformité ou d'accréditation CLIA. Ce test est destiné aux professionnels de santé ou aux opérateurs compétents pour réaliser des tests au chevet du patient.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.