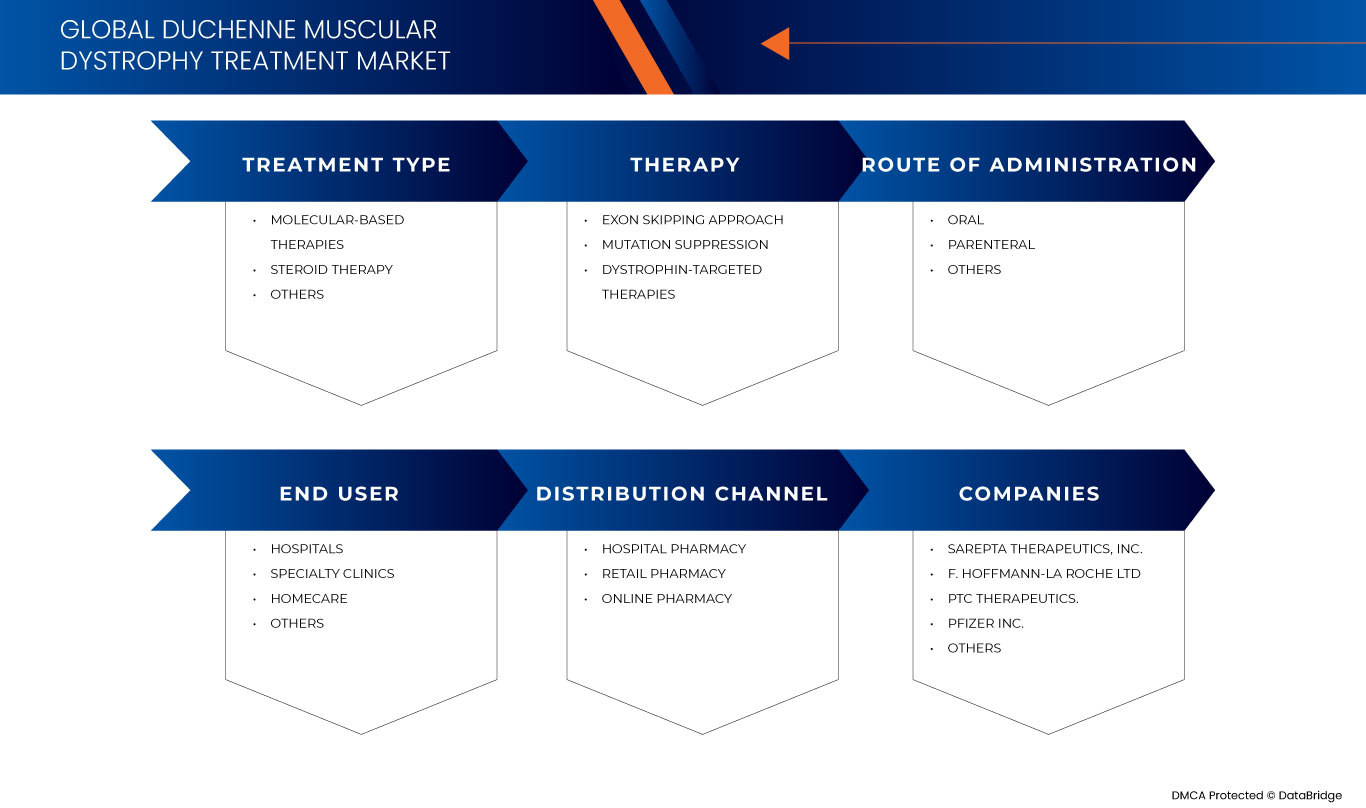
Marché mondial du traitement de la dystrophie musculaire de Duchenne, par type de traitement (thérapies moléculaires, thérapie aux stéroïdes et autres), thérapie (approche par saut d'exon, suppression des mutations et thérapies ciblant la dystrophine), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile et autres), canal de distribution (pharmacie hospitalière, pharmacie de détail et pharmacie en ligne) - Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et taille du marché du traitement de la dystrophie musculaire de Duchenne
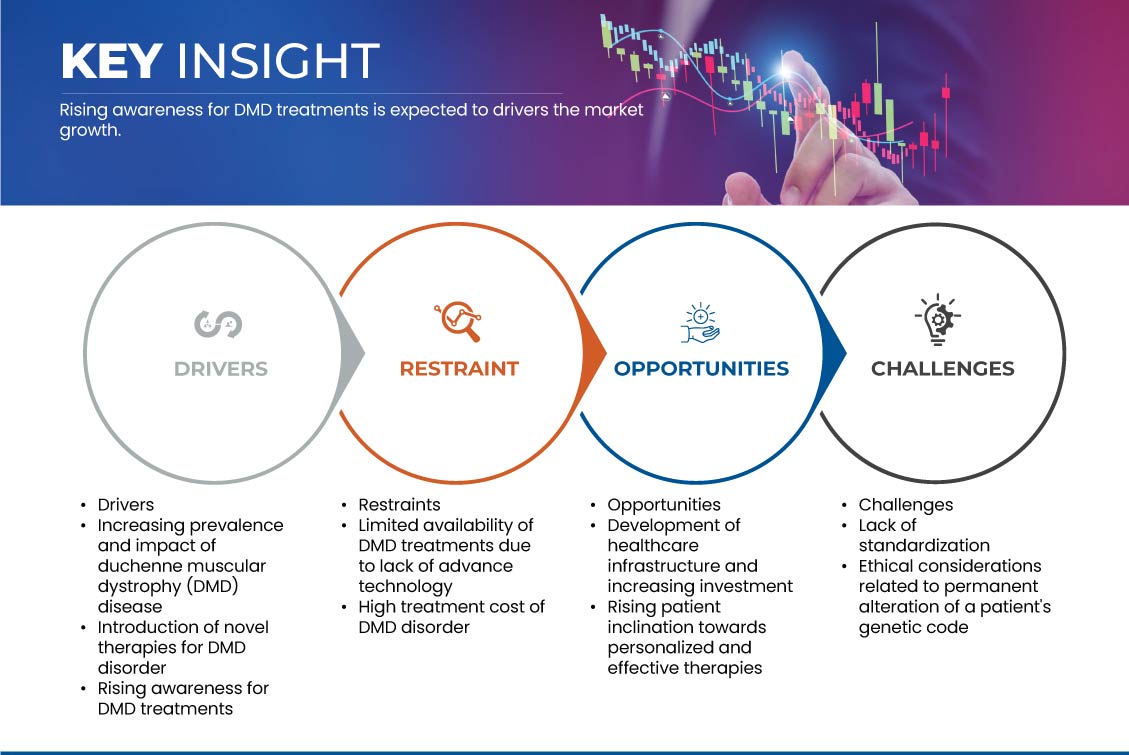
Les principaux facteurs qui devraient stimuler la croissance du marché sont la sensibilisation croissante au traitement de la DMD et l'introduction de nouvelles thérapies pour le trouble de DMD. En outre, la prévalence et l'impact croissants du trouble de DMD sont un autre facteur clé qui devrait stimuler la croissance du marché. L'augmentation du nombre d'essais cliniques est une tendance récente qui devrait également stimuler la croissance du marché. Cependant, la disponibilité limitée du traitement de la DMD en raison de la technologie avancée et le coût élevé du traitement du trouble de DMD devraient freiner la croissance du marché.
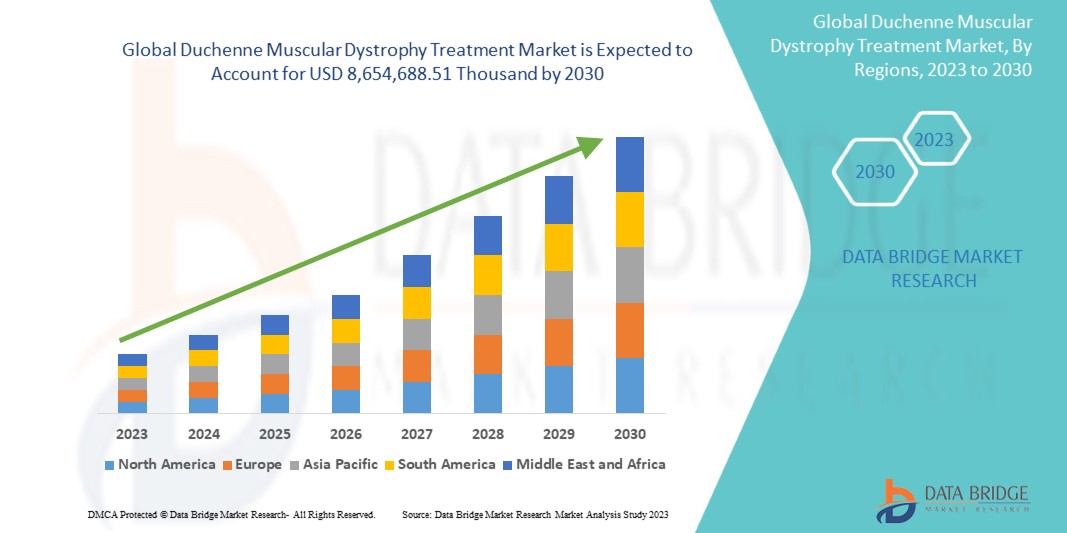
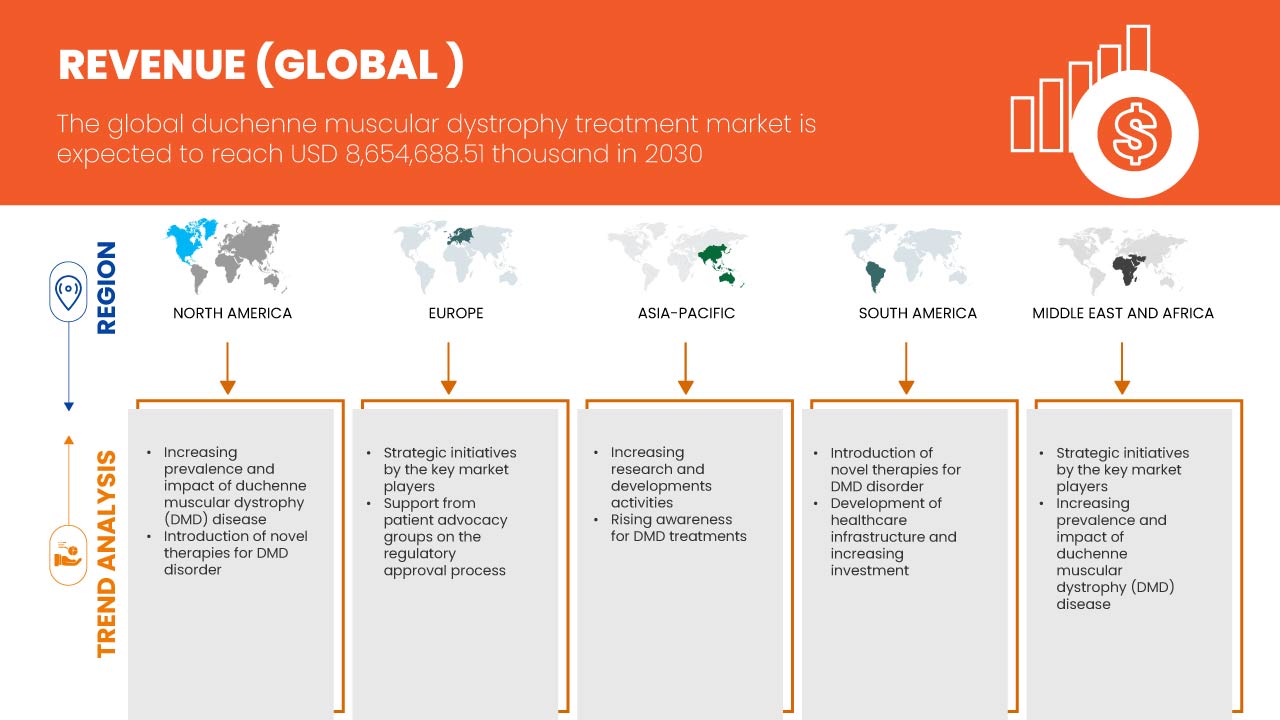
Data Bridge Market Research estime que le marché mondial du traitement de la dystrophie musculaire de Duchenne devrait atteindre 8 654 688,51 milliers USD d'ici 2030, à un TCAC de 16,8 % au cours de la période de prévision 2023-2030. Ce rapport de marché couvre également en profondeur l'analyse des prix et les avancées technologiques.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable de 2015 à 2020) |
|
Unités quantitatives |
Chiffre d'affaires en milliers de dollars américains |
|
Segments couverts |
Type de traitement (thérapies moléculaires, thérapie aux stéroïdes et autres), thérapie (approche par saut d'exon, suppression des mutations et thérapies ciblant la dystrophine), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile et autres), canal de distribution (pharmacie hospitalière, pharmacie de détail et pharmacie en ligne) |
|
Pays couverts |
États-Unis, Canada, Mexique, Allemagne, France, Royaume-Uni, Italie, Espagne, Russie, Turquie, Belgique, Pays-Bas, Suisse, Reste de l'Europe, Japon, Chine, Corée du Sud, Inde, Australie, Singapour, Thaïlande, Malaisie, Indonésie, Philippines, Reste de l'Asie-Pacifique, Brésil, Argentine, Reste de l'Amérique du Sud, Afrique du Sud, Arabie saoudite, Émirats arabes unis, Égypte, Israël et Reste du Moyen-Orient et de l'Afrique |
|
Acteurs du marché couverts |
Sarepta Therapeutics, Inc., GSK plc., Capricor Therapeutics, Inc., Dyne Therapeutics, Solid Biosciences Inc., BioMarin, Stealth BioTherapeutics Inc., Avidity Biosciences, ReveraGen BioPharma, Inc. PTC Therapeutics., NS Pharma, Inc., ITALFARMACO SpA, FibroGen, Inc., SANTHERA PHARMACEUTICALS, Pfizer Inc., F. Hoffmann-La Roche Ltd, Akashi RX et TAIHO PHARMACEUTICAL CO., LTD, entre autres |
Définition du marché
La dystrophie musculaire de Duchenne (DMD) est une maladie génétique rare caractérisée par une dégénérescence et une faiblesse musculaires progressives. Le traitement principal de la DMD consiste à utiliser des corticostéroïdes, tels que la prednisone ou le déflazacort. Ces médicaments aident à réduire l'inflammation et à retarder la dégénérescence musculaire, prolongeant ainsi la capacité à marcher et préservant la fonction musculaire. Il a été démontré que le traitement aux corticostéroïdes améliore la force musculaire, la fonction respiratoire et la qualité de vie globale des personnes atteintes de DMD.
Dynamique du marché mondial du traitement de la dystrophie musculaire de Duchenne
Cette section traite de la compréhension des moteurs, des avantages, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
- Augmentation de la prévalence et de l'impact de la dystrophie musculaire de Duchenne (DMD)
La dystrophie musculaire de Duchenne (DMD) est une maladie génétique rare et invalidante caractérisée par une faiblesse musculaire progressive et une perte de fonction. Elle touche principalement les hommes, les symptômes apparaissant généralement dans la petite enfance. Le nombre de personnes diagnostiquées avec la DMD est également en augmentation, et cette prévalence croissante a créé un besoin de traitements et de thérapies efficaces, alors que la population mondiale continue de croître.
La prévalence croissante de la DMD a également accru les efforts de sensibilisation des patients et des professionnels de la santé. Ces groupes jouent un rôle essentiel dans la sensibilisation à la DMD, la défense du financement de la recherche et l'approbation plus rapide des traitements potentiels. La communauté DMD est en pleine croissance, par conséquent, l'avancement des technologies et des investissements dans ce domaine de la santé est également important.
- Introduction de nouvelles thérapies pour le trouble DMD
L’émergence d’approches thérapeutiques innovantes conduit à une augmentation des soins pour la DMD et contribue de manière significative à l’expansion du marché. Les options de traitement traditionnelles se concentraient principalement sur la gestion des symptômes et les soins de soutien. Cependant, l’introduction de nouvelles thérapies représente un changement de paradigme car elles visent à s’attaquer aux mutations génétiques fondamentales responsables de la DMD.
De plus, les médicaments sautant des exons sont apparus comme une autre classe prometteuse de thérapies pour la DMD. Ces médicaments sont conçus pour « sauter » des exons spécifiques dans le gène de la dystrophine, permettant la production d'une protéine dystrophine tronquée mais partiellement fonctionnelle. Ces médicaments peuvent ralentir considérablement la progression de la maladie et améliorer la qualité de vie des personnes atteintes de DMD. Le développement et l'approbation de ces thérapies ont suscité de nouvelles attentes chez les patients atteints de DMD et devraient encore stimuler la croissance du marché.
Opportunité
- Développement des infrastructures de santé et augmentation des investissements
Les patients atteints de DMD peuvent être diagnostiqués plus tôt grâce à une infrastructure de soins de santé plus solide, notamment des hôpitaux et des installations de diagnostic bien équipés. Un diagnostic précoce permet une intervention et un traitement rapides, ce qui peut ralentir la progression de la maladie et améliorer les résultats pour les patients. Les systèmes de santé développés disposent souvent de centres et de cliniques spécialisés dédiés aux maladies rares, telles que la DMD. Ces centres offrent des soins complets, notamment l'accès à des professionnels de santé spécialisés, à la physiothérapie et à des services de soutien, qui peuvent améliorer la qualité de vie des patients atteints de DMD.
Les systèmes de santé développés proposent souvent une large gamme de services de soutien, tels que la physiothérapie et l’ergothérapie , les appareils d’assistance et les conseils, qui peuvent améliorer considérablement la qualité de vie des patients atteints de DMD. Les gouvernements, les investisseurs privés et les organisations philanthropiques sont plus susceptibles d’investir dans le développement de médicaments pour les maladies rares, comme la DMD, lorsqu’il existe une infrastructure de soins de santé bien établie. Cet investissement peut soutenir la recherche, les essais cliniques et le développement de thérapies innovantes.
Retenue/Défi
- Disponibilité limitée des traitements contre la DMD en raison du manque de technologie avancée
Les options de traitement disponibles pour la DMD sont limitées, laissant aux patients et à leurs familles peu d’options pour ralentir la progression de la maladie ou améliorer leur bien-être général.
Les progrès technologiques se traduisent souvent par la mise au point de traitements hautement spécialisés et innovants pour la DMD, tels que les thérapies géniques ou les approches de médecine personnalisée. Ces thérapies de pointe sont complexes à développer, à fabriquer et à administrer. Le développement des thérapies géniques, en particulier, implique des processus complexes et des techniques de fabrication spécialisées. Ces thérapies nécessitent la modification ou le remplacement de gènes défectueux pour traiter la cause sous-jacente de la DMD, ce qui entraîne des coûts de traitement élevés. En outre, le nombre limité d'installations de fabrication capables de produire des thérapies géniques contribue encore davantage à leur disponibilité limitée.
La complexité des traitements avancés contre la DMD peut rendre leur fabrication et leur administration difficiles, ce qui contribue encore davantage à leur disponibilité limitée. Des installations et une expertise spécialisées peuvent être nécessaires pour la production, et l'administration de ces traitements peut exiger des professionnels de santé spécialisés, qui peuvent être rares dans certaines régions.
Développements récents
- En mars 2023, Dyne Therapeutics a annoncé que DYNE-251, un traitement expérimental pour les mutations de la dystrophie musculaire de Duchenne (DMD) sensibles au saut de l'exon 51, avait obtenu la désignation de médicament orphelin et de maladie pédiatrique rare de la FDA américaine. DYNE-251 est en cours d'évaluation dans le cadre de l'essai clinique de phase 1/2 DELIVER. Cela aide l'organisation à augmenter sa catégorie de produits et son chiffre d'affaires global.
- En août 2022, FibroGen, Inc. a annoncé les principales données de l'essai de phase 3 LELANTOS-2 sur le pamrevlumab pour le traitement des patients ambulatoires atteints de DMD sous corticostéroïdes systémiques de fond. Cela a aidé l'entreprise à renforcer son portefeuille de produits en cours de développement.
Portée du marché mondial du traitement de la dystrophie musculaire de Duchenne
Le marché mondial du traitement de la dystrophie musculaire de Duchenne est segmenté en cinq segments notables en fonction du type de traitement, de la thérapie, de la voie d'administration, de l'utilisateur final et du canal de distribution. La croissance de ces segments vous aidera à analyser les segments de croissance limités dans les industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour les aider à prendre des décisions stratégiques pour identifier les principales applications du marché.
Type de traitement
- Thérapies moléculaires
- Thérapie aux stéroïdes
- Autres
En fonction du type de traitement, le marché est segmenté en thérapies moléculaires, thérapie aux stéroïdes et autres.
Thérapie
- Approche par saut d'exon
- Suppression des mutations
- Thérapies ciblant la dystrophine
Sur la base de la thérapie, le marché est segmenté en approche de saut d’exon, de suppression de mutation et de thérapies ciblant la dystrophine.
Voie d'administration
- Oral
- Parentérale
- Autres
Sur la base de la voie d’administration, le marché est segmenté en voie orale, parentérale et autres.
Utilisateur final
- Hôpitaux
- Soins à domicile
- Cliniques spécialisées
- Autres
Sur la base de l’utilisateur final, le marché est segmenté en hôpitaux, soins de santé à domicile, cliniques spécialisées et autres.
Canal de distribution
- Pharmacie de l'hôpital
- Pharmacie en ligne
- Pharmacie de détail
Sur la base du canal de distribution, le marché est segmenté en pharmacie hospitalière, pharmacie en ligne et pharmacie de détail .
Analyse/perspectives régionales du marché mondial du traitement de la dystrophie musculaire de Duchenne
Le marché mondial du traitement de la dystrophie musculaire de Duchenne est segmenté en cinq segments notables en fonction du type de traitement, de la thérapie, de la voie d’administration, de l’utilisateur final et du canal de distribution.
Les pays couverts dans ce rapport sur le marché mondial du traitement de la dystrophie musculaire de Duchenne sont les États-Unis, le Canada, le Mexique, l'Allemagne, la France, le Royaume-Uni, l'Italie, l'Espagne, la Russie, la Turquie, la Belgique, les Pays-Bas, la Suisse, le reste de l'Europe, le Japon, la Chine, la Corée du Sud, l'Inde, l'Australie, Singapour, la Thaïlande, la Malaisie, l'Indonésie, les Philippines, le reste de l'Asie-Pacifique, le Brésil, l'Argentine, le reste de l'Amérique du Sud, l'Afrique du Sud, l'Arabie saoudite, les Émirats arabes unis, l'Égypte, Israël et le reste du Moyen-Orient et de l'Afrique.
Les États-Unis devraient dominer l'Amérique du Nord en raison de la sensibilisation croissante et du dépistage de la DMD. L'Allemagne devrait dominer l'Europe en raison de la prévalence croissante de la dystrophie musculaire et de la pénétration de nouvelles avancées technologiques. La Chine devrait dominer l'Asie-Pacifique, car les initiatives stratégiques des principaux acteurs du marché se développent considérablement.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que l'analyse de la chaîne de valeur en aval et en amont, les tendances techniques, l'analyse des cinq forces de Porter et les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques mondiales et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des tarifs nationaux et les routes commerciales sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché mondiales du traitement de la dystrophie musculaire de Duchenne
Le paysage concurrentiel du marché mondial du traitement de la dystrophie musculaire de Duchenne fournit des détails sur le concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les nouvelles initiatives du marché, la présence mondiale, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises par rapport au marché.
Français Certains des principaux acteurs du marché opérant sur le marché mondial du traitement de la dystrophie musculaire de Duchenne sont Sarepta Therapeutics, Inc., GSK plc., Capricor Therapeutics, Inc., Dyne Therapeutics, Solid Biosciences Inc., BioMarin, Stealth BioTherapeutics Inc., Avidity Biosciences, ReveraGen BioPharma, Inc. PTC Therapeutics., NS Pharma, Inc, ITALFARMACO SpA, FibroGen, Inc, SANTHERA PHARMACEUTICALS, Pfizer Inc., F. Hoffmann-La Roche Ltd, Akashi RX et TAIHO PHARMACEUTICAL CO., LTD, entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 TREATMENT TYPE SEGMENT LIFELINE CURVE
2.8 MARKET END USER COVERAGE GRID
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL'S MODEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 PRICING ANALYSIS
5 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: REGULATIONS
5.1 REGULATIONS IN U.S.
5.2 REGULATTIONS IN EUROPE
5.3 REGULATTIONS IN AUSTRALIA
5.4 REGULATIONS IN SOUTH AFRICA
5.5 REGULATIONS IN BRAZIL
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND IMPACT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) DISEASE
6.1.2 INTRODUCTION OF NOVEL THERAPIES FOR DMD DISORDER
6.1.3 RISING AWARENESS FOR DMD TREATMENTS
6.1.4 INCREASE IN THE NUMBER OF CLINICAL TRIALS IS A RECENT TREND
6.2 RESTRAINTS
6.2.1 LIMITED AVAILABILITY OF DMD TREATMENTS DUE TO LACK OF ADVANCE TECHNOLOGY
6.2.2 HIGH TREATMENT COST OF DMD DISORDER
6.3 OPPORTUNITIES
6.3.1 DEVELOPMENT OF HEALTHCARE INFRASTRUCTURE AND INCREASING INVESTMENT
6.3.2 RISING PATIENT INCLINATION TOWARDS PERSONALIZED AND EFFECTIVE THERAPIES
6.3.3 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYERS
6.3.4 SUPPORT FROM PATIENT ADVOCACY GROUPS ON THE REGULATORY APPROVAL PROCESS
6.4 CHALLENGES
6.4.1 LACK OF STANDARDIZATION IN DMD DIAGNOSIS
6.4.2 ETHICAL CONSIDERATIONS RELATED TO PERMANENT ALTERATION OF A PATIENT'S GENETIC CODE
7 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE
7.1 OVERVIEW
7.2 MOLECULAR-BASED THERAPIES
7.2.1 ANTISENSE OLIGONUCLEOTIDE THERAPY
7.2.1.1 EXONDYS 51
7.2.1.2 AMONDYS 45
7.2.1.3 VYONDYS 53
7.3 NONSENSE MUTATION
7.3.1.1 Translarna
7.4 STEROID THERAPY
7.4.1 PREDNISONE
7.4.2 DEFLAZACORT
7.5 OTHERS
8 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY
8.1 OVERVIEW
8.2 EXON SKIPPING APPROACH
8.2.1 MULTI-EXON SKIPPING APPROACH
8.2.2 SINGLE-EXON SKIPPING APPROACH
8.3 MUTATION SUPPRESSION
8.4 DYSTROPHIN-TARGETED THERAPIES
8.4.1 GENE THERAPIES
8.4.2 CELL THERAPIES
8.4.2.1 Gene Editing
8.4.2.2 Gene Addition
9 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.3 ORAL
9.4 OTHERS
10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOMECARE
10.5 OTHERS
11 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 HOSPITAL PHARMACY
11.3 RETAIL PHARMACY
11.4 ONLINE PHARMACY
12 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION
12.1 OVERVIEW
12.2 NORTH AMERICA
12.2.1 U.S.
12.2.2 CANADA
12.2.3 MEXICO
12.3 EUROPE
12.3.1 GERMANY
12.3.2 FRANCE
12.3.3 U.K.
12.3.4 ITALY
12.3.5 SPAIN
12.3.6 RUSSIA
12.3.7 TURKEY
12.3.8 BELGIUM
12.3.9 NETHERLANDS
12.3.10 SWITZERLAND
12.3.11 REST OF EUROPE
12.4 ASIA-PACIFIC
12.4.1 CHINA
12.4.2 JAPAN
12.4.3 INDIA
12.4.4 SOUTH KOREA
12.4.5 AUSTRALIA
12.4.6 SINGAPORE
12.4.7 THAILAND
12.4.8 MALAYSIA
12.4.9 INDONESIA
12.4.10 PHILIPPINES
12.4.11 REST OF ASIA-PACIFIC
12.5 SOUTH AMERICA
12.5.1 BRAZIL
12.5.2 ARGENTINA
12.5.3 REST OF SOUTH AMERICA
12.6 MIDDLE EAST AND AFRICA
12.6.1 SOUTH AFRICA
12.6.2 SAUDI ARABIA
12.6.3 U.A.E
12.6.4 EGYPT
12.6.5 ISRAEL
12.6.6 REST OF MIDDLE EAST AND AFRICA
13 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: GLOBAL
14 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
15 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
17 SWOT ANALYSIS
18 COMPANY PROFILES
18.1 SAREPTA THERAPEUTICS, INC.
18.1.1 COMPANY SNAPSHOT
18.1.1 REVENUE ANALYSIS
18.1.2 COMPANY SHARE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 PIPELINE PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 F. HOFFMANN-LA ROCHE LTD
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 PIPELINE PORTFOLIO
18.2.6 RECENT DEVELOPMENTS
18.3 PTC THERAPEUTICS.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENT
18.4 PFIZER INC.
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PIPELINE PORTFOLIO
18.4.5 PRODUCT PORTFOLIO
18.4.6 RECENT DEVELOPMENT
18.5 AKASHI RX
18.5.1 COMPANY SNAPSHOT
18.5.2 PIPELINE PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 AVIDITY BIOSCIENCES
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PIPELINE PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 BIOMARIN
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PIPELINE PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 CAPRICOR THERAPEUTICS, INC.
18.8.1 COMPANY SNAPSHOT
18.8.2 PIPELINE PORTFOLIO
18.8.3 RECENT DEVELOPMENTS
18.9 DYNE THERAPEUTICS
18.9.1 COMPANY SNAPSHOT
18.9.2 PIPELINE PORTFOLIO
18.9.3 RECENT DEVELOPMENTS
18.1 FIBROGEN, INC.
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PIPELINE PORTFOLIO
18.10.4 RECENT DEVELOPMENT
18.11 ITALFARMACO S.P.A.
18.11.1 COMPANY SNAPSHOT
18.11.2 PIPELINE PORTFOLIO
18.11.3 RECENT DEVELOPMENT
18.12 NS PHARMA, INC.
18.12.1 COMPANY SNAPSHOT
18.12.2 PIPELINE PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 REVERAGEN BIOPHARMA, INC.
18.13.1 COMPANY SNAPSHOT
18.13.2 PIPELINE PORTFOLIO
18.13.3 RECENT DEVELOPMENT
18.14 SANTHERA PHARMACEUTICALS
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PIPELINE PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 TAIHO PHARMACEUTICAL CO., LTD.
18.15.1 COMPANY SNAPSHOT
18.15.2 PIPELINE PORTFOLIO
18.15.3 RECENT DEVELOPMENTS
18.16 SOLID BIOSCIENCES INC.
18.16.1 COMPANY SNAPSHOT
18.16.2 PIPELINE PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 STEALTH BIOTHERAPEUTICS INC
18.17.1 COMPANY SNAPSHOT
18.17.2 PIPELINE PORTFOLIO
18.17.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
Liste des tableaux
TABLE 1 LIST OF PRICES FOR APPROVED DRUGS OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
TABLE 2 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE , 2021-2030 (USD THOUSAND)
TABLE 3 GLOBAL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 4 GLOBAL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 5 GLOBAL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 6 GLOBAL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 7 GLOBAL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 8 GLOBAL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 9 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 11 GLOBAL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 12 GLOBAL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 13 GLOBAL MUTATION SUPPRESSION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 14 GLOBAL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 15 GLOBAL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 16 GLOBAL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 17 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION , 2021-2030 (USD THOUSAND)
TABLE 18 GLOBAL PARENTERAL IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 19 GLOBAL ORAL IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 20 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 21 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USERS, 2021-2030 (USD THOUSAND)
TABLE 22 GLOBAL HOSPITALS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 23 GLOBAL SPECIALITY CLINICS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 24 GLOBAL HOMECARE IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 25 GLOBAL OTHERS IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 26 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 27 GLOBAL HOSPITAL PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 28 GLOBAL RETAIL PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 29 GLOBAL ONLINE PHARMACY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 30 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY REGION, 2021-2030 (USD THOUSAND)
TABLE 31 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND) COUNTRY
TABLE 32 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 33 NORTH AMERICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 34 NORTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 35 NORTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 36 NORTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 37 NORTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 38 NORTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 39 NORTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 40 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 41 NORTH AMERICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 42 NORTH AMERICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 43 NORTH AMERICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 44 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 45 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 46 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 47 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 48 U.S. MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 49 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 50 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 51 U.S. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 52 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 53 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 54 U.S. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 55 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 56 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 57 U.S. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 58 U.S.DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 59 U.S. EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 60 U.S. DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 61 U.S. CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 62 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 63 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 64 U.S. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 65 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 66 CANADA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 67 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 68 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 69 CANADA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 70 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 71 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 72 CANADA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 73 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 74 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 75 CANADA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 76 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 77 CANADA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 78 CANADA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 79 CANADA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 80 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 81 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 82 CANADA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 83 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 84 MEXICO MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 85 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 86 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 87 MEXICO ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 88 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 89 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 90 MEXICO NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 91 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 92 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 93 MEXICO STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 94 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 95 MEXICO EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 96 MEXICO DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 97 MEXICO CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 98 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 99 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 100 MEXICO DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 101 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 102 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 103 EUROPE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 104 EUROPE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 105 EUROPE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 106 EUROPE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 107 EUROPE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 108 EUROPE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 109 EUROPE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 110 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 111 EUROPE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 112 EUROPE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 113 EUROPE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 114 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 115 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 116 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 117 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 118 GERMANY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 119 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 120 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 121 GERMANY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 122 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 123 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 124 GERMANY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 125 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 126 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 127 GERMANY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 128 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 129 GERMANY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 130 GERMANY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 131 GERMANY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 132 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 133 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 134 GERMANY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 135 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 136 FRANCE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 137 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 138 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 139 FRANCE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 140 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 141 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 142 FRANCE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 143 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 144 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 145 FRANCE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 146 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 147 FRANCE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 148 FRANCE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 149 FRANCE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 150 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 151 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 152 FRANCE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 153 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 154 U.K. MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 155 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 156 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 157 U.K. ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 158 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 159 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 160 U.K. NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 161 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 162 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 163 U.K. STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 164 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 165 U.K. EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 166 U.K. DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 167 U.K. CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 168 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 169 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 170 U.K. DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 171 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 172 ITALY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 173 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 174 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 175 ITALY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 176 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 177 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 178 ITALY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 179 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 180 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 181 ITALY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 182 ITALYDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 183 ITALY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 184 ITALY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 185 ITALY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 186 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 187 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 188 ITALY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 189 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 190 SPAIN MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 191 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 192 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 193 SPAIN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 194 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 195 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 196 SPAIN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 197 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 198 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 199 SPAIN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 200 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 201 SPAIN EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 202 SPAIN DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 203 SPAIN CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 204 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 205 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 206 SPAIN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 207 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 208 RUSSIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 209 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 210 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 211 RUSSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 212 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 213 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 214 RUSSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 215 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 216 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 217 RUSSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 218 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 219 RUSSIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 220 RUSSIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 221 RUSSIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 222 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 223 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 224 RUSSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 225 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 226 TURKEY MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 227 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 228 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 229 TURKEY ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 230 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 231 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 232 TURKEY NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 233 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 234 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 235 TURKEY STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 236 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 237 TURKEY EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 238 TURKEY DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 239 TURKEY CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 240 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 241 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 242 TURKEY DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 243 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 244 BELGIUM MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 245 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 246 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 247 BELGIUM ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 248 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 249 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 250 BELGIUM NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 251 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 252 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 253 BELGIUM STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 254 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 255 BELGIUM EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 256 BELGIUM DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 257 BELGIUM CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 258 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 259 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 260 BELGIUM DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 261 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 262 NETHERLANDS MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 263 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 264 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 265 NETHERLANDS ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 266 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 267 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 268 NETHERLANDS NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 269 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 270 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 271 NETHERLANDS STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 272 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 273 NETHERLANDS EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 274 NETHERLANDS DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 275 NETHERLANDS CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 276 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 277 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 278 NETHERLANDS DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 279 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 280 SWITZERLAND MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 281 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 282 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 283 SWITZERLAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 284 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 285 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 286 SWITZERLAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 287 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 288 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 289 SWITZERLAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 290 SWITZERLANDDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 291 SWITZERLAND EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 292 SWITZERLAND DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 293 SWITZERLAND CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 294 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 295 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 296 SWITZERLAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 297 REST OF EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 298 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 299 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 300 ASIA-PACIFIC MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 301 ASIA-PACIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 302 ASIA-PACIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 303 ASIA-PACIFIC NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 304 ASIA-PACIFIC NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 305 ASIA-PACIFIC STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 306 ASIA-PACIFIC STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 307 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 308 ASIA-PACIFIC EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 309 ASIA-PACIFIC DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 310 ASIA-PACIFIC CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 311 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 312 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 313 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 314 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 315 CHINA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 316 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 317 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 318 CHINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 319 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 320 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 321 CHINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 322 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 323 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 324 CHINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 325 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 326 CHINA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 327 CHINA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 328 CHINA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 329 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 330 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 331 CHINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 332 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 333 JAPAN MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 334 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 335 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 336 JAPAN ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 337 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 338 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 339 JAPAN NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 340 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 341 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 342 JAPAN STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 343 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 344 JAPAN EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 345 JAPAN DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 346 JAPAN CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 347 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 348 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 349 JAPAN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 350 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 351 INDIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 352 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 353 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 354 INDIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 355 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 356 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 357 INDIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 358 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 359 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 360 INDIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 361 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 362 INDIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 363 INDIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 364 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 365 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 366 INDIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 367 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 368 SOUTH KOREA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 369 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 370 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 371 SOUTH KOREA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 372 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 373 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 374 SOUTH KOREA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 375 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 376 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 377 SOUTH KOREA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 378 SOUTH KOREADUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 379 SOUTH KOREA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 380 SOUTH KOREA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 381 SOUTH KOREA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 382 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 383 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 384 SOUTH KOREA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 385 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 386 AUSTRALIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 387 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 388 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 389 AUSTRALIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 390 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 391 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 392 AUSTRALIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 393 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 394 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 395 AUSTRALIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 396 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 397 AUSTRALIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 398 AUSTRALIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 399 AUSTRALIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 400 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 401 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 402 AUSTRALIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 403 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 404 SINGAPORE MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 405 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 406 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 407 SINGAPORE ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 408 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 409 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 410 SINGAPORE NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 411 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 412 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 413 SINGAPORE STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 414 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 415 SINGAPORE EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 416 SINGAPORE DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 417 SINGAPORE CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 418 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 419 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 420 SINGAPORE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 421 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 422 THAILAND MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 423 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 424 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 425 THAILAND ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 426 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 427 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 428 THAILAND NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 429 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 430 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 431 THAILAND STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 432 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 433 THAILAND EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 434 THAILAND DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 435 THAILAND CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 436 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 437 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 438 THAILAND DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 439 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 440 MALAYSIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 441 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 442 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 443 MALAYSIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 444 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 445 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 446 MALAYSIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 447 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 448 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 449 MALAYSIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 450 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 451 MALAYSIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 452 MALAYSIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 453 MALAYSIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 454 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 455 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 456 MALAYSIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 457 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 458 INDONESIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 459 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 460 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 461 INDONESIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 462 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 463 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 464 INDONESIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 465 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 466 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 467 INDONESIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 468 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 469 INDONESIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 470 INDONESIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 471 INDONESIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 472 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 473 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 474 INDONESIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 475 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 476 PHILIPPINES MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 477 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 478 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 479 PHILIPPINES ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 480 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 481 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 482 PHILIPPINES NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 483 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 484 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 485 PHILIPPINES STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 486 PHILIPPINESDUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 487 PHILIPPINES EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 488 PHILIPPINES DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 489 PHILIPPINES CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 490 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 491 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 492 PHILIPPINES DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 493 REST OF ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 494 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 495 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 496 SOUTH AMERICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 497 SOUTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 498 SOUTH AMERICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 499 SOUTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 500 SOUTH AMERICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 501 SOUTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 502 SOUTH AMERICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 503 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 504 SOUTH AMERICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 505 SOUTH AMERICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 506 SOUTH AMERICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 507 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 508 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 509 SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 510 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 511 BRAZIL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 512 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 513 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 514 BRAZIL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 515 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 516 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 517 BRAZIL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 518 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 519 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 520 BRAZIL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 521 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 522 BRAZIL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 523 BRAZIL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 524 BRAZIL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 525 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 526 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 527 BRAZIL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 528 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 529 ARGENTINA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 530 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 531 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 532 ARGENTINA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 533 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 534 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 535 ARGENTINA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 536 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 537 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 538 ARGENTINA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 539 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 540 ARGENTINA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 541 ARGENTINA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 542 ARGENTINA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 543 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 544 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 545 ARGENTINA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 546 REST OF SOUTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 547 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY COUNTRY, 2021-2030 (USD THOUSAND)
TABLE 548 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 549 MIDDLE EAST AND AFRICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 550 MIDDLE EAST AND AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 551 MIDDLE EAST AND AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 552 MIDDLE EAST AND AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 553 MIDDLE EAST AND AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 554 MIDDLE EAST AND AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 555 MIDDLE EAST AND AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 556 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 557 MIDDLE EAST AND AFRICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 558 MIDDLE EAST AND AFRICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 559 MIDDLE EAST AND AFRICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 560 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 561 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 562 MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 563 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 564 SOUTH AFRICA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 565 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 566 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 567 SOUTH AFRICA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 568 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 569 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 570 SOUTH AFRICA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 571 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 572 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 573 SOUTH AFRICA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 574 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 575 SOUTH AFRICA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 576 SOUTH AFRICA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 577 SOUTH AFRICA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 578 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 579 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 580 SOUTH AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 581 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 582 SAUDI ARABIA MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 583 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 584 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 585 SAUDI ARABIA ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 586 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 587 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 588 SAUDI ARABIA NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 589 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 590 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 591 SAUDI ARABIA STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 592 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 593 SAUDI ARABIA EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 594 SAUDI ARABIA DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 595 SAUDI ARABIA CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 596 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 597 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 598 SAUDI ARABIA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 599 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 600 U.A.E MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 601 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 602 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 603 U.A.E ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 604 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 605 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 606 U.A.E NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 607 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 608 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 609 U.A.E STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 610 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 611 U.A.E EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 612 U.A.E DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 613 U.A.E CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 614 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 615 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 616 U.A.E DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 617 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 618 EGYPT MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 619 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 620 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 621 EGYPT ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 622 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 623 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 624 EGYPT NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 625 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 626 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 627 EGYPT STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 628 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 629 EGYPT EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 630 EGYPT DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 631 EGYPT CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 632 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 633 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 634 EGYPT DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 635 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 636 ISRAEL MOLECULAR-BASED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 637 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 638 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 639 ISRAEL ANTISENSE OLIGONUCLEOTIDE THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 640 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 641 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 642 ISRAEL NONSENSE MUTATION IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 643 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
TABLE 644 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (VOLUME)
TABLE 645 ISRAEL STEROID THERAPY IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (ASP)
TABLE 646 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 647 ISRAEL EXON SKIPPING APPROACH IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 648 ISRAEL DYSTROPHIN-TARGETED THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 649 ISRAEL CELL THERAPIES IN DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY THERAPY, 2021-2030 (USD THOUSAND)
TABLE 650 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD THOUSAND)
TABLE 651 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY END USER, 2021-2030 (USD THOUSAND)
TABLE 652 ISRAEL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD THOUSAND)
TABLE 653 REST OF MIDDLE EAST AND AFRICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET, BY TREATMENT TYPE, 2021-2030 (USD THOUSAND)
Liste des figures
FIGURE 1 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: SEGMENTATION
FIGURE 2 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DATA TRIANGULATION
FIGURE 3 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE AND IMPACT OF DUCHENNE MUSCULAR DYSTROPHY (DMD) AND INTRODUCTION OF NOVEL THERAPIES FOR DMD DISORDER ARE EXPECTED TO DRIVE THE GROWTH OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET FROM 2023 TO 2030
FIGURE 12 MOLECULAR-BASED THERAPIES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET
FIGURE 14 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE , 2022
FIGURE 15 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE, 2023-2030 (USD THOUSAND)
FIGURE 16 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE , CAGR (2023-2030)
FIGURE 17 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY TREATMENT TYPE, LIFELINE CURVE
FIGURE 18 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, 2022
FIGURE 19 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, 2023-2030 (USD THOUSAND)
FIGURE 20 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, CAGR (2023-2030)
FIGURE 21 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 22 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 23 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD THOUSAND)
FIGURE 24 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 25 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 26 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, 2022
FIGURE 27 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, 2023-2030 (USD THOUSAND)
FIGURE 28 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD THOUSAND)
FIGURE 32 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET SNAPSHOT
FIGURE 35 GLOBAL DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 36 NORTH AMERICA DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 37 EUROPE DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)
FIGURE 38 ASIA-PACIFIC DUCHENNE MUSCULAR DYSTROPHY TREATMENT MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.