Europe Sarcopenia Treatment Market
Taille du marché en milliards USD
TCAC :
% 
 USD
789.43 Million
USD
1,131.28 Million
2024
2032
USD
789.43 Million
USD
1,131.28 Million
2024
2032
| 2025 –2032 | |
| USD 789.43 Million | |
| USD 1,131.28 Million | |
|
|
|
|
Segmentation du marché européen du traitement de la sarcopénie, par type de traitement (médicaments, vitamines/compléments alimentaires et autres), type (sarcopénie primaire et sarcopénie secondaire), stades (pré-sarcopénie, sarcopénie et sarcopénie sévère), voie d'administration (orale, injectable et autres), sexe (homme et femme), utilisateur final (hôpitaux, cliniques spécialisées, soins à domicile et autres), canal de distribution (achat direct, vente au détail et autres) - Tendances et prévisions du secteur jusqu'en 2032
Taille du marché européen du traitement de la sarcopénie
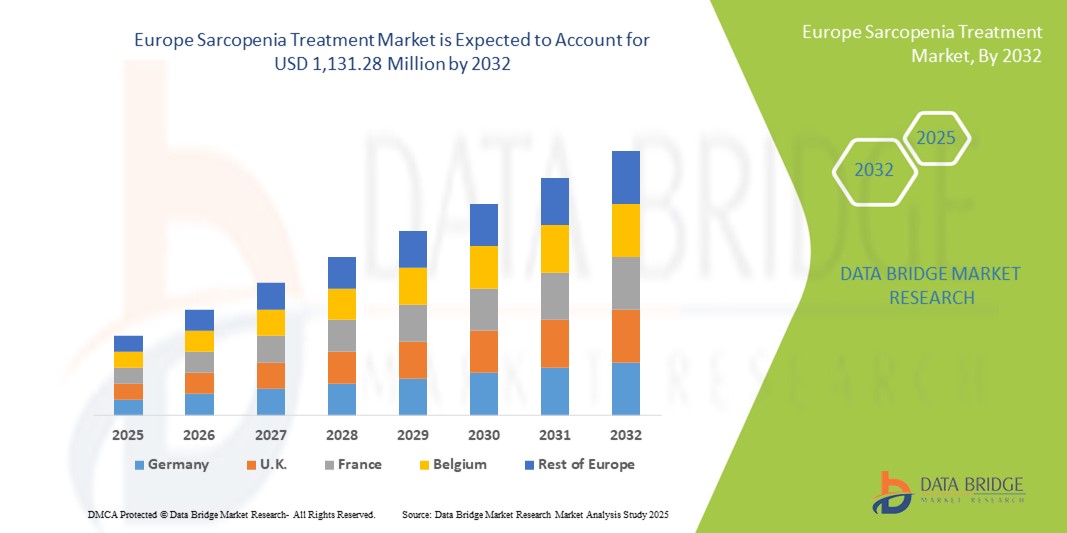
- La taille du marché européen du traitement de la sarcopénie était évaluée à 789,43 millions USD en 2024 et devrait atteindre 1 131,28 millions USD d'ici 2032 , à un TCAC de 4,6 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par la prévalence croissante de la malnutrition, des carences en vitamines et de la perte musculaire liée à l'âge, associée à une prise de conscience croissante de l'impact de la sarcopénie sur la santé et la qualité de vie.
- De plus, la demande croissante d'options thérapeutiques efficaces, telles que les compléments alimentaires, la kinésithérapie et les médicaments, fait des traitements contre la sarcopénie un élément essentiel pour les populations vieillissantes. Ces facteurs convergents accélèrent l'adoption de solutions thérapeutiques, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché européen du traitement de la sarcopénie
- Les traitements de la sarcopénie, comprenant des compléments nutritionnels, une thérapie physique et des interventions pharmacologiques, deviennent de plus en plus essentiels pour gérer la perte musculaire liée à l'âge et maintenir l'indépendance fonctionnelle de la population âgée en Europe en raison de leur efficacité pour améliorer la masse musculaire, la force et la qualité de vie globale.
- La demande croissante de traitements contre la sarcopénie est principalement due au vieillissement de la population européenne, à la sensibilisation croissante aux impacts de la sarcopénie sur la santé et à l'importance croissante accordée aux soins préventifs et aux programmes de vieillissement en bonne santé.
- L'Allemagne a dominé le marché du traitement de la sarcopénie avec la plus grande part de revenus de 23,9 % en 2024, caractérisée par une infrastructure de soins de santé avancée, des dépenses de santé élevées et des initiatives gouvernementales proactives favorisant les soins aux personnes âgées, avec une adoption massive de programmes de supplémentation nutritionnelle et de physiothérapie.
- L'Italie devrait être le pays qui connaîtra la croissance la plus rapide sur le marché du traitement de la sarcopénie au cours de la période de prévision en raison de l'augmentation des investissements dans les soins de santé, de la croissance de la population gériatrique et de la sensibilisation croissante à la perte musculaire liée à l'âge.
- Le segment des compléments alimentaires a dominé le marché du traitement de la sarcopénie avec une part de marché de 39,2 % en 2024, grâce au rôle bien établi des protéines, de la vitamine D et du calcium dans le maintien et la récupération musculaires chez les personnes âgées.
Portée du rapport et segmentation du marché européen du traitement de la sarcopénie
|
Attributs |
Aperçu du marché du traitement de la sarcopénie en Europe |
|
Segments couverts |
|
|
Pays couverts |
Europe
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché européen du traitement de la sarcopénie
Intégration de la santé numérique et de la télémédecine
- Une tendance importante et croissante sur le marché européen du traitement de la sarcopénie est l'intégration de solutions de santé numériques et de plateformes de télémédecine, permettant aux patients âgés de surveiller leur santé musculaire, de suivre leur nutrition et de recevoir des conseils à distance de professionnels de la santé.
- Par exemple, le programme de télésanté NutriTrack permet aux patients d'enregistrer leur apport alimentaire et leurs exercices de renforcement musculaire, tandis que les physiothérapeutes fournissent des recommandations en temps réel pour les ajustements thérapeutiques.
- L'intégration de la santé numérique permet un suivi continu des progrès du patient, en proposant des plans de traitement personnalisés et des rappels pour la supplémentation et les exercices, améliorant ainsi l'observance et les résultats.
- La combinaison transparente de capteurs portables , d'applications mobiles et de téléconsultations facilite la gestion centralisée du traitement de la sarcopénie, permettant aux cliniciens de coordonner efficacement les interventions nutritionnelles, thérapeutiques et pharmacologiques.
- Cette tendance vers des soins basés sur la technologie remodèle les attentes en matière de gestion de la sarcopénie, avec des entreprises telles que PhysioPlus développant des plateformes qui intègrent les données des patients, la supervision des exercices à distance et le coaching numérique.
- La demande de traitements de la sarcopénie assistés par la santé numérique augmente rapidement dans les hôpitaux, les cliniques externes et les soins à domicile, car les patients accordent de plus en plus d'importance à la commodité, à la surveillance et aux interventions personnalisées.
Dynamique du marché européen du traitement de la sarcopénie
Conducteur
Augmentation de la population gériatrique et sensibilisation à la perte musculaire liée à l'âge
- Le vieillissement croissant de la population en Europe, combiné à une plus grande prise de conscience de l'impact de la sarcopénie sur la mobilité et la qualité de vie, est un facteur majeur de la demande accrue de traitements efficaces.
- Par exemple, en 2024, l’Allemagne a lancé le « Programme de vieillissement en bonne santé », qui promeut la supplémentation nutritionnelle et les interventions en matière d’exercice physique dans les établissements de soins pour personnes âgées afin d’atténuer la prévalence de la sarcopénie.
- Alors que les personnes âgées présentent un risque accru de chutes, de fragilité et de déclin fonctionnel, les prestataires de soins de santé et les soignants recherchent de plus en plus des interventions qui améliorent la masse musculaire et la force.
- L’accent croissant mis sur les soins de santé préventifs, notamment le diagnostic précoce et le traitement rapide de la sarcopénie, accélère l’adoption de suppléments nutritionnels, de thérapies physiques et d’options pharmacologiques.
- Les campagnes de santé publique et les programmes gériatriques sensibilisent à la sarcopénie, stimulant ainsi la demande des patients et l'engagement des prestataires de soins de santé dans la fourniture de solutions de traitement ciblées.
Retenue/Défi
Accessibilité limitée et coûts de traitement élevés
- Les inquiétudes concernant l’accessibilité et le caractère abordable des traitements contre la sarcopénie constituent un défi important pour une adoption plus large du marché en Europe.
- Par exemple, malgré les preuves à l’appui de la supplémentation en protéines et de la physiothérapie , certains patients âgés d’Europe de l’Est ont un accès limité à ces interventions en raison des coûts élevés ou d’une infrastructure de soins de santé insuffisante.
- La variabilité des politiques de remboursement des soins de santé d’un pays à l’autre peut restreindre l’accès des patients aux compléments nutritionnels, aux médicaments ou aux séances de physiothérapie spécialisées.
- Bien que l’efficacité du traitement soit bien documentée, le manque de directives standardisées et la sensibilisation limitée des soignants peuvent réduire l’observance et l’adoption des thérapies.
- Les essais cliniques sur les traitements de la sarcopénie peuvent utiliser des critères diagnostiques différents, ce qui complique la comparaison des résultats. Ce manque de standardisation peut entraver le développement et l'approbation de nouveaux traitements par les organismes de réglementation.
- Surmonter ces défis grâce à des programmes soutenus par le gouvernement, des options thérapeutiques abordables et des initiatives éducatives pour les patients et les soignants sera essentiel pour une croissance soutenue du marché.
Portée du marché européen du traitement de la sarcopénie
Le marché est segmenté en fonction du type de traitement, du type de sarcopénie, des stades, de la voie d’administration, du sexe, de l’utilisateur final et du canal de distribution.
- Par type de traitement
Selon le type de traitement, le marché du traitement de la sarcopénie est segmenté en médicaments, vitamines et compléments alimentaires, entre autres. Ce segment a dominé le marché avec la plus grande part de chiffre d'affaires (39,2 %) en 2024, grâce à l'utilisation généralisée de suppléments en protéines, vitamine D et calcium chez les personnes âgées pour prévenir la perte musculaire. Les compléments alimentaires sont privilégiés pour leur innocuité, leur facilité d'administration et leur capacité à maintenir la masse et la fonction musculaires. La sensibilisation accrue aux interventions nutritionnelles et les programmes gouvernementaux de soutien favorisent encore leur adoption. Les patients reçoivent souvent des compléments alimentaires en première intention, tant pour la sarcopénie primaire que secondaire. La demande sur le marché est soutenue par la disponibilité d'aliments enrichis et de formules spécialisées riches en protéines. Les professionnels de santé recommandent les compléments alimentaires dans le cadre des soins préventifs et thérapeutiques des personnes âgées.
Le segment des médicaments devrait connaître la croissance la plus rapide, soit 11,8 % entre 2025 et 2032, grâce aux nouveaux traitements pharmacologiques ciblant l'atrophie musculaire, aux thérapies hormonales et aux inhibiteurs de la myostatine. Les médicaments sont de plus en plus prescrits aux patients atteints de sarcopénie sévère ou de sarcopénie secondaire liée à des maladies chroniques. L'augmentation des investissements en R&D et des autorisations réglementaires en Europe accélère leur adoption par le marché. Les médicaments offrent des bénéfices ciblés et cliniquement validés pour l'amélioration de la masse et de la force musculaires. Des campagnes de sensibilisation encouragent leur utilisation en complément des compléments alimentaires et de la physiothérapie. Les thérapies innovantes offrent un potentiel pour des stratégies thérapeutiques combinées, stimulant ainsi la croissance du segment.
- Par type
Le marché du traitement de la sarcopénie est segmenté en deux catégories : la sarcopénie primaire et la sarcopénie secondaire. La sarcopénie primaire a dominé le marché avec une part de chiffre d'affaires de 55,3 % en 2024, tirée par la perte musculaire liée à l'âge qui touche les personnes âgées en Europe. Les programmes de prévention et d'intervention précoce ciblant le déclin lié à l'âge sont courants dans des pays comme l'Allemagne et la France. La sarcopénie primaire est souvent prise en charge par des changements de mode de vie, des compléments alimentaires et de la physiothérapie. Les campagnes de sensibilisation et les programmes de soins gériatriques favorisent une large adoption. La prévalence de la sarcopénie primaire chez les personnes âgées assure une demande constante. Les hôpitaux et les cliniques intègrent des mesures préventives, maintenant ainsi une position dominante sur le marché.
Le segment de la sarcopénie secondaire devrait connaître la croissance la plus rapide, soit 12,3 % entre 2025 et 2032, alimenté par l'incidence croissante de la sarcopénie due à des maladies chroniques telles que le diabète, la BPCO et le cancer. Le traitement nécessite souvent une combinaison de médicaments, de suppléments et de kinésithérapie supervisée. Le développement de la recherche sur la sarcopénie liée à la maladie favorise le développement de thérapies ciblées. La prévalence croissante des maladies chroniques et liées au mode de vie en Europe favorise leur adoption. Les programmes cliniques incluent de plus en plus d'interventions contre la sarcopénie secondaire. La sensibilisation des professionnels de santé et des patients contribue à accélérer l'adoption des thérapies.
- Par étapes
Sur la base des stades, le marché du traitement de la sarcopénie est segmenté en pré-sarcopénie, sarcopénie et sarcopénie sévère. Le stade de la sarcopénie dominait le marché avec la plus grande part de chiffre d'affaires (47,8 %) en 2024, les patients étant généralement diagnostiqués à un stade précoce ou modéré. Les interventions telles que la nutrition, l'exercice et les médicaments sont particulièrement efficaces à ce stade. Un dépistage régulier et des programmes gériatriques favorisent une adoption précoce du traitement. Les hôpitaux et les cliniques spécialisées privilégient les interventions rapides pour prévenir la progression de la maladie. Les campagnes de sensibilisation et les stratégies de gestion proactives contribuent à la domination du marché. L'adoption du traitement est favorisée par l'amélioration des résultats pour les patients et la réduction du risque de déclin de la mobilité.
Le segment de la sarcopénie sévère devrait connaître la croissance la plus rapide, soit 13,1 % entre 2025 et 2032, en raison de l'augmentation du nombre de patients âgés présentant une perte musculaire avancée. Des thérapies combinées intensives, incluant médicaments, suppléments et physiothérapie, sont nécessaires à ce stade. Les professionnels de santé se concentrent sur l'amélioration de la mobilité, la réduction des risques de chute et l'amélioration de la qualité de vie. La prévalence croissante de la sarcopénie sévère chez les personnes âgées stimule la demande d'interventions efficaces. Des programmes de prise en charge avancée sont mis en œuvre dans les hôpitaux et les cliniques. L'augmentation de la population gériatrique assure une croissance soutenue du marché dans ce segment.
- Par voie d'administration
Selon la voie d'administration, le marché du traitement de la sarcopénie est segmenté en formes orales, injectables et autres. Le segment oral a dominé le marché avec la plus grande part de chiffre d'affaires (53,7 %) en 2024, grâce à la facilité d'administration des compléments alimentaires et des médicaments par voie orale. Les patients privilégient les traitements oraux pour leur praticité, leur observance et leur sécurité. Les compléments et les médicaments sont largement disponibles sans ordonnance et recommandés pour la sarcopénie primaire et secondaire. Les produits oraux comprennent des aliments enrichis, des gélules et des poudres adaptés aux personnes âgées. Leur adoption est soutenue par des programmes de soins à domicile et des recommandations cliniques. La croissance du marché est également stimulée par des campagnes de sensibilisation du public promouvant la supplémentation orale.
Le segment des injectables devrait connaître la croissance la plus rapide, soit 14,2 % entre 2025 et 2032, grâce au développement de thérapies injectables ciblant la sarcopénie sévère et les traitements anabolisants. Les médicaments injectables offrent un dosage précis et une efficacité plus rapide dans les cas avancés. Les hôpitaux et les cliniques spécialisées adoptent de plus en plus les options injectables pour améliorer les résultats des patients. Les thérapies innovantes permettent une récupération musculaire ciblée chez les patients atteints de sarcopénie chronique ou sévère. Les essais cliniques et les autorisations de mise sur le marché sur les marchés européens soutiennent leur adoption. La sensibilisation croissante des professionnels de santé et des patients à l'efficacité des traitements stimule la croissance du segment.
- Par sexe
Le marché du traitement de la sarcopénie est segmenté selon le sexe, hommes et femmes. Le segment féminin a dominé le marché avec la plus grande part de chiffre d'affaires (51,5 %) en 2024, en raison d'une prévalence plus élevée de la sarcopénie chez les femmes âgées, notamment après la ménopause, en raison des changements hormonaux affectant la masse musculaire. Des interventions nutritionnelles, de la physiothérapie et des médicaments sont couramment recommandés aux femmes. Les initiatives de santé publique axées sur la santé des femmes favorisent l'adoption de ces traitements. Les campagnes de sensibilisation ciblent le dépistage et la prise en charge précoces chez les femmes. Les prestataires de soins privilégient les interventions personnalisées pour les femmes âgées. La combinaison de soins préventifs et de traitements thérapeutiques garantit une demande soutenue.
Le segment masculin devrait connaître la croissance la plus rapide, soit 10,9 % entre 2025 et 2032, grâce à une sensibilisation croissante à la sarcopénie chez les hommes âgés et à une participation accrue aux programmes de soins préventifs. Les interventions sur le mode de vie, la supplémentation et les thérapies cliniques gagnent en popularité auprès des hommes. Les programmes publics et privés favorisent le dépistage précoce chez les hommes. L'accent croissant mis sur la mobilité, la force et l'autonomie fonctionnelle favorise l'adoption. Les recherches cliniques mettant en évidence la sarcopénie chez les hommes soutiennent la croissance. L'augmentation de la population masculine âgée assure l'expansion du marché dans ce segment.
- Par utilisateur final
En fonction de l'utilisateur final, le marché du traitement de la sarcopénie est segmenté entre hôpitaux, cliniques spécialisées, soins à domicile et autres. Les hôpitaux ont dominé le marché avec la plus grande part de chiffre d'affaires (46,2 %) en 2024, offrant une prise en charge complète de la sarcopénie, incluant diagnostics, supplémentation, médicaments et programmes de physiothérapie. Les hôpitaux ont mis en place des programmes de soins gériatriques facilitant l'adoption des traitements. Le dépistage précoce et la surveillance continue en milieu hospitalier garantissent des résultats optimaux pour les patients. Les hôpitaux collaborent souvent avec les soins à domicile et les cliniques pour étendre les soins. Les hôpitaux publics et privés promeuvent activement les initiatives de soins préventifs. L'approche centralisée des traitements en milieu hospitalier garantit une demande constante du marché.
Le segment des soins à domicile devrait connaître la croissance la plus rapide, soit 15,3 % entre 2025 et 2032, grâce à la demande croissante de soins à domicile pour les personnes âgées et d'interventions nutritionnelles et de kinésithérapie à domicile. Les programmes de télésurveillance et de télémédecine permettent une prise en charge efficace en dehors du cadre hospitalier. Les programmes personnalisés de soins à domicile améliorent l'observance et les résultats des traitements. Cette croissance est soutenue par une sensibilisation croissante des patients âgés aux questions de confort et d'autonomie. Les prestataires de soins à domicile intègrent des outils numériques pour un suivi en temps réel. Ce segment bénéficie des politiques de santé favorisant le vieillissement sur place.
- Par canal de distribution
En fonction du canal de distribution, le marché du traitement de la sarcopénie est segmenté en vente directe, vente au détail et autres. Le segment de la vente au détail a dominé le marché avec la plus grande part de chiffre d'affaires (49,6 %) en 2024, grâce à la large disponibilité des compléments alimentaires et des médicaments en pharmacie, supermarchés et plateformes de commerce électronique. La facilité d'accès, la disponibilité des médicaments en vente libre et la commodité soutiennent cette domination du marché. Les consommateurs privilégient la vente au détail pour des achats fréquents et un accès immédiat. L'expansion de la vente au détail en zones urbaines et semi-urbaines contribue à la croissance du chiffre d'affaires. Les campagnes marketing des marques ciblant les aidants et les personnes âgées renforcent leur notoriété. Ce segment bénéficie de réseaux de distribution établis et de la visibilité des marques.
Le segment des appels d'offres directs devrait connaître la croissance la plus rapide, soit 12,7 % entre 2025 et 2032, grâce à l'augmentation des achats de produits pour le traitement de la sarcopénie par les hôpitaux, les cliniques spécialisées et les programmes gouvernementaux de soins aux personnes âgées. Les commandes groupées par appels d'offres garantissent un approvisionnement régulier pour les établissements. La sensibilisation croissante aux soins préventifs et aux programmes de prise en charge du vieillissement favorise la distribution par appels d'offres. Les initiatives gouvernementales en matière de santé reposent de plus en plus sur l'approvisionnement direct de compléments alimentaires et de médicaments. L'adoption institutionnelle garantit des volumes de vente plus importants et des revenus durables. Les partenariats avec les fabricants et les distributeurs accélèrent la croissance du marché sur ce canal.
Analyse régionale du marché européen du traitement de la sarcopénie
- L'Allemagne a dominé le marché du traitement de la sarcopénie avec la plus grande part de revenus de 23,9 % en 2024, caractérisée par une infrastructure de soins de santé avancée, des dépenses de santé élevées et des initiatives gouvernementales proactives favorisant les soins aux personnes âgées, avec une adoption massive de programmes de supplémentation nutritionnelle et de physiothérapie.
- Les patients et les prestataires de soins de santé du pays adoptent de plus en plus de suppléments nutritionnels, de physiothérapie et de médicaments pour une intervention précoce et une gestion de la sarcopénie
- L’adoption généralisée est en outre soutenue par des programmes gouvernementaux proactifs, des campagnes de santé publique et des systèmes de soins gériatriques bien établis, faisant des traitements contre la sarcopénie un élément essentiel des soins de santé aux personnes âgées.
Aperçu du marché allemand du traitement de la sarcopénie
En 2024, le marché allemand du traitement de la sarcopénie affichait la plus grande part de chiffre d'affaires en Europe, grâce à des établissements de santé de pointe et à des programmes de soins gériatriques bien établis. Les professionnels de santé privilégient le dépistage et la prise en charge précoces de la sarcopénie par le biais de compléments alimentaires, de kinésithérapie et de médicaments. Les campagnes de santé publique et les initiatives de soins aux personnes âgées soutenues par l'État favorisent la sensibilisation et l'adoption des traitements. L'accent mis par l'Allemagne sur la prévention, conjugué à des dépenses de santé élevées, encourage l'intégration d'approches multimodales de prise en charge de la sarcopénie. Le marché connaît une croissance dans les hôpitaux, les cliniques et les soins à domicile, témoignant d'une large acceptation des solutions thérapeutiques. Les patients bénéficient d'options thérapeutiques accessibles et d'une intégration technologique dans le suivi et les soins.
Aperçu du marché français du traitement de la sarcopénie
Le marché français du traitement de la sarcopénie devrait connaître une croissance significative au cours de la période de prévision, principalement portée par le vieillissement de la population et la sensibilisation croissante aux bonnes pratiques du vieillissement. Les professionnels de santé français recommandent de plus en plus de compléments alimentaires et de kinésithérapie pour prévenir et gérer la sarcopénie. Les initiatives gouvernementales et les programmes de soins préventifs ciblant les personnes âgées soutiennent la croissance du marché. L'intégration de la télémédecine et des plateformes de santé numérique améliore l'observance du traitement et le suivi des patients. Les soins de santé en établissement, en ambulatoire et à domicile connaissent une adoption croissante. L'accent mis sur la qualité de vie et l'autonomie fonctionnelle des personnes âgées est un moteur essentiel du marché en France.
Aperçu du marché italien du traitement de la sarcopénie
Le marché italien du traitement de la sarcopénie devrait connaître une croissance soutenue, portée par l'augmentation de la population gériatrique et une sensibilisation croissante aux risques pour la santé liés à la perte musculaire. Les professionnels de santé italiens encouragent une intervention précoce par le biais de suppléments, de programmes d'exercices et de médicaments. Les initiatives soutenues par le gouvernement et les campagnes de prévention favorisent l'adoption de ces traitements, notamment dans les structures de soins de proximité et à domicile. Les patients s'engagent de plus en plus dans des programmes d'autosoins, soutenus par la santé numérique et la télésurveillance. Les hôpitaux et les cliniques spécialisées continuent d'élargir leur offre de services pour lutter contre la sarcopénie de manière globale. La croissance du marché est portée par l'effet combiné de l'accent mis sur les soins préventifs, de l'accessibilité aux soins et de la sensibilisation des patients.
Aperçu du marché britannique du traitement de la sarcopénie
Le marché britannique du traitement de la sarcopénie devrait connaître une croissance TCAC considérable au cours de la période de prévision, stimulé par une sensibilisation croissante à la détérioration musculaire liée à l'âge et par les programmes de soins préventifs. Les professionnels de santé recommandent des compléments alimentaires, de la kinésithérapie et des médicaments pour une intervention précoce. Les initiatives gouvernementales en faveur de la santé des personnes âgées et des pratiques de vieillissement en bonne santé favorisent une adoption accrue. L'intégration de la santé numérique, notamment la télésurveillance et la télémédecine, améliore l'engagement des patients et l'observance du traitement. L'adoption de programmes de prise en charge de la sarcopénie, tant en milieu résidentiel qu'en milieu clinique, est en constante expansion. Globalement, l'accent mis par le Royaume-Uni sur les soins préventifs, l'autonomie fonctionnelle et les interventions centrées sur le patient stimule la croissance du marché.
Part de marché du traitement de la sarcopénie en Europe
L'industrie européenne du traitement de la sarcopénie est principalement dirigée par des entreprises bien établies, notamment :
- Biophytis (France)
- Novartis AG (Suisse)
- Sanofi (France)
- Bayer AG (Allemagne)
- UCB SA (Belgique)
- Teva Pharmaceutical Industries Ltd. (Israël)
- H. Lundbeck A/S (Danemark)
- Galapagos NV (Belgique)
- Ipsen SA (France)
- Almirall SA (Espagne)
- Orion Corporation (Finlande)
- Biocryst Pharmaceuticals, Inc. (États-Unis)
- Haplogen Pharmaceuticals AG (Suisse)
- Medtronic (Irlande)
- AbbVie Inc. (États-Unis)
- Pfizer Inc. (États-Unis)
- Lilly USA, LLC (États-Unis)
- Bristol-Myers Squibb Company (États-Unis)
- AstraZeneca (Royaume-Uni)
Quels sont les développements récents sur le marché européen du traitement de la sarcopénie ?
- En septembre 2025, Biophytis a dévoilé sa stratégie d'essai de phase 2 pour Sarconeos (BIO101) ciblant la sarcopénie liée à l'obésité. L'essai sera mené en Europe et au Brésil et vise à évaluer l'efficacité de BIO101 dans l'amélioration de la force et de la fonction musculaires chez les patients obèses. Cette extension à la sarcopénie liée à l'obésité souligne la polyvalence de BIO101 comme traitement potentiel de multiples pathologies liées à la sarcopénie.
- En août 2025, Biophytis a annoncé avoir reçu l'approbation de l'Agence européenne des médicaments (EMA) et des autorités réglementaires belges pour lancer la première partie de son essai clinique de phase III sur Sarconeos (BIO101), un candidat médicament contre la sarcopénie. Cette approbation marque une étape importante dans le développement de traitements pharmacologiques contre la sarcopénie en Europe.
- En juin 2025, Biophytis a annoncé le lancement de l'essai clinique SARA de phase III pour Sarconeos (BIO101), un candidat médicament destiné au traitement de la sarcopénie chez les personnes âgées. Cet essai pivot, mené dans plusieurs pays européens, vise à évaluer l'efficacité et la sécurité de Sarconeos pour améliorer les performances physiques et la force musculaire des patients sarcopéniques. Les résultats de cet essai pourraient ouvrir la voie au premier traitement pharmacologique de la sarcopénie en Europe.
- En avril 2025, une initiative européenne menée par le groupe COMET (Core Outcome Measures in Effectiveness Trials) a élaboré un ensemble de critères de jugement principaux (COS) pour la sarcopénie. Cet ensemble vise à standardiser les critères de jugement mesurés dans les essais cliniques et la pratique clinique courante, garantissant ainsi la cohérence et la comparabilité entre les études. La mise en place de cet ensemble constitue une avancée significative vers l'amélioration de la qualité des données probantes et des stratégies de traitement de la sarcopénie en Europe.
- En août 2024, TNF Pharmaceuticals a annoncé son intention de lancer un essai clinique de phase 2b sur l'efficacité de l'isomyosamine dans la sarcopénie et la fragilité au début du premier trimestre 2025. Cet essai vise à explorer davantage l'efficacité du médicament dans la sarcopénie/fragilité, en s'appuyant sur les résultats positifs statistiquement significatifs d'une précédente étude clinique de phase 2.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.