Global Medical Device Regulatory Affairs Outsourcing Market
Taille du marché en milliards USD
TCAC :
% 
 USD
7.36 Billion
USD
19.30 Billion
2024
2032
USD
7.36 Billion
USD
19.30 Billion
2024
2032
| 2025 –2032 | |
| USD 7.36 Billion | |
| USD 19.30 Billion | |
|
|
|
|
Segmentation du marché mondial de l'externalisation des affaires réglementaires des dispositifs médicaux, par services (services d'affaires réglementaires, conseil qualité, rédaction et publication réglementaires, soumissions réglementaires, demandes d'essais cliniques, enregistrements de produits, représentation juridique et rédaction médicale), produit (produits finis, électronique et matières premières), type de dispositif (classes I, II et III), catégorie (produits biologiques, médicaments et dispositifs médicaux), application (cardiologie, imagerie diagnostique, orthopédie, diagnostic in vitro, ophtalmologie, chirurgie générale et plastique, administration de médicaments, dentisterie, endoscopie, soins du diabète, etc.), utilisateur final (petite entreprise de dispositifs médicaux, entreprise moyenne de dispositifs médicaux, sociétés pharmaceutiques et biotechnologiques, fabricant de dispositifs médicaux et grande entreprise de dispositifs médicaux) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché de l'externalisation des affaires réglementaires des dispositifs médicaux
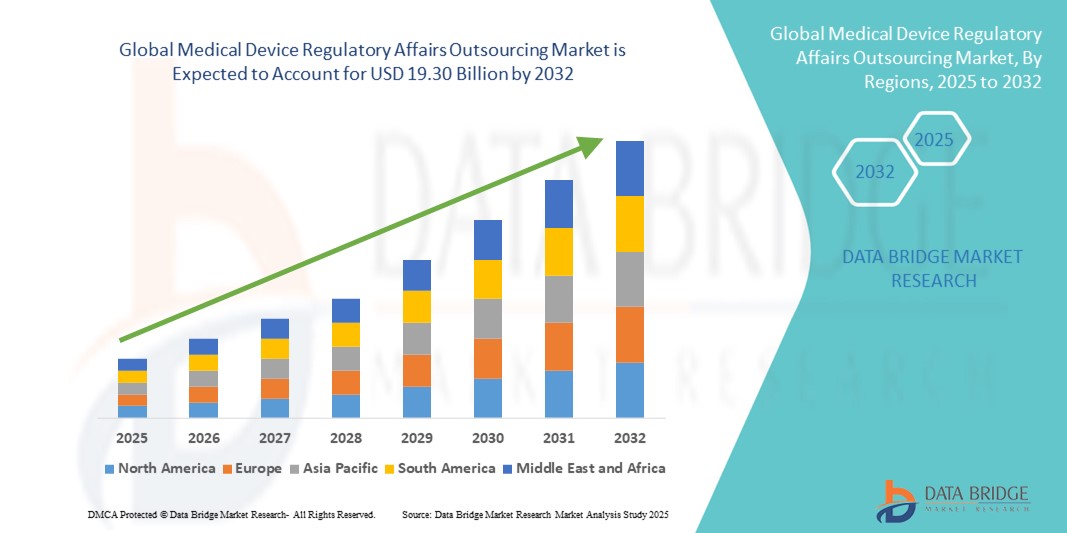
- La taille du marché mondial de l'externalisation des affaires réglementaires des dispositifs médicaux était évaluée à 7,36 milliards USD en 2024 et devrait atteindre 19,30 milliards USD d'ici 2032 , à un TCAC de 12,8 % au cours de la période de prévision.
- La croissance du marché est principalement tirée par la complexité croissante des exigences réglementaires mondiales et la demande croissante d'approbations de produits plus rapides, ce qui incite les fabricants de dispositifs médicaux à s'appuyer sur des services d'externalisation réglementaire spécialisés.
- De plus, l'essor des activités de R&D, les mises à jour fréquentes des normes de conformité et la pression pour réduire les délais de mise sur le marché incitent les entreprises à s'associer à des experts réglementaires externes. Ces facteurs accélèrent l'adoption de solutions d'externalisation, contribuant ainsi significativement à l'expansion du marché.
Analyse du marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
- L'externalisation des affaires réglementaires des dispositifs médicaux implique de déléguer des tâches réglementaires telles que l'enregistrement des produits, la documentation de conformité et la liaison avec les autorités à des sociétés tierces spécialisées, devenant une stratégie cruciale pour les fabricants qui cherchent à naviguer dans des environnements réglementaires mondiaux de plus en plus complexes de manière efficace et rentable.
- La demande croissante d'externalisation réglementaire est principalement motivée par les mises à jour continues des exigences de conformité mondiales, la nécessité d'accélérer les approbations de marché et le manque d'expertise réglementaire interne parmi les fabricants de dispositifs de petite et moyenne taille.
- L'Amérique du Nord a dominé le marché de l'externalisation des affaires réglementaires des dispositifs médicaux avec la plus grande part de revenus de 39,2 % en 2024, soutenue par la maturité de l'industrie des dispositifs médicaux de la région, un cadre réglementaire solide et une forte pénétration de l'externalisation parmi les fabricants basés aux États-Unis qui recherchent des approbations plus rapides de la FDA.
- L'Asie-Pacifique devrait connaître la croissance la plus rapide du marché au cours de la période de prévision en raison de l'expansion de la base de fabrication de dispositifs médicaux de la région, de l'évolution des cadres réglementaires et des avantages en termes de coûts offerts par les CRO et les cabinets de conseil en réglementation locaux.
- Le segment de la rédaction et de la publication réglementaires a dominé le marché avec une part de 42 % en 2024, attribuée à son rôle essentiel pour garantir des soumissions claires et conformes aux organismes de réglementation, ce qui a un impact direct sur les délais d'approbation et le succès du lancement des produits.
Portée du rapport et segmentation du marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
|
Attributs |
Externalisation des affaires réglementaires des dispositifs médicaux : informations clés sur le marché |
|
Segments couverts |
|
|
Pays couverts |
Amérique du Nord
Europe
Asie-Pacifique
Moyen-Orient et Afrique
Amérique du Sud
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
« Transformation numérique et solutions réglementaires basées sur l'IA »
- L'adoption croissante des technologies numériques et de l'intelligence artificielle (IA) pour améliorer les processus réglementaires et l'efficacité de la conformité est une tendance majeure et croissante sur le marché mondial de l'externalisation des affaires réglementaires des dispositifs médicaux. Cette transition numérique rationalise les opérations et transforme la façon dont les fabricants abordent les demandes réglementaires complexes et multi-pays.
- Par exemple, les fournisseurs de services réglementaires tels que Freyr et IQVIA déploient des plateformes basées sur l'IA pour l'automatisation des documents réglementaires, le suivi des soumissions en temps réel et l'intelligence réglementaire prédictive, permettant une prise de décision plus rapide et plus précise.
- L'intégration de l'IA permet la compilation automatisée des dossiers techniques, l'identification des lacunes réglementaires et la mise en conformité proactive avec l'évolution des normes mondiales. Ces fonctionnalités réduisent considérablement les délais de mise sur le marché et la charge de travail des fabricants.
- L'utilisation de plateformes numériques centralisées améliore encore la collaboration entre les partenaires d'externalisation et les équipes réglementaires internes, garantissant la transparence, la cohérence et la facilité d'accès aux documents de soumission mis à jour.
- Cette tendance vers des solutions réglementaires intelligentes remodèle les attentes au sein du secteur, les clients exigeant des délais d'exécution plus rapides, une précision des données améliorée et un support de conformité évolutif.
- À mesure que la complexité des réglementations mondiales augmente, la demande de services d'externalisation réglementaire intelligents, automatisés et axés sur la technologie augmente sur les marchés des dispositifs médicaux matures et émergents, les entreprises considérant de plus en plus ces partenariats comme essentiels à leurs stratégies d'expansion mondiale.
Dynamique du marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Conducteur
« Complexité croissante des cadres réglementaires mondiaux et innovation produit »
- La complexité croissante des normes réglementaires internationales et l’innovation continue dans les technologies des dispositifs médicaux sont des facteurs clés qui accélèrent la demande de services d’externalisation des affaires réglementaires.
- Par exemple, en janvier 2024, Parexel a étendu ses services mondiaux de conseil réglementaire pour aider les entreprises à naviguer dans les nouveaux cadres MDR et IVDR de l'UE, soulignant le besoin croissant de conseils d'experts dans des environnements de conformité complexes.
- Les fabricants de dispositifs médicaux sont soumis à la pression de devoir suivre le rythme de l'évolution des exigences mondiales tout en garantissant un accès rapide au marché, ce qui rend les partenaires réglementaires externes essentiels pour maintenir à la fois la rapidité et la précision.
- L'essor des solutions de santé numériques, des appareils dotés d'IA et des produits combinés a ajouté des niveaux supplémentaires de contrôle réglementaire, obligeant les entreprises à s'appuyer sur des experts tiers pour les soumissions stratégiques, la conformité continue et la surveillance du marché.
- En externalisant ces tâches, les fabricants peuvent se concentrer sur l'innovation et la commercialisation tout en réduisant les charges de ressources internes, en accélérant les délais d'approbation et en se développant plus efficacement sur de nouveaux marchés.
Retenue/Défi
« Préoccupations liées à la confidentialité des données et pénurie de talents »
- Les préoccupations croissantes en matière de confidentialité des données et de cybersécurité représentent un défi majeur sur le marché mondial de l'externalisation des affaires réglementaires des dispositifs médicaux. Face au transfert transfrontalier de données sensibles sur les patients et d'informations exclusives sur les produits, les entreprises doivent garantir une conformité totale avec les réglementations en matière de protection des données, telles que le RGPD et la loi HIPAA.
- Par exemple, toute violation ou mauvaise gestion de la documentation réglementaire par des fournisseurs tiers pourrait exposer les fabricants à des risques juridiques, financiers et de réputation importants, retardant potentiellement l'approbation des produits ou déclenchant des sanctions réglementaires.
- En outre, le marché est confronté à une pénurie persistante de professionnels de la réglementation qualifiés possédant une connaissance approfondie des réglementations et des normes de qualité spécifiques à chaque région, ce qui entraîne des goulots d'étranglement et des défis en matière de contrôle qualité pour les entreprises d'externalisation.
- Alors que certaines entreprises investissent dans le développement de la main-d'œuvre et dans l'infrastructure de conformité numérique, combler ce fossé en matière de talents et de sécurité est essentiel pour gagner et conserver la confiance des clients.
- La résolution de ces problèmes par le biais de protocoles de gouvernance des données améliorés, d'une mise à niveau continue des compétences et de partenariats régionaux stratégiques sera essentielle pour garantir une externalisation des affaires réglementaires sécurisée, efficace et conforme à long terme.
Portée du marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le marché est segmenté sur la base des services, du produit, du type d’appareil, de la catégorie, de l’application et de l’utilisateur final.
- Par services
Sur la base des services, le marché de l'externalisation des affaires réglementaires des dispositifs médicaux est segmenté en services d'affaires réglementaires, conseil qualité, rédaction et publication réglementaires, soumissions réglementaires, demandes d'essais cliniques, enregistrements de produits, représentation juridique et rédaction médicale. Le segment de la rédaction et de la publication réglementaires a dominé le marché avec une part de chiffre d'affaires de 42 % en 2024, grâce à son rôle essentiel dans la préparation et la soumission de documents réglementaires conformes et détaillés auprès des agences mondiales. La rédaction réglementaire garantit la clarté, l'exactitude et la cohérence des soumissions, ce qui est essentiel pour obtenir les approbations de produits dans les délais et maintenir la conformité tout au long du cycle de vie du produit.
Le segment des demandes d'essais cliniques devrait connaître sa croissance la plus rapide entre 2025 et 2032, alimenté par le nombre croissant de dispositifs innovants entrant en développement clinique. Face au durcissement des cadres réglementaires, l'externalisation des tâches d'application clinique à des prestataires de services réglementaires expérimentés permet de garantir l'exactitude et la conformité, réduisant ainsi les délais d'approbation.
- Par produit
En termes de produits, le marché de l'externalisation des affaires réglementaires des dispositifs médicaux est segmenté en produits finis, électronique et matières premières. En 2024, le segment des produits finis a représenté la plus grande part de chiffre d'affaires du marché, en raison du volume important de dispositifs médicaux finaux nécessitant une documentation, des tests et des approbations réglementaires approfondis avant leur commercialisation. Ces produits impliquent souvent des stratégies de soumission mondiales complètes et nécessitent un soutien en matière de surveillance post-commercialisation, ce qui stimule la demande d'externalisation.
Le secteur de l'électronique devrait connaître le TCAC le plus élevé entre 2025 et 2032, porté par l'intégration croissante des technologies numériques, de l'IA et des composants logiciels dans les dispositifs médicaux. La conformité réglementaire des composants électroniques, notamment ceux des appareils connectés et portables, requiert une expertise pointue en cybersécurité et en préparation de dossiers techniques, ce qui stimule la demande d'externalisation.
- Par type d'appareil
Selon le type de dispositif, le marché de l'externalisation des affaires réglementaires des dispositifs médicaux est segmenté en classes I, II et III. Le segment de classe II a dominé le marché avec la plus grande part de chiffre d'affaires en 2024, en raison du grand nombre de dispositifs médicaux moyennement complexes relevant de cette classification. Ces dispositifs nécessitent une documentation réglementaire importante et la conformité du système qualité, ce qui incite les fabricants à rechercher un soutien externe pour obtenir des autorisations efficaces et rapides.
Le segment des dispositifs de classe III devrait connaître la croissance la plus rapide entre 2025 et 2032, car ces dispositifs à haut risque nécessitent des autorisations préalables à la mise sur le marché approfondies, des essais cliniques rigoureux et des données de surveillance à long terme. La complexité et le niveau de risque de ces dispositifs font de l'externalisation un choix stratégique pour les fabricants souhaitant répondre aux exigences réglementaires mondiales.
- Par catégorie
Le marché de l'externalisation des affaires réglementaires des dispositifs médicaux est segmenté en catégories : produits biologiques, médicaments et dispositifs médicaux. Ce segment a dominé le marché avec la plus grande part de chiffre d'affaires en 2024, porté par l'innovation croissante et le volume croissant de nouveaux dispositifs développés à l'échelle mondiale. L'évolution constante des exigences réglementaires spécifiques aux dispositifs médicaux incite les fabricants à externaliser leurs activités auprès de prestataires spécialisés pour une meilleure conformité et une meilleure efficacité.
Le secteur des produits biologiques devrait connaître la croissance la plus rapide entre 2025 et 2032, portée par l'essor des produits combinés et des thérapies personnalisées. Ces produits nécessitent des approches réglementaires multidisciplinaires, rendant l'externalisation essentielle pour maîtriser les processus d'approbation complexes et garantir une conformité coordonnée.
- Par application
En fonction des applications, le marché de l'externalisation des affaires réglementaires des dispositifs médicaux est segmenté en cardiologie, imagerie diagnostique, orthopédie, diagnostic in vitro (DIV), ophtalmologie, chirurgie générale et plastique, administration de médicaments, dentisterie, endoscopie, diabète, etc. Le segment du DIV a représenté la plus grande part de chiffre d'affaires du marché en 2024, soutenu par l'essor des tests diagnostiques et la mise en œuvre de cadres réglementaires stricts tels que le Règlement européen sur les dispositifs médicaux (RDIV). L'externalisation réglementaire est essentielle pour garantir l'exactitude de la classification, de la documentation technique et des données d'évaluation des performances de ces produits.
Le secteur des soins du diabète devrait connaître le TCAC le plus élevé au cours de la période de prévision, grâce à la prolifération d'appareils innovants de surveillance de la glycémie et de solutions de santé numériques. Ces technologies nécessitent des stratégies réglementaires spécifiques et un suivi continu de la conformité, ce qui accroît le recours aux partenaires d'externalisation.
- Par utilisateur final
En fonction de l'utilisateur final, le marché de l'externalisation des affaires réglementaires des dispositifs médicaux est segmenté en petites et moyennes entreprises de dispositifs médicaux, en sociétés pharmaceutiques et biotechnologiques, en fabricants de dispositifs médicaux et en grandes entreprises de dispositifs médicaux. En 2024, ces dernières ont dominé le marché avec la plus grande part de chiffre d'affaires. Ces entreprises gèrent des portefeuilles diversifiés sur plusieurs marchés mondiaux et s'appuient sur l'externalisation réglementaire pour maintenir leur efficacité opérationnelle et leur cohérence réglementaire.
Le segment des petites entreprises de dispositifs médicaux devrait connaître la croissance la plus rapide entre 2025 et 2032, en raison de ressources réglementaires internes limitées et du besoin de conseils d'experts pour respecter des normes de conformité complexes. Ces entreprises externalisent souvent l'intégralité des tâches réglementaires afin d'accélérer leur entrée sur le marché et de réduire leurs charges opérationnelles.
Analyse régionale du marché de l'externalisation des affaires réglementaires des dispositifs médicaux
- L'Amérique du Nord a dominé le marché de l'externalisation des affaires réglementaires des dispositifs médicaux avec la plus grande part de revenus de 39,2 % en 2024, soutenue par la maturité de l'industrie des dispositifs médicaux de la région, un cadre réglementaire solide et une forte pénétration de l'externalisation parmi les fabricants basés aux États-Unis qui recherchent des approbations plus rapides de la FDA.
- Les entreprises de la région accordent la priorité aux approbations de produits en temps opportun et à l'expansion stratégique mondiale, ce qui alimente la dépendance à l'égard des partenaires d'externalisation pour gérer les soumissions complexes et les réglementations en constante évolution telles que le QMSR de la FDA américaine et le soutien à la transition vers le MDR de l'UE.
- Cette forte position sur le marché est également soutenue par un écosystème bien établi de cabinets de conseil en réglementation, des investissements importants en R&D et une forte concentration de fabricants de dispositifs médicaux, faisant de l'Amérique du Nord une plaque tournante clé pour les services réglementaires externalisés à toutes les étapes du développement et de la commercialisation des produits.
Aperçu du marché américain de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le marché américain de l'externalisation des affaires réglementaires des dispositifs médicaux a représenté la plus grande part de chiffre d'affaires en Amérique du Nord (84 %) en 2024, grâce à la complexité des processus réglementaires de la FDA et au recours croissant à l'expertise externe pour accélérer les approbations. Face à l'important volume d'innovations dans le domaine des dispositifs médicaux et à la pression croissante pour une entrée sur le marché plus rapide, les fabricants américains externalisent des tâches telles que la préparation des soumissions, la stratégie réglementaire et la conformité post-commercialisation. De plus, l'évolution continue de la réglementation de la FDA, notamment la mise à jour du QMSR, accentue le besoin de services de conseil réglementaire spécialisés pour les entreprises de toutes tailles.
Aperçu du marché européen de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le marché européen de l'externalisation des affaires réglementaires des dispositifs médicaux devrait connaître une croissance annuelle moyenne (TCAC) substantielle tout au long de la période de prévision, principalement stimulée par la mise en œuvre des cadres européens MDR et IVDR. Ces réglementations ont considérablement renforcé les exigences en matière de documentation, de preuves cliniques et de surveillance, stimulant ainsi la demande de partenaires d'externalisation possédant une expertise réglementaire spécifique à l'UE. La forte base de fabricants de dispositifs médicaux de petite et moyenne taille dans la région contribue également à l'adoption de l'externalisation, notamment dans des pays clés comme l'Allemagne, la France et les Pays-Bas, où l'obtention rapide du marquage CE est essentielle à la réussite commerciale.
Aperçu du marché britannique de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le marché britannique de l'externalisation des affaires réglementaires des dispositifs médicaux devrait connaître une croissance annuelle moyenne (TCAC) remarquable au cours de la période de prévision, soutenue par les évolutions réglementaires post-Brexit et la nécessité croissante de gérer les procédures de conformité UKCA et internationales. Les fabricants britanniques externalisent de plus en plus leurs tâches réglementaires afin de garantir leur conformité aux exigences de la MHRA tout en cherchant à accéder au marché mondial. La présence de cabinets de conseil réglementaire hautement spécialisés et d'un écosystème medtech solide fait du Royaume-Uni un contributeur clé à la croissance du marché régional.
Aperçu du marché allemand de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le marché allemand de l'externalisation des affaires réglementaires des dispositifs médicaux devrait connaître une croissance annuelle moyenne (TCAC) considérable au cours de la période de prévision, porté par son positionnement de pôle majeur de fabrication de dispositifs médicaux et un environnement réglementaire strict. Soucieux de se conformer aux exigences complexes du RDM de l'UE, les fabricants allemands se tournent vers des sociétés d'externalisation pour obtenir de l'aide en matière de documentation, d'analyse des écarts et d'évaluations cliniques. L'accent mis sur la précision, la qualité et la durabilité dans le secteur des technologies médicales allemandes encourage également la collaboration avec des partenaires réglementaires experts afin de maintenir leur compétitivité mondiale.
Analyse du marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux en Asie-Pacifique
Le marché de l'externalisation des affaires réglementaires des dispositifs médicaux en Asie-Pacifique devrait connaître une croissance annuelle composée (TCAC) record de 25 % sur la période de prévision 2025-2032, portée par l'expansion de la production de dispositifs médicaux, la hausse des exportations internationales et la sophistication croissante des cadres réglementaires dans des pays comme la Chine, le Japon et l'Inde. Les gouvernements régionaux renforcent leur surveillance et s'alignent sur les normes mondiales, ce qui incite les entreprises locales et internationales à externaliser les tâches de conformité. De plus, la rentabilité et l'évolutivité rapide des solutions d'externalisation en Asie-Pacifique attirent les fabricants multinationaux de dispositifs médicaux en quête d'homologation et de distribution régionales.
Aperçu du marché japonais de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le marché japonais de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux prend de l'ampleur grâce à la complexité des processus d'approbation PMDA et au fort taux d'innovation technologique du secteur des technologies médicales. Les fabricants japonais recourent de plus en plus à l'externalisation réglementaire pour la classification des produits, le soutien aux essais cliniques et la documentation bilingue afin de garantir des approbations locales et internationales rapides. La demande d'expertise réglementaire dans des domaines tels que les dispositifs basés sur l'IA et les produits combinés stimule également la croissance du marché au Japon.
Aperçu du marché indien de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
En 2024, le marché indien de l'externalisation des affaires réglementaires des dispositifs médicaux représentait la plus grande part de chiffre d'affaires en Asie-Pacifique, grâce à l'expansion de la base de fabrication de dispositifs médicaux du pays, à une conformité accrue aux directives CDSCO et à la présence croissante de cabinets de conseil en réglementation nationaux. La poussée vers le « Made in India » et l'intelligente infrastructure de santé ont entraîné une forte augmentation du développement de produits, nécessitant un soutien réglementaire efficace. La disponibilité de professionnels qualifiés et les avantages en termes de coûts font de l'Inde une plaque tournante pour l'externalisation réglementaire locale et internationale.
Part de marché de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux
Le secteur de l'externalisation des affaires réglementaires relatives aux dispositifs médicaux est principalement dirigé par des entreprises bien établies, notamment :
- Parexel International (MA) Corporation (États-Unis)
- North American Science Associates, LLC (États-Unis)
- SGS Société Générale de Surveillance SA. (Suisse)
- Pace (États-Unis)
- Trilogy Writing & Consulting GmbH (Allemagne)
- Creganna (Irlande)
- Intertek Group plc (Royaume-Uni)
- WuXi AppTec (Chine)
- Charles River Laboratories (États-Unis)
- Celestica Inc. (Canada)
- Freyr (États-Unis)
- Cactus Communications (Inde)
- In.Corp Indonesia (Indonésie)
- Eurofins Scientifique (Luxembourg)
- Plexus Corp. (États-Unis)
- Sanmina Corporation (États-Unis)
- OMRON Corporation (Japon)
Quels sont les développements récents sur le marché mondial de l’externalisation des affaires réglementaires des dispositifs médicaux ?
- En avril 2023, Parexel International Corporation a étendu ses services mondiaux de conseil réglementaire afin d'aider les développeurs de dispositifs médicaux et de produits combinés à s'adapter aux exigences de plus en plus complexes du Règlement européen sur les dispositifs médicaux (RDM UE) et du Règlement sur les diagnostics in vitro (RDIV). Cette amélioration stratégique reflète l'engagement de Parexel à aider ses clients à accélérer leur accès au marché en s'appuyant sur une expertise réglementaire approfondie et une présence mondiale, notamment dans un contexte de durcissement des exigences de conformité européennes.
- En mars 2023, ICON plc a annoncé le lancement d'une nouvelle plateforme de veille réglementaire conçue pour aider les entreprises de dispositifs médicaux à gérer l'évolution de la réglementation mondiale et leurs stratégies de soumission. Cette solution numérique offre un accès en temps réel aux mises à jour réglementaires, aux délais de conformité et aux modèles de documents, permettant ainsi de simplifier les processus de préparation et d'approbation. Cette innovation souligne l'importance accordée par ICON à l'intégration de la technologie au conseil réglementaire afin d'optimiser l'efficacité et la précision pour ses clients.
- En mars 2023, Freyr Solutions a ouvert un nouveau pôle mondial de services réglementaires en Asie-Pacifique, afin d'accompagner les entreprises de dispositifs médicaux dans leur conformité complète aux homologations nationales. Ce nouveau centre renforce la capacité de Freyr à gérer la demande croissante des marchés émergents et renforce sa position de leader de l'externalisation des affaires réglementaires en proposant des solutions évolutives et adaptées à chaque région.
- En février 2023, IQVIA a annoncé un partenariat stratégique avec un fabricant européen de dispositifs médicaux afin de soutenir la préparation des dossiers réglementaires et la production de rapports d'évaluation clinique dans le cadre du RDM de l'UE. Cette collaboration souligne la capacité d'IQVIA à fournir des services réglementaires complets et personnalisés et reflète une tendance plus générale des fabricants à recourir à l'externalisation pour gérer les complexités du RDM et atténuer les risques.
- En janvier 2023, Medistri SA, fournisseur suisse de services de technologies médicales, a lancé une solution intégrée de conseil en affaires réglementaires et en gestion de la qualité destinée aux fabricants de dispositifs médicaux de petite et moyenne taille. Cette offre vise à simplifier le parcours de conformité des start-ups et des jeunes entreprises, en leur fournissant un accompagnement économique pour le marquage CE, la préparation des dossiers techniques et la surveillance post-commercialisation. Cette initiative témoigne de l'intérêt croissant du secteur pour des services d'externalisation évolutifs et accessibles, adaptés aux entreprises disposant de ressources réglementaires internes limitées.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
- INTRODUCTION
- OBJECTIVES OF THE STUDY
- MARKET DEFINITION
- OVERVIEW OF GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET
- LIMITATIONS
- MARKETS COVERED
- MARKET SEGMENTATION
- MARKETS COVERED
- GEOGRAPHICAL SCOPE
- YEARS CONSIDERED FOR THE STUDY
- CURRENCY AND PRICING
- DBMR TRIPOD DATA VALIDATION MODEL
- MULTIVARIATE MODELLING
- SERVICES LIFELINE CURVE
- PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
- DBMR MARKET POSITION GRID
- VENDOR SHARE ANALYSIS
- MARKET APPLICATION COVERAGE GRID
- SECONDARY SOURCES
- ASSUMPTIONS
- EXECUTIVE SUMMARY
- PREMIUM INSIGHT
- MARKET OVERVIEW
- DRIVERS
- INCREASING GEOGRAPHICAL EXPANSION ACTIVITIES BY THE MEDICAL DEVICE COMPANIES
- INCREASING ADOPTION OF OUTSOURCING MODELS FOR REGULATORY SERVICES
- INCREASE IN R&D ACTIVITIES BY THE MEDICAL DEVICE COMPANIES
- RISING NUMBER OF CLINICAL TRIALS
- INCREASING NUMBER OF PATENT EXPIRATIONS
- RESTRAINTS
- FLUCTUATION IN PRICES
- HIGH COMPETITION AMONG KEY MARKET PLAYERS
- OPPORTUNITIES
- INCREASING SPENDING ON HEALTHCARE
- END-TO-END REGULATORY EXPERTISE
- AWARDS AND RECOGNITION
- CHALLENGES
- LACK OF ACCESSIBILITY
- PANDEMIC OUTBREAK OF COVID-19
- IMPACT OF COVID-19 ON THE GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET
- IMPACT ON PRICE
- IMPACT ON DEMAND
- IMPACT ON SUPPLY CHAIN
- STRATEGIC DECISIONS OF GOVERNMENT AND MANUFACTURERS
- CONCLUSION
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES
- OVERVIEW
- REGULATORY AFFAIRS SERVICES
- CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS
- CERTIFICATION SERVICES
- ISO 13485 AUDITS
- NOTIFIED BODY (EU)
- GLOBAL MARKET ACCESS
- CB SCHEME
- NRL & SCC (US & CAN)
- MDSAP
- REGULATORY WRITING AND PUBLISHING
- LEGAL REPRESENTATION
- OTHERS
- QUALITY CONSULTING
- MEDICAL WRITING
- CLINICAL WRITING
- REGULATORY WRITING
- SCIENTIFIC WRITING
- OTHERS
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY PRODUCT
- OVERVIEW
- FINISHED GOODS
- ELECTRONICS
- RAW MATERIALS
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY DEVICE TYPE
- OVERVIEW
- CLASS I
- CLASS II
- CLASS III
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY APPLICATION
- OVERVIEW
- CARDIOLOGY
- CLASS III
- CLASS I
- CLASS II
- GENERAL AND PLASTIC SURGERY
- CLASS I
- CLASS II
- CLASS III
- IVD
- CLASS I
- CLASS III
- CLASS II
- ORTHOPAEDIC
- CLASS III
- CLASS I
- CLASS II
- DIAGNOSTIC IMAGING
- CLASS II
- CLASS I
- CLASS III
- DENTAL
- CLASS I
- CLASS III
- CLASS II
- OPHTHALMIC
- CLASS I
- CLASS II
- CLASS III
- ENDOSCOPY
- CLASS II
- CLASS I
- CLASS III
- DIABETES CARE
- CLASS I
- CLASS II
- CLASS III
- DRUG DELIVERY
- CLASS II
- CLASS III
- CLASS I
- OTHERS
- CLASS I
- CLASS II
- CLASS III
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY END USER
- OVERVIEW
- MEDIUM MEDICAL DEVICE COMPANY
- SMALL MEDICAL DEVICE COMPANY
- LARGE MEDICAL DEVICE COMPANY
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY GEOGRAPHY
- OVERVIEW
- NORTH AMERICA
- U.S.
- CANADA
- MEXICO
- EUROPE
- GERMANY
- FRANCE
- U.K.
- ITALY
- SPAIN
- RUSSIA
- TURKEY
- IRELAND
- BELGIUM
- NETHERLANDS
- SWITZERLAND
- REST OF EUROPE
- ASIA-PACIFIC
- JAPAN
- CHINA
- SOUTH KOREA
- INDIA
- AUSTRALIA
- SINGAPORE
- THAILAND
- MALAYSIA
- INDONESIA
- PHILIPPINES
- REST OF ASIA-PACIFIC
- SOUTH AMERICA
- BRAZIL
- ARGENTINA
- REST OF SOUTH AMERICA
- MIDDLE EAST AND AFRICA
- SOUTH AFRICA
- SAUDI ARABIA
- U.A.E.
- EGYPT
- ISRAEL
- REST OF MIDDLE EAST AND AFRICA
- GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: COMPANY LANDSCAPE
- COMPANY SHARE ANALYSIS: GLOBAL
- COMPANY SHARE ANALYSIS: NORTH AMERICA
- COMPANY SHARE ANALYSIS: EUROPE
- COMPANY SHARE ANALYSIS: ASIA-PACIFIC
- SWOT ANALYSIS
- COMPANY PROFILE
- PLEXUS CORP.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANLYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- CELESTICA INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANLYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- FLEX LTD.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- TE CONNECTIVITY
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- COMPANY SHARE ANLYSIS
- INDUSTRY & SOLUTION PORTFOLIO
- RECENT DEVELOPMENTS
- TECOMET, INC.
- COMPANY SNAPSHOT
- COMPANY SHARE ANLYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PAREXEL INTERNATIONAL CORPORATION
- COMPANY SNAPSHOT
- SOLUTION PORTFOLIO
- RECENT DEVELOPMENTS
- SANMINA CORPORATION
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- INDUSTRY PORTFOLIO
- RECENT DEVELOPMENTS
- EUROFINS SCIENTIFIC
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENT
- AMERICAN PRECLINICAL SERVICES, LLC
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- CACTUS COMMUNICATIONS
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- CEKINDO BUSINESS INTERNATIONAL
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- CHARLES RIVER LABORATORIES
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT & SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- COVANCE (A SUBSIDIARY OF LABORATORY CORPORATION OF AMERICA HOLDINGS)
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- CREGANNA (A SUBSIDIARY OF TE CONNECTIVITY)
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT & SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- EAST WEST MANUFACTURING
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- FREYR
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- HERAEUS HOLDING
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- INTEGER HOLDINGS CORPORATION
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- INTERTEK GROUP PLC
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- IQVIA
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- NORTH AMERICAN SCIENCE ASSOCIATES, INC.
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- NORTECH SYSTEMS, INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- OMICS INTERNATIONAL
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- OMRON CORPORATION
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- PACE ANALYTICAL SERVICES, LLC
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENTS
- JABIL INC.
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- SGS SA
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- STERIGENICS U.S., LLC – A SOTERA HEALTH COMPANY
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- TRILOGY WRITING & CONSULTING GMBH
- COMPANY SNAPSHOT
- PRODUCT PORTFOLIO
- RECENT DEVELOPMENT
- TÜV SÜD
- COMPANY SNAPSHOT
- SERVICE PORTFOLIO
- RECENT DEVELOPMENT
- WUXI APPTEC
- COMPANY SNAPSHOT
- REVENUE ANALYSIS
- SERVICE PORTFOLIO
- RECENT DEVELOPMENTS
- QUESTIONNAIRE
- RELATED REPORTS
Liste des tableaux
TABLE 1 Global Medical device regulatory affairs outsourcing market, By services, 2019-2028 (USD million)
TABLE 2 Global Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD Million)
TABLE 3 Global Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 4 Global Clinical Trials Applications and Product Registrations IN Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 5 Global Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 6 Global Quality Consulting service in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD Million)
TABLE 7 Global Medical Writing service in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD Million)
TABLE 8 Global Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 9 Global Medical device regulatory affairs outsourcing market, By product, 2019-2028 (USD million)
TABLE 10 Global Finished Goods products in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 11 Global Electronics products in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 12 Global Raw Materials products in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 13 Global Medical device regulatory affairs outsourcing market, By device type, 2019-2028 (USD million)
TABLE 14 Global class i Device in Medical device regulatory affairs outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 15 Global Class II Device in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 16 Global Class III Device in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 17 Global Medical device regulatory affairs outsourcing market, By application, 2019-2028 (USD million)
TABLE 18 Global Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 19 Global Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 20 Global General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 21 Global General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 22 Global IVD in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 23 Global IVD in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 24 Global Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 25 Global Orthopedic in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 26 Global Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 27 Global Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 28 Global Dental in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 29 Global dental in Medical device regulatory affairs outsourcing market, By device type, 2019-2028 (USD million)
TABLE 30 Global Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 31 Global Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 32 Global Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 33 Global Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 34 Global Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 35 Global Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 36 Global Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 37 Global Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD million)
TABLE 38 Global Others in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 39 Global Others in Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD million)
TABLE 40 Global Medical device regulatory affairs outsourcing market, By end user, 2019-2028 (USD million)
TABLE 41 Global Medium Medical Device Company in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 42 Global Small Medical Device Company in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 43 Global Large Medical Device Company in Medical Device Regulatory Affairs Outsourcing Market, By Region, 2019-2028 (USD million)
TABLE 44 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 45 North America medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD million)
TABLE 46 North America Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 47 North America Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 48 North America CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 49 North America CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 50 North America Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 51 North America Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 52 North America Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 53 North America Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 54 North America Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD Million)
TABLE 55 North America General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 56 North America IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 57 North America Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 58 North America Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 59 North America Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 60 North America Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 61 North America Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 62 North America Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 63 North America Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 64 North America Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 65 North America Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 66 U.S. Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 67 U.S. Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 68 U.S. CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 69 U.S. CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 70 U.S. Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 71 U.S. Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 72 U.S. Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 73 U.S. Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 74 U.S. Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 75 U.S. General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 76 U.S. IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 77 U.S. Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 78 U.S. Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 79 U.S. Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 80 U.S. Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 81 U.S. Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 82 U.S. Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 83 U.S. Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 84 U.S. Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 85 U.S. Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 86 CANADA Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 87 CANADA Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 88 CANADA CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 89 CANADA CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 90 CANADA Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 91 CANADA Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 92 CANADA Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 93 CANADA Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 94 CANADA Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 95 CANADA General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 96 CANADA IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 97 CANADA Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 98 CANADA Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 99 CANADA Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 100 CANADA Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 101 CANADA Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 102 CANADA Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 103 CANADA Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 104 CANADA Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 105 CANADA Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 106 MEXICO Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 107 MEXICO Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 108 MEXICO CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 109 MEXICO CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 110 MEXICO Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 111 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 112 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 113 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 114 MEXICO Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 115 MEXICO General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 116 MEXICO IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 117 MEXICO Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 118 MEXICO Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 119 MEXICO Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 120 MEXICO Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 121 MEXICO Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 122 MEXICO Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 123 MEXICO Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 124 MEXICO Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 125 MEXICO Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 126 Europe medical device regulatory affairs outsourcing Market, By COUNTRY, 2021-2028 (USD million)
TABLE 127 Europe Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 128 Europe Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 129 Europe CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 130 Europe CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 131 Europe Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 132 Europe Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 133 Europe Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 134 Europe Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 135 Europe Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 136 Europe General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 137 Europe IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 138 Europe Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 139 Europe Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 140 Europe Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 141 Europe Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 142 Europe Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 143 Europe Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 144 Europe Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 145 Europe Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 146 Europe Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 147 Germany Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 148 Germany Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 149 Germany CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 150 Germany CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 151 Germany Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 152 Germany Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 153 Germany Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 154 Germany Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 155 Germany Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 156 Germany General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 157 Germany IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 158 Germany Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 159 Germany Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 160 Germany Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 161 Germany Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 162 Germany Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 163 Germany Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 164 Germany Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 165 Germany Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 166 Germany Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 167 France Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 168 France Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 169 France CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 170 France CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 171 France Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 172 France Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 173 France Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 174 France Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 175 France Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 176 France General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 177 France IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 178 France Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 179 France Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 180 France Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 181 France Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 182 France Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 183 France Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 184 France Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 185 France Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 186 France Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 187 U.K. Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 188 U.K. Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 189 U.K. CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 190 U.K. CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 191 U.K. Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 192 U.K. Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 193 U.K. Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 194 U.K. Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 195 U.K. Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 196 U.K. General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 197 U.K. IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 198 U.K. Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 199 U.K. Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 200 U.K. Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 201 U.K. Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 202 U.K. Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 203 U.K. Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 204 U.K. Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 205 U.K. Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 206 U.K. Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 207 Italy Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 208 Italy Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 209 Italy CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 210 Italy CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 211 Italy Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 212 Italy Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 213 Italy Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 214 Italy Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 215 Italy Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 216 Italy General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 217 Italy IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 218 Italy Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 219 Italy Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 220 Italy Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 221 Italy Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 222 Italy Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 223 Italy Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 224 Italy Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 225 Italy Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 226 Italy Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 227 Spain Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 228 Spain Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 229 Spain CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 230 Spain CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 231 Spain Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 232 Spain Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 233 Spain Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 234 Spain Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 235 Spain Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 236 Spain General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 237 Spain IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 238 Spain Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 239 Spain Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 240 Spain Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 241 Spain Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 242 Spain Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 243 Spain Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 244 Spain Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 245 Spain Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 246 Spain Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 247 Russia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 248 Russia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 249 Russia CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 250 Russia CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 251 Russia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 252 Russia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 253 Russia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 254 Russia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 255 Russia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 256 Russia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 257 Russia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 258 Russia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 259 Russia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 260 Russia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 261 Russia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 262 Russia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 263 Russia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 264 Russia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 265 Russia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 266 Russia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 267 Turkey Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 268 Turkey Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 269 Turkey CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 270 Turkey CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 271 Turkey Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 272 Turkey Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 273 Turkey Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 274 Turkey Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 275 Turkey Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 276 Turkey General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 277 Turkey IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 278 Turkey Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 279 Turkey Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 280 Turkey Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 281 Turkey Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 282 Turkey Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 283 Turkey Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 284 Turkey Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 285 Turkey Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 286 Turkey Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 287 Ireland Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 288 Ireland Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 289 Ireland CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 290 Ireland CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 291 Ireland Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 292 Ireland Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 293 Ireland Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 294 Ireland Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 295 Ireland Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 296 Ireland General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 297 Ireland IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 298 Ireland Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 299 Ireland Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 300 Ireland Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 301 Ireland Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 302 Ireland Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 303 Ireland Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 304 Ireland Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 305 Ireland Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 306 Ireland Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 307 Belgium Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 308 Belgium Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 309 Belgium CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 310 Belgium CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 311 Belgium Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 312 Belgium Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 313 Belgium Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 314 Belgium Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 315 Belgium Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 316 Belgium General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 317 Belgium IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 318 Belgium Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 319 Belgium Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 320 Belgium Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 321 Belgium Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 322 Belgium Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 323 Belgium Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 324 Belgium Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 325 Belgium Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 326 Belgium Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 327 Netherlands Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 328 Netherlands Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 329 Netherlands CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 330 Netherlands CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 331 Netherlands Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 332 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 333 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 334 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 335 Netherlands Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 336 Netherlands General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 337 Netherlands IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 338 Netherlands Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 339 Netherlands Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 340 Netherlands Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 341 Netherlands Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 342 Netherlands Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 343 Netherlands Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 344 Netherlands Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 345 Netherlands Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 346 Netherlands Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 347 Switzerland Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 348 Switzerland Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 349 Switzerland CLINICAL TRIALS APPLICATIONS AND PRODUCT REGISTRATIONS IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 350 Switzerland CERTIFICATION SERVICES IN MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET, BY SERVICES, 2019-2028 (USD MILLION)
TABLE 351 Switzerland Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 352 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 353 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 354 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 355 Switzerland Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 356 Switzerland General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 357 Switzerland IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 358 Switzerland Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 359 Switzerland Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 360 Switzerland Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 361 Switzerland Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 362 Switzerland Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 363 Switzerland Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 364 Switzerland Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 365 Switzerland Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 366 Switzerland Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 367 REST OF EUROPE Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 368 Asia-Pacific medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD MILLION)
TABLE 369 Asia-Pacific Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 370 Asia-Pacific Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 371 Asia-Pacific Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 372 Asia-Pacific Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 373 Asia-Pacific Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 374 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 375 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 376 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 377 Asia-Pacific Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By device type, 2019-2028 (USD Million)
TABLE 378 Asia-Pacific General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 379 Asia-Pacific IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 380 Asia-Pacific Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 381 Asia-Pacific Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 382 Asia-Pacific Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 383 Asia-Pacific Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 384 Asia-Pacific Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 385 Asia-Pacific Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 386 Asia-Pacific Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 387 Asia-Pacific Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 388 Asia-Pacific Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 389 Japan Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 390 Japan Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 391 Japan Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 392 Japan Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 393 Japan Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 394 Japan Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 395 Japan Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 396 Japan Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 397 Japan Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 398 Japan General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 399 Japan IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 400 Japan Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 401 Japan Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 402 Japan Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 403 Japan Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 404 Japan Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 405 Japan Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 406 Japan Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 407 Japan Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 408 Japan Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 409 China Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 410 China Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 411 China Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 412 China Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 413 China Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 414 China Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 415 China Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 416 China Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 417 China Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 418 China General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 419 China IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 420 China Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 421 China Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 422 China Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 423 China Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 424 China Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 425 China Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 426 China Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 427 China Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 428 China Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 429 South Korea Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 430 South Korea Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 431 South Korea Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 432 South Korea Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 433 South Korea Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 434 South Korea Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 435 South Korea Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 436 South Korea Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 437 South Korea Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 438 South Korea General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 439 South Korea IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 440 South Korea Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 441 South Korea Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 442 South Korea Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 443 South Korea Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 444 South Korea Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 445 South Korea Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 446 South Korea Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 447 South Korea Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 448 South Korea Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 449 India Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 450 India Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 451 India Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 452 India Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 453 India Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 454 India Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 455 India Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 456 India Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 457 India Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 458 India General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 459 India IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 460 India Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 461 India Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 462 India Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 463 India Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 464 India Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 465 India Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 466 India Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 467 India Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 468 India Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 469 Australia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 470 Australia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 471 Australia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 472 Australia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 473 Australia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 474 Australia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 475 Australia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 476 Australia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 477 Australia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 478 Australia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 479 Australia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 480 Australia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 481 Australia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 482 Australia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 483 Australia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 484 Australia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 485 Australia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 486 Australia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 487 Australia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 488 Australia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 489 Singapore Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 490 Singapore Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 491 Singapore Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 492 Singapore Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 493 Singapore Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 494 Singapore Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 495 Singapore Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 496 Singapore Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 497 Singapore Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 498 Singapore General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 499 Singapore IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 500 Singapore Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 501 Singapore Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 502 Singapore Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 503 Singapore Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 504 Singapore Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 505 Singapore Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 506 Singapore Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 507 Singapore Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 508 Singapore Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 509 Thailand Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 510 Thailand Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 511 Thailand Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 512 Thailand Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 513 Thailand Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 514 Thailand Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 515 Thailand Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 516 Thailand Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 517 Thailand Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 518 Thailand General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 519 Thailand IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 520 Thailand Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 521 Thailand Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 522 Thailand Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 523 Thailand Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 524 Thailand Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 525 Thailand Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 526 Thailand Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 527 Thailand Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 528 Thailand Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 529 Malaysia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 530 Malaysia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 531 Malaysia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 532 Malaysia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 533 Malaysia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 534 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 535 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 536 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 537 Malaysia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 538 Malaysia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 539 Malaysia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 540 Malaysia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 541 Malaysia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 542 Malaysia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 543 Malaysia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 544 Malaysia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 545 Malaysia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 546 Malaysia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 547 Malaysia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 548 Malaysia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 549 Indonesia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 550 Indonesia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 551 Indonesia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 552 Indonesia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 553 Indonesia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 554 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 555 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 556 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 557 Indonesia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 558 Indonesia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 559 Indonesia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 560 Indonesia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 561 Indonesia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 562 Indonesia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 563 Indonesia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 564 Indonesia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 565 Indonesia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 566 Indonesia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 567 Indonesia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 568 Indonesia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 569 Philippines Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 570 Philippines Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 571 Philippines Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 572 Philippines Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 573 Philippines Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 574 Philippines Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 575 Philippines Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 576 Philippines Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 577 Philippines Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 578 Philippines General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 579 Philippines IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 580 Philippines Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 581 Philippines Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 582 Philippines Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 583 Philippines Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 584 Philippines Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 585 Philippines Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 586 Philippines Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 587 Philippines Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 588 Philippines Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 589 Rest of Asia-Pacific Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 590 South America medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD million)
TABLE 591 South America Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 592 South America Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 593 South America Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 594 South America Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 595 South America Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 596 South America Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 597 South America Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 598 South America Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 599 South America Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 600 South America General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 601 South America IVD in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 602 South America Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 603 South America Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 604 South America Dental in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 605 South America Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 606 South America Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 607 South America Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 608 South America Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 609 South America Others in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 610 South America Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 611 Brazil Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 612 Brazil Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 613 Brazil Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 614 Brazil Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 615 Brazil Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 616 Brazil Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 617 Brazil Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 618 Brazil Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 619 Brazil Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 620 Brazil General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 621 Brazil IVD in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 622 Brazil Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 623 Brazil Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 624 Brazil Dental in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 625 Brazil Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 626 Brazil Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 627 Brazil Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 628 Brazil Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 629 Brazil Others in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 630 Brazil Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 631 Argentina Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 632 Argentina Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 633 Argentina Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 634 Argentina Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 635 Argentina Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 636 Argentina Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 637 Argentina Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 638 Argentina Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 639 Argentina Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 640 Argentina General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 641 Argentina IVD in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 642 Argentina Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 643 Argentina Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 644 Argentina Dental in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 645 Argentina Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 646 Argentina Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 647 Argentina Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 648 Argentina Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 649 Argentina Others in Medical Device Regulatory Affairs Outsourcing Market, By DEVICE TYPE, 2019-2028 (USD Million)
TABLE 650 Argentina Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 651 Rest of South America Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 652 Middle East and Africa medical device regulatory affairs outsourcing Market, By COUNTRY, 2019-2028 (USD million)
TABLE 653 Middle East and Africa Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 654 Middle East and Africa Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 655 Middle East and Africa Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 656 Middle East and Africa Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 657 Middle East and Africa Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 658 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 659 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 660 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 661 Middle East and Africa Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 662 Middle East and Africa General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 663 Middle East and Africa IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 664 Middle East and Africa Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 665 Middle East and Africa Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 666 Middle East and Africa Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 667 Middle East and Africa Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 668 Middle East and Africa Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 669 Middle East and Africa Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 670 Middle East and Africa Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 671 Middle East and Africa Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 672 Middle East and Africa Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 673 South Africa Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 674 South Africa Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 675 South Africa Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 676 South Africa Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 677 South Africa Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 678 South Africa Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 679 South Africa Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 680 South Africa Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 681 South Africa Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 682 South Africa General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 683 South Africa IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 684 South Africa Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 685 South Africa Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 686 South Africa Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 687 South Africa Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 688 South Africa Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 689 South Africa Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 690 South Africa Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 691 South Africa Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 692 South Africa Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 693 Saudi Arabia Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 694 Saudi Arabia Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 695 Saudi Arabia Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 696 Saudi Arabia Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 697 Saudi Arabia Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 698 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 699 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 700 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 701 Saudi Arabia Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 702 Saudi Arabia General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 703 Saudi Arabia IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 704 Saudi Arabia Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 705 Saudi Arabia Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 706 audi Arabia Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 707 Saudi Arabia Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 708 Saudi Arabia Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 709 Saudi Arabia Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 710 Saudi Arabia Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 711 Saudi Arabia Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 712 Saudi Arabia Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 713 U.A.E. Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 714 U.A.E. Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 715 U.A.E. Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 716 U.A.E. Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 717 U.A.E. Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 718 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 719 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 720 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 721 U.A.E. Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 722 U.A.E. General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 723 U.A.E. IVD in Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 724 U.A.E. Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 725 U.A.E. Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 726 U.A.E. Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 727 U.A.E. Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 728 U.A.E. Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 729 U.A.E. Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 730 U.A.E. Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 731 U.A.E. Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 732 U.A.E. Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 733 Egypt Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 734 Egypt Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 735 Egypt Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 736 Egypt Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 737 Egypt Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 738 Egypt Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 739 Egypt Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 740 Egypt Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 741 Egypt Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 742 Egypt General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 743 Egypt IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 744 Egypt Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 745 Egypt Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 746 Egypt Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 747 Egypt Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 748 Egypt Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 749 Egypt Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 750 Egypt Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 751 Egypt Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 752 Egypt Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 753 Israel Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 754 Israel Regulatory Affairs Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 755 Israel Clinical Trials Applications and Product Registrations in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 756 Israel Certification Services in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 757 Israel Medical Writing in Medical Device Regulatory Affairs Outsourcing Market, By Services, 2019-2028 (USD Million)
TABLE 758 Israel Medical Device Regulatory Affairs Outsourcing Market, By Product, 2019-2028 (USD Million)
TABLE 759 Israel Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 760 Israel Medical Device Regulatory Affairs Outsourcing Market, By Application, 2019-2028 (USD Million)
TABLE 761 Israel Cardiology in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 762 Israel General and Plastic Surgery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 763 Israel IVD in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 764 Israel Orthopaedic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 765 Israel Diagnostic Imaging in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 766 Israel Dental in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 767 Israel Ophthalmic in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 768 Israel Endoscopy in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 769 Israel Diabetes Care in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 770 Israel Drug Delivery in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 771 Israel Others in Medical Device Regulatory Affairs Outsourcing Market, By Device Type, 2019-2028 (USD Million)
TABLE 772 Israel Medical Device Regulatory Affairs Outsourcing Market, By End User, 2019-2028 (USD Million)
TABLE 773 Rest of Middle East and Africa Medical device regulatory affairs outsourcing Market, By Services, 2019-2028 (USD Million)
Liste des figures
FIGURE 1 Global MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: SEGMENTATION
FIGURE 2 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: DATA TRIANGULATION
FIGURE 3 global Medical device regulatory affairs outsourcing market: DROC ANALYSIS
FIGURE 4 Global Medical device regulatory affairs outsourcing market: Global VS. REGIONAL MARKET ANALYSIS
FIGURE 5 Global Medical device regulatory affairs outsourcing market: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL Medical device regulatory affairs outsourcing market: MULTIVARIATE MODELLING
FIGURE 7 GLOBAL Medical device regulatory affairs outsourcing market: INTERVIEW DEMOGRAPHICS
FIGURE 8 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL medical device regulatory affairs outsourcing market: MARKET application COVERAGE GRID
FIGURE 11 Global Medical device regulatory affairs outsourcing market: SEGMENTATION
FIGURE 12 NORTH AMERICA is expected to DOMINATE THE GLOBAL Medical device regulatory affairs outsourcing market, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 13 Increasing adoption of outsourcing models for regulatory services is DRIVing THE global Medical device regulatory affairs outsourcing market IN THE FORECAST PERIOD OF 2021 to 2028
FIGURE 14 Regulatory Affairs Services SEGMENT is expected to account for the largest share of the global MEDICAL DEVICE REGULATORY AFFAIRS OUTSOURCING MARKET in 2021 & 2028
FIGURE 15 ASIA-PACIFIC is the fastest growing market FOR the Medical device regulatory affairs outsourcing MANUFACTURERS IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGEs OF global Medical Device Regulatory Affairs Outsourcing Market
FIGURE 17 Global Medical device regulatory affairs outsourcing market: By services, 2020
FIGURE 18 Global Medical device regulatory affairs outsourcing market: By services, 2021-2028 (USD Million)
FIGURE 19 Global Medical device regulatory affairs outsourcing market: By services, CAGR (2021-2028)
FIGURE 20 Global Medical device regulatory affairs outsourcing market: By services, LIFELINE CURVE
FIGURE 21 Global Medical device regulatory affairs outsourcing market: By product, 2020
FIGURE 22 Global Medical device regulatory affairs outsourcing market: By product, 2021-2028 (USD million)
FIGURE 23 Global Medical device regulatory affairs outsourcing market: By product, CAGR (2021-2028)
FIGURE 24 Global Medical device regulatory affairs outsourcing market: By product, LIFELINE CURVE
FIGURE 25 Global Medical device regulatory affairs outsourcing market: By device type, 2020
FIGURE 26 Global Medical device regulatory affairs outsourcing market: By device type, 2021-2028 (USD million)
FIGURE 27 Global Medical device regulatory affairs outsourcing market: By device type, CAGR (2021-2028)
FIGURE 28 Global Medical device regulatory affairs outsourcing market: By device type, LIFELINE CURVE
FIGURE 29 Global Medical device regulatory affairs outsourcing market: By application, 2020
FIGURE 30 Global Medical device regulatory affairs outsourcing market: By application, 2021-2028 (USD million)
FIGURE 31 Global Medical device regulatory affairs outsourcing market: By application, CAGR (2021-2028)
FIGURE 32 Global Medical device regulatory affairs outsourcing market: By application, LIFELINE CURVE
FIGURE 33 Global Medical device regulatory affairs outsourcing market: By end user, 2020
FIGURE 34 Global Medical device regulatory affairs outsourcing market: By end user, 2021-2028 (USD million)
FIGURE 35 Global Medical device regulatory affairs outsourcing market: By end user, CAGR (2021-2028)
FIGURE 36 Global Medical device regulatory affairs outsourcing market: By end user, LIFELINE CURVE
FIGURE 37 GLobal medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 38 global medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 39 global medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2022 & 2028)
FIGURE 40 global medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 41 global medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 42 North America medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 43 North America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 44 North America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 45 North America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 46 North America medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 47 Europe medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 48 Europe medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 49 Europe medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 50 Europe medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 51 Europe medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 52 Asia-Pacific medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 53 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 54 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 55 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 56 Asia-Pacific medical device regulatory affairs outsourcing MARKET: BY SERVICES (2021-2028)
FIGURE 57 South America medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 58 South America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 59 South America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 60 South America medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 61 South America medical device regulatory affairs outsourcing MARKET: BY services (2021-2028)
FIGURE 62 Middle East and Africa medical device regulatory affairs outsourcing MARKET: SNAPSHOT (2020)
FIGURE 63 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020)
FIGURE 64 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2021 & 2028)
FIGURE 65 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY COUNTRY (2020 & 2028)
FIGURE 66 Middle East and Africa medical device regulatory affairs outsourcing MARKET: BY Services (2021-2028)
FIGURE 67 Global Medical device regulatory affairs outsourcing Market: company share 2020 (%)
FIGURE 68 North America Medical device regulatory affairs outsourcing Market: company share 2020 (%)
FIGURE 69 Europe Medical device regulatory affairs outsourcing Market: company share 2020 (%)
FIGURE 70 Asia-Pacific Medical device regulatory affairs outsourcing Market: company share 2020 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.