Marché européen du diagnostic des accidents vasculaires cérébraux, par gravité (modérée, grave, légère), type (tomodensitométrie (CT Scan), angiographie par tomodensitométrie (CTA), imagerie par résonance magnétique (IRM), angiographie par résonance magnétique (ARM), échographie Doppler transcrânienne , test d'impulsion vidéo de la tête (VHIT), autres), application (accident vasculaire cérébral ischémique, accident vasculaire cérébral hémorragique, attaques ischémiques transitoires (TIAS)), utilisateur final (hôpitaux, cliniques, centres de chirurgie ambulatoire, soins à domicile), canal de distribution (appel d'offres direct, distributeurs tiers, autres), stade (préopératoire, périopératoire, postopératoire), pays (Allemagne, France, Royaume-Uni, Italie, Espagne, Russie, Pays-Bas, Suisse, Turquie, Autriche, Belgique et reste de l'Europe), tendances et prévisions de l'industrie jusqu'en 2028
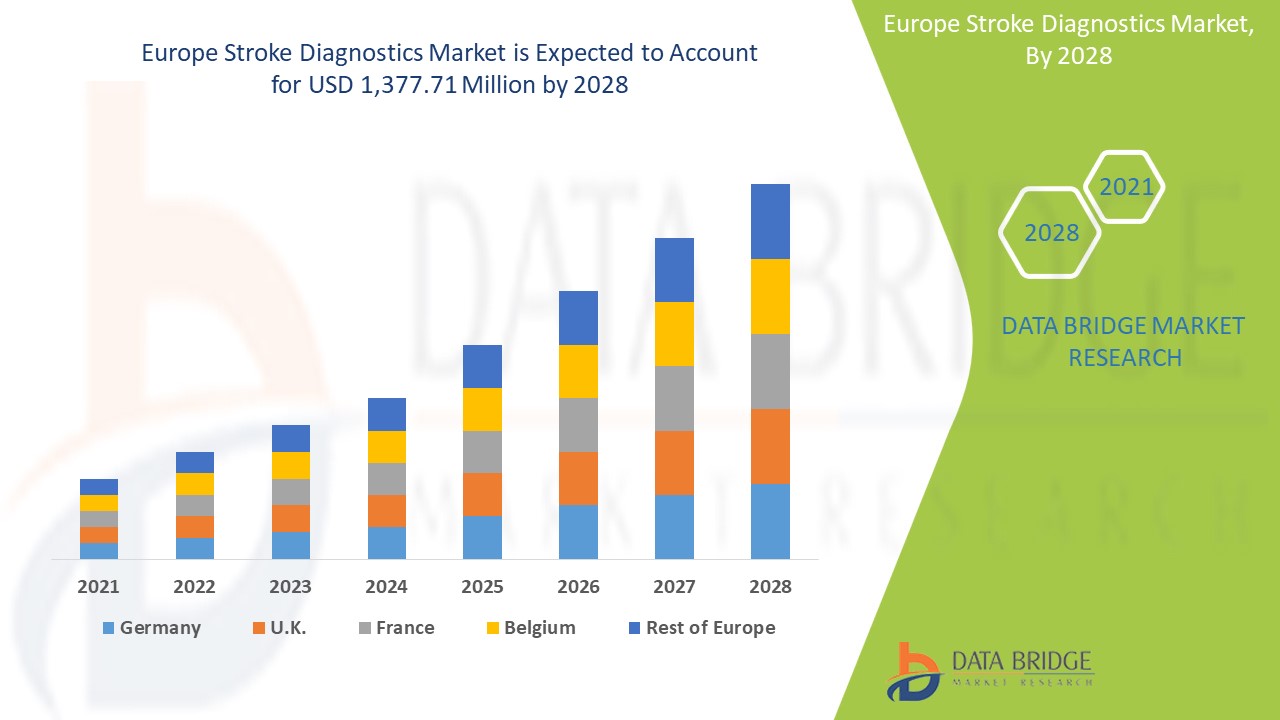 Analyse et perspectives du marché : marché européen du diagnostic des accidents vasculaires cérébraux
Analyse et perspectives du marché : marché européen du diagnostic des accidents vasculaires cérébraux
Le marché européen du diagnostic des accidents vasculaires cérébraux devrait connaître une croissance exponentielle au cours de la période de prévision de 2021 à 2028. Data Bridge Market Research analyse que le marché connaît une croissance de 6,0 % au cours de la période de prévision de 2021 à 2028 et devrait atteindre 1 377,71 millions USD d'ici 2028. L'augmentation du développement de produits et la demande croissante de diagnostic précoce sont les principaux moteurs de la demande du marché au cours de la période de prévision. Cependant, des réglementations strictes peuvent entraver la croissance du marché.
Un accident vasculaire cérébral survient lorsque l'apport sanguin au cerveau est diminué ou complètement bloqué, ce qui empêche le tissu cérébral de recevoir de l'oxygène et des nutriments. Différents types d'appareils de diagnostic sont utilisés pour détecter un accident vasculaire cérébral et ses premiers symptômes, par exemple la tomodensitométrie (TDM), l'angiographie par tomodensitométrie (CTA), l'imagerie par résonance magnétique (IRM), l'angiographie par résonance magnétique (ARM), l'échographie Doppler transcrânienne et d'autres.
L'incidence croissante des accidents vasculaires cérébraux et des maladies cardiovasculaires et neurologiques a accru la demande de diagnostics d'accidents vasculaires cérébraux dans les pays en développement. En outre, l'augmentation des dépenses de santé stimule la croissance du marché. D'un autre côté, le coût élevé du diagnostic peut freiner la croissance du marché. La présence d'acteurs sur le marché et le lancement de nouveaux produits offrent une opportunité. Cependant, le manque de professionnels qualifiés peut constituer un défi pour le marché.
Le rapport sur le marché du diagnostic des accidents vasculaires cérébraux fournit des détails sur la part de marché, les nouveaux développements, l'impact des acteurs du marché national et local, l'analyse des opportunités en termes de poches de revenus émergentes, les changements dans la réglementation du marché, les approbations de produits , les décisions stratégiques, les lancements de produits, les expansions géographiques et les innovations technologiques sur le marché. Pour comprendre l'analyse et le scénario du marché, contactez-nous pour un briefing d'analyste. Notre équipe vous aidera à créer une solution d'impact sur les revenus pour atteindre votre objectif souhaité.
 Portée et taille du marché du diagnostic des accidents vasculaires cérébraux en Europe
Portée et taille du marché du diagnostic des accidents vasculaires cérébraux en Europe
Le marché européen du diagnostic des accidents vasculaires cérébraux est divisé en six segments notables qui sont basés sur la gravité, le type, l'application, l'utilisateur final, le canal de distribution et le stade. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
- En fonction de la gravité, le marché européen du diagnostic des accidents vasculaires cérébraux est segmenté en modéré, sévère et léger. En 2021, le segment modéré devrait dominer le marché en raison de l'augmentation des dépenses de santé et des établissements de santé.
- Sur la base du type, le marché européen du diagnostic des accidents vasculaires cérébraux est segmenté en tomodensitométrie (CT Scan), angiographie par tomodensitométrie (CTA), imagerie par résonance magnétique (IRM), angiographie par résonance magnétique (ARM), échographie Doppler transcrânienne, test d'impulsion vidéo de la tête (VHIT), autres. En 2021, le segment de la tomodensitométrie (CT Scan) devrait dominer le marché grâce à l'amélioration croissante de la technologie, qui fournit des résultats précis en moins de temps.
- En fonction des applications, le marché européen du diagnostic des accidents vasculaires cérébraux est segmenté en accidents vasculaires cérébraux ischémiques, accidents vasculaires cérébraux hémorragiques et accidents ischémiques transitoires (AIT). En 2021, l'AVC ischémique devrait dominer le marché grâce à l'amélioration croissante de la technologie de diagnostic des AVC.
- Sur la base des utilisateurs finaux, le marché européen du diagnostic des accidents vasculaires cérébraux est segmenté en hôpitaux, soins à domicile, centres de chirurgie ambulatoire et cliniques. En 2021, le segment hospitalier devrait dominer le marché avec la présence de tous les appareils de diagnostic pour le diagnostic.
- Sur la base du canal de distribution, le marché européen du diagnostic des accidents vasculaires cérébraux est segmenté en appels d'offres directs, distributeurs tiers et autres. En 2021, les appels d'offres directs devraient dominer le marché en raison de la présence de divers acteurs majeurs du marché.
- En fonction du stade de développement, le marché européen du diagnostic des accidents vasculaires cérébraux est segmenté en préopératoire, périopératoire et postopératoire. En 2021, le préopératoire devrait dominer le marché en raison de l'incidence croissante des accidents vasculaires cérébraux et des maladies cardiovasculaires.
Analyse du marché européen du diagnostic des accidents vasculaires cérébraux (AVC) au niveau des pays
Le marché européen du diagnostic des accidents vasculaires cérébraux est analysé et des informations sur la taille du marché sont fournies en six segments notables, à savoir la gravité, le type, l’application, l’utilisateur final, le canal de distribution et le stade.
Les pays couverts dans le rapport sur le marché du diagnostic des accidents vasculaires cérébraux sont l'Allemagne, le Royaume-Uni, la France, l'Italie, l'Espagne, les Pays-Bas, la Russie, la Suisse, la Belgique, la Turquie, l'Autriche, la Norvège, la Hongrie, la Lituanie, l'Irlande, la Pologne et le reste de l'Europe.
Le segment du diagnostic des accidents vasculaires cérébraux en Europe devrait connaître le taux de croissance le plus élevé au cours de la période de prévision de 2021 à 2028 en raison de la demande croissante de diagnostics des accidents vasculaires cérébraux. L'Allemagne devrait dominer le marché européen du diagnostic des accidents vasculaires cérébraux en raison de la croissance de la R&D dans le secteur de la santé.
La section par pays du rapport fournit également des facteurs d'impact sur les marchés individuels et des changements de réglementation sur le marché national qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie des pays, les actes réglementaires et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques européennes et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des canaux de vente sont pris en compte tout en fournissant une analyse prévisionnelle des données nationales.
L'augmentation des dépenses de santé stimule la croissance du marché des diagnostics d'AVC
Le marché du diagnostic des accidents vasculaires cérébraux vous fournit également une analyse de marché détaillée de la croissance de chaque pays dans le secteur du diagnostic des accidents vasculaires cérébraux avec les ventes de médicaments de diagnostic des accidents vasculaires cérébraux, l'impact des progrès de la technologie de diagnostic des accidents vasculaires cérébraux et les changements dans les scénarios réglementaires avec leur soutien au marché du diagnostic des accidents vasculaires cérébraux. Les données sont disponibles pour la période historique de 2010 à 2019.
Analyse du paysage concurrentiel et des parts de marché du diagnostic des accidents vasculaires cérébraux
Le paysage concurrentiel du marché du diagnostic des accidents vasculaires cérébraux fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, les sites et installations de production, les forces et les faiblesses de l'entreprise, le lancement du produit, les pipelines d'essais de produits, les approbations de produits, les brevets, la largeur et l'étendue du produit, la domination des applications, la courbe de survie technologique. Les points de données ci-dessus fournis ne concernent que l'orientation de l'entreprise vers le marché du diagnostic des accidents vasculaires cérébraux.
Les principales entreprises présentes sur le marché européen du diagnostic des accidents vasculaires cérébraux sont Koninklijke Philips NV, Stryker, Hologic, Inc., ESAOTE SPA, Shimadzu Corporation, Aspect Imaging Ltd, Siemens, Neusoft Corporation, Medfield diagnostics AB, FUJIFILM Holdings Corporation, General Electric Company, ALPINION MEDICAL SYSTEMS Co., Ltd, Shenzhen Mindray Bio-Medical Electronics Co., Ltd, Analogic Corporation, Carestream Health, BPL Medical Technologies, IMRIS, Deerfield Imaging Inc., Canon Inc., SAMSUNG, SternMed GmbH, entre autres.
De nombreux lancements de produits et accords sont également initiés par des entreprises du monde entier, ce qui accélère également le marché du diagnostic des accidents vasculaires cérébraux.
Par exemple,
- En mai 2021, Siemens a annoncé le lancement du nouveau Somatom X.ceed1, un nouveau scanner CT (tomodensitométrie) haute résolution et haute vitesse conçu spécifiquement pour les domaines cliniques les plus difficiles. Il a permis à l'entreprise d'élargir son portefeuille de produits
- En juillet 2021, Koninklijke Philips NV a annoncé son partenariat avec Cognizant pour développer des solutions de santé numériques de bout en bout qui permettront aux organisations de soins de santé et aux entreprises des sciences de la vie d'améliorer les soins aux patients et d'accélérer les essais cliniques. Cela renforcera leur avancée technologique et augmentera leurs ventes
La collaboration, le lancement de produits, l'expansion commerciale, les récompenses et la reconnaissance, les coentreprises et d'autres stratégies des acteurs du marché améliorent le marché de l'entreprise sur le marché du diagnostic des accidents vasculaires cérébraux, ce qui offre également à l'organisation l'avantage d'améliorer son offre de diagnostic des accidents vasculaires cérébraux.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE STROKE DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 EUROPE STROKE DIAGNOSTICS MARKET: REGULATIONS
6.1 REGULATION IN THE U.S.
6.2 REGULATION IN EUROPE
6.3 REGULATION IN CHINA
6.4 REGULATION IN JAPAN
6.5 REGULATION IN SOUTH AFRICA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 TECHNOLOGICAL ADVANCEMENT
7.1.2 INCREASE IN THE INCIDENCE OF STROKE
7.1.3 INCREASE IN THE GERIATRIC POPULATION
7.1.4 INCREASE IN NUMBER OF PATIENTS WITH HYPERTENSION AND CORONARY HEART DISEASES
7.2 RESTRAINTS
7.2.1 HIGH COST OF DIAGNOSIS
7.2.2 INCREASE IN PRODUCT RECALL
7.3 OPPORTUNITIES
7.3.1 RISE IN HEALTHCARE SPENDING
7.3.2 INCREASE IN DIABETIC POPULATION AND OBESITY
7.3.3 INCREASE IN FDA APPROVAL AND PRODUCT LAUNCH
7.3.4 RISE IN AWARENESS REGARDING HEALTH AND STROKE
7.4 CHALLENGES
7.4.1 UNFAVORABLE REIMBURSEMENT SCENARIO
7.4.2 COMPLICATION RELATED TO DIAGNOSTIC DEVICES
8 IMPACT OF COVID-19 ON EUROPE STROKE DIAGNOSTICS MARKET
8.1 IMPACT ON PRICE
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC INITIATIVES
8.5 CONCLUSION
9 EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY
9.1 OVERVIEW
9.2 MODERATE
9.3 SEVERE
9.4 MILD
10 EUROPE STROKE DIAGNOSTICS MARKET, BY TYPE
10.1 OVERVIEW
10.2 COMPUTED TOMOGRAPHY (CT SCAN)
10.3 COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA)
10.4 MAGNETIC RESONANCE IMAGING (MRI)
10.5 MAGNETIC RESONANCE ANGIOGRAPHY (MRA)
10.6 TRANSCRANIAL DOPPLER ULTRASOUND
10.7 VIDEO HEAD IMPULSE TEST (VHIT)
10.8 OTHERS
10.8.1 CAROTID ULTRASOUND
10.8.2 CAROTID ANGIOGRAPHY
10.8.3 ELECTROCARDIOGRAPHY (EKG)
10.8.4 ECHOCARDIOGRAPHY
10.8.5 BLOOD TESTS
10.8.6 NUCLEAR NEUROIMAGING
10.8.7 OTHERS
11 EUROPE STROKE DIAGNOSTICS MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 ISCHEMIC STROKE
11.2.1 THROMBOTIC STROKES
11.2.2 EMBOLIC STROKES
11.3 HEMORRHAGIC STROKE
11.3.1 INTRACEREBRAL HEMORRHAGE
11.3.2 SUBARACHNOID HEMORRHAGE
11.4 TRANSIENT ISCHEMIC ATTACKS (TIAS)
12 EUROPE STROKE DIAGNOSTICS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.3 CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 HOME HEALTHCARE
12.6 OTHERS
13 EUROPE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTORS
13.4 OTHERS
14 EUROPE STROKE DIAGNOSTICS MARKET, BY STAGE
14.1 OVERVIEW
14.2 PRE OPERATIVE
14.3 PERI OPERATIVE
14.4 POST OPERATIVE
15 EUROPE STROKE DIAGNOSTICS MARKET, BY REGION
15.1 EUROPE
15.1.1 GERMANY
15.1.2 FRANCE
15.1.3 U.K.
15.1.4 ITALY
15.1.5 SPAIN
15.1.6 RUSSIA
15.1.7 NETHERLANDS
15.1.8 SWITZERLAND
15.1.9 TURKEY
15.1.10 AUSTRIA
15.1.11 BELGIUM
15.1.12 REST OF EUROPE
16 STROKE DIAGNOSTICS MARKET COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: EUROPE
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 SIEMENS
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 KONINKLIJKE PHILIPS N.V.
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GENERAL ELECTRIC COMPANY
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENTS
18.4 CANON INC
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
18.6 ALPINION MEDICAL SYSTEMS CO., LTD
18.6.1 COMPANY SNAPSHOT
18.6.2 PRODUCT PORTFOLIO
18.6.3 RECENT DEVELOPMENTS
18.7 ANALOGIC CORPORATION
18.7.1 COMPANY SNAPSHOT
18.7.2 PRODUCT PORTFOLIO
18.7.3 RECENT DEVELOPMENT
18.8 ASPECT IMAGING LTD
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BPL MEDICAL TECHNOLOGIES
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENT
18.1 CARESTREAM HEALTH
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENTS
18.11 ESAOTE SPA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 FONAR CORP
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENT
18.13 FUJIFILM HOLDINGS CORPORATION
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 HOLOGIC, INC
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENTS
18.15 IMRIS, DEERFIELD IMAGING INC.
18.15.1 COMPANY SNAPSHOT
18.15.2 PRODUCT PORTFOLIO
18.15.3 RECENT DEVELOPMENT
18.16 MEDFIELD DIAGNOSTICS AB
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MEDTRON AG
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENT
18.18 NEUSOFT CORPORATION
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 SAMSUNG
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 PRODUCT PORTFOLIO
18.19.4 RECENT DEVELOPMENTS
18.2 SHENXHEN ANKE HIGH-TECH CO., LTD.
18.20.1 COMPANY SNAPSHOT
18.20.2 PRODUCT PORTFOLIO
18.20.3 RECENT DEVELOPMENT
18.21 SHIMADZU CORPORATION
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENTS
18.22 SIUI
18.22.1 COMPANY SNAPSHOT
18.22.2 PRODUCT PORTFOLIO
18.22.3 RECENT DEVELOPMENTS
18.23 STERNMED GMBH
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 STRYKER
18.24.1 COMPANY SNAPSHOT
18.24.2 REVENUE ANALYSIS
18.24.3 PRODUCT PORTFOLIO
18.24.4 RECENT DEVELOPMENTS
18.25 TERASON DIVISION TERATECH CORPORATION
18.25.1 COMPANY SNAPSHOT
18.25.2 PRODUCT PORTFOLIO
18.25.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
Liste des tableaux
TABLE 1 EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 2 EUROPE MODERATE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 3 EUROPE SEVERE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 4 EUROPE MILD IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 5 EUROPE STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 6 EUROPE COMPUTED TOMOGRAPHY (CT SCAN) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 7 EUROPE COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 8 EUROPE MAGNETIC RESONANCE IMAGING (MRI) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 9 EUROPE MAGNETIC RESONANCE ANGIOGRAPHY (MRA) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 10 EUROPE TRANSCRANIAL DOPPLER ULTRASOUND IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 11 EUROPE VIDEO HEAD IMPULSE TEST (VHIT) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 12 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 13 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 14 EUROPE STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 15 EUROPE ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 16 EUROPE ISCHEMIC STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 17 EUROPE HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 18 EUROPE HEMORRHAGIC STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 19 EUROPE TRANSIENT ISCHEMIC ATTACKS (TIAS) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 20 EUROPE STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 21 EUROPE HOSPITAL IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 22 EUROPE CLINICS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 23 EUROPE AMBULATORY SURGICAL CENTERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 24 EUROPE HOME HEALTHCARE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 25 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 26 EUROPE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 27 EUROPE DIRECT TENDER IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 28 EUROPE THIRD PARTY DISTRIBUTORS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 29 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 30 EUROPE STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 31 EUROPE PRE OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 32 EUROPE PERI OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 33 EUROPE POST OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 34 EUROPE STROKE DIAGNOSTICS MARKET, BY COUNTRY, 2019-2028 (USD MILLION)
TABLE 35 EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 36 EUROPE STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 37 EUROPE OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 38 EUROPE STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 39 EUROPE ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 40 EUROPE HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 41 EUROPE STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 42 EUROPE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 43 EUROPE STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 44 GERMANY STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 45 GERMANY STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 46 GERMANY OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 47 GERMANY STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 48 GERMANY ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 49 GERMANY HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 50 GERMANY STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 51 GERMANY STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 52 GERMANY STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 53 FRANCE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 54 FRANCE STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 55 FRANCE OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 56 FRANCE STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 57 FRANCE ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 58 FRANCE HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 59 FRANCE STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 60 FRANCE STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 61 FRANCE STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 62 U.K. STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 63 U.K. STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 64 U.K. OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 65 U.K. STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 66 U.K. ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 67 U.K. HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 68 U.K. STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 69 U.K. STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 70 U.K. STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 71 ITALY STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 72 ITALY STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 73 ITALY OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 74 ITALY STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 75 ITALY ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 76 ITALY HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 77 ITALY STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 78 ITALY STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 79 ITALY STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 80 SPAIN STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 81 SPAIN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 82 SPAIN OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 83 SPAIN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 84 SPAIN ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 85 SPAIN HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 86 SPAIN STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 87 SPAIN STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 88 SPAIN STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 89 RUSSIA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 90 RUSSIA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 91 RUSSIA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 92 RUSSIA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 93 RUSSIA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 94 RUSSIA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 95 RUSSIA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 96 RUSSIA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 97 RUSSIA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 98 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 99 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 100 NETHERLANDS OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 101 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 102 NETHERLANDS ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 103 NETHERLANDS HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 104 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 105 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 106 NETHERLANDS STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 107 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 108 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 109 SWITZERLAND OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 110 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 111 SWITZERLAND ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 112 SWITZERLAND HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 113 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 114 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 115 SWITZERLAND STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 116 TURKEY STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 117 TURKEY STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 118 TURKEY OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 119 TURKEY STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 120 TURKEY ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 121 TURKEY HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 122 TURKEY STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 123 TURKEY STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 124 TURKEY STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 125 AUSTRIA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 126 AUSTRIA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 127 AUSTRIA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 128 AUSTRIA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 129 AUSTRIA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 130 AUSTRIA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 131 AUSTRIA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 132 AUSTRIA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 133 AUSTRIA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 134 BELGIUM STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 135 BELGIUM STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 136 BELGIUM OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 137 BELGIUM STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 138 BELGIUM ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 139 BELGIUM HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 140 BELGIUM STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 141 BELGIUM STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 142 BELGIUM STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 143 REST OF EUROPE STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE STROKE DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 EUROPE STROKE DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE STROKE DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE STROKE DIAGNOSTICS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE STROKE DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE STROKE DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE STROKE DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE STROKE DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE STROKE DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE STROKE DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR STROKE DIAGNOSTISIS EXPECTED TO DRIVE THE EUROPE STROKE DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 12 MODERATE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE STROKE DIAGNOSTICS MARKET IN 2021 & 2028
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE STROKE DIAGNOSTICS MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE STROKE DIAGNOSTICS MARKET
FIGURE 15 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, 2020
FIGURE 16 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, 2020-2028 (USD MILLION)
FIGURE 17 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, CAGR (2021-2028)
FIGURE 18 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY, LIFELINE CURVE
FIGURE 19 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, 2020
FIGURE 20 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, 2020-2028 (USD MILLION)
FIGURE 21 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 22 EUROPE STROKE DIAGNOSTICS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 23 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, 2020
FIGURE 24 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, 2020-2028 (USD MILLION)
FIGURE 25 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 26 EUROPE STROKE DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 27 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, 2020
FIGURE 28 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, 2020-2028 (USD MILLION)
FIGURE 29 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, CAGR (2021-2028)
FIGURE 30 EUROPE STROKE DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2020
FIGURE 32 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2020-2028 (USD MILLION)
FIGURE 33 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 34 EUROPE STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 35 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, 2020
FIGURE 36 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, 2020-2028 (USD MILLION)
FIGURE 37 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, CAGR (2021-2028)
FIGURE 38 EUROPE STROKE DIAGNOSTICS MARKET: BY STAGE, LIFELINE CURVE
FIGURE 39 EUROPE STROKE DIAGNOSTICS MARKET: SNAPSHOT (2020)
FIGURE 40 EUROPE STROKE DIAGNOSTICS MARKET: BY COUNTRY (2020)
FIGURE 41 EUROPE STROKE DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2028)
FIGURE 42 EUROPE STROKE DIAGNOSTICS MARKET: BY COUNTRY (2020 & 2028)
FIGURE 43 EUROPE STROKE DIAGNOSTICS MARKET: BY SEVERITY (2021-2028)
FIGURE 44 STROKE DIAGNOSTICS MARKET COMPANY SHARE 2020 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.













