Europe Lymphangioleiomyomatosis Lam Market
Taille du marché en milliards USD
TCAC :
% 
 USD
39.90 Million
USD
55.03 Million
2024
2032
USD
39.90 Million
USD
55.03 Million
2024
2032
| 2025 –2032 | |
| USD 39.90 Million | |
| USD 55.03 Million | |
|
|
|
|
Segmentation du marché européen de la lymphangioleiomyomatose (LAM) par type de maladie (LAM associée à la sclérose tubéreuse et LAM sporadique), type (diagnostic et traitement), complications (pneumothorax, chylothorax, tumeur rénale, épanchements pleuraux, œdème et accumulation de liquide, et autres), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, centres de diagnostic, soins à domicile et autres), canal de distribution (appel d'offres direct, pharmacies hospitalières, pharmacies de détail, pharmacies en ligne et autres) - Tendances du secteur et prévisions jusqu'en 2032
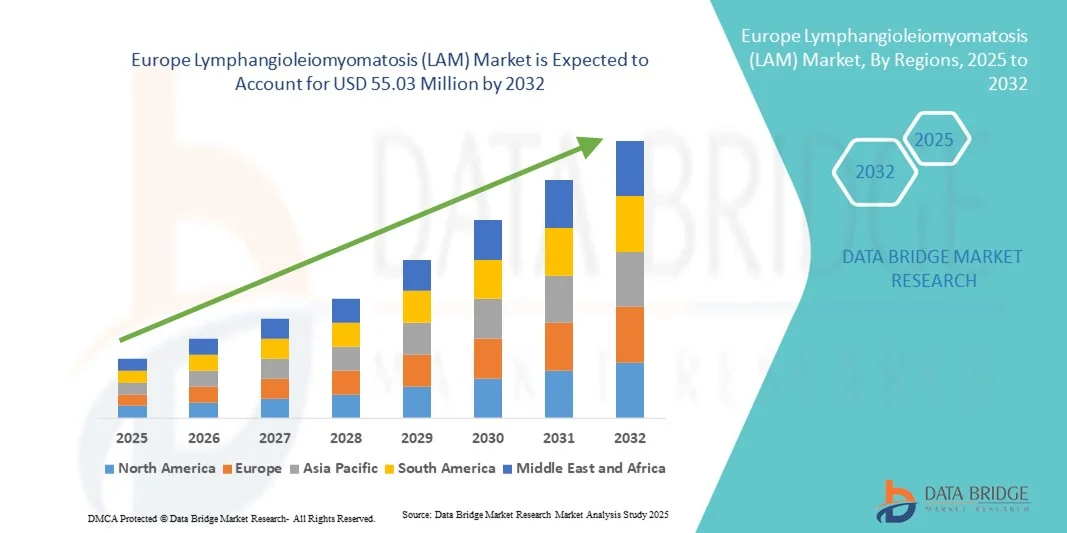
Taille du marché européen de la lymphangioleiomyomatose (LAM)
- Le marché européen de la lymphangioléiomyomatose (LAM) était évalué à 39,90 millions de dollars américains en 2024 et devrait atteindre 55,03 millions de dollars américains d'ici 2032 , avec un TCAC de 4,10 % au cours de la période de prévision.
- La croissance du marché est principalement due à l'augmentation de la sensibilisation et des taux de diagnostic des maladies pulmonaires rares dans les pays européens, ainsi qu'aux progrès de la médecine de précision et du diagnostic moléculaire.
- De plus, le développement des collaborations de recherche, l'amélioration de l'accès aux médicaments orphelins et l'expansion des essais cliniques pour les thérapies ciblées contribuent à améliorer les résultats des traitements et la prise en charge des patients. L'ensemble de ces facteurs accélère l'adoption des thérapies innovantes contre la LAM, renforçant ainsi la croissance globale du marché régional.
Analyse du marché européen de la lymphangioléiomyomatose (LAM)
- La lymphangioleiomyomatose (LAM), une maladie pulmonaire rare et progressive touchant principalement les femmes, fait l'objet d'une attention croissante en Europe grâce à une meilleure sensibilisation clinique, aux progrès de la recherche sur les maladies rares et à l'amélioration des techniques de diagnostic, notamment l'imagerie à haute résolution et les tests génétiques.
- Le besoin croissant de thérapies efficaces contre la LAM est motivé par un accès élargi aux traitements à base de sirolimus, par l'augmentation des investissements dans les programmes de lutte contre les maladies rares et par la recherche collaborative soutenue par les institutions universitaires et les entreprises pharmaceutiques.
- Le Royaume-Uni a dominé le marché européen du LAM avec la plus grande part de revenus (35,6 %) en 2024, grâce à une infrastructure clinique solide, des organisations de patients actives telles que LAM Action et un soutien gouvernemental important au développement des médicaments orphelins et aux registres des maladies rares.
- L'Allemagne devrait connaître la croissance la plus rapide sur le marché européen du LAM au cours de la période de prévision, grâce à l'intensification des essais cliniques, au développement de réseaux hospitaliers spécialisés dans les maladies pulmonaires et à l'adoption croissante de traitements personnalisés.
- Le segment des traitements a dominé le marché avec la plus grande part de marché (62,3 %) en 2024, grâce à l'adoption croissante de thérapies ciblant les symptômes et la progression de la LAM, ainsi qu'à la disponibilité accrue de soins spécialisés dans les hôpitaux et les cliniques spécialisées à travers l'Europe.
Portée du rapport et segmentation du marché européen de la lymphangioléiomyomatose (LAM)
|
Attributs |
Lymphangioleiomyomatose (LAM) en Europe : Principales informations sur le marché |
|
Segments couverts |
|
|
Pays couverts |
Europe
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur du marché, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché élaborés par Data Bridge Market Research incluent également une analyse approfondie par des experts, des données épidémiologiques sur les patients, une analyse des projets en développement, une analyse des prix et un aperçu du cadre réglementaire. |
Tendances du marché européen de la lymphangioléiomyomatose (LAM)
Importance croissante accordée au diagnostic précoce et à la sensibilisation aux maladies rares
- Une tendance significative et croissante sur le marché européen de la LAM est l'importance accrue accordée au diagnostic précoce grâce à l'imagerie à haute résolution, aux tests génétiques et aux registres de patients, permettant des interventions opportunes et une amélioration des résultats pour les patients.
- Par exemple, les réseaux nationaux de maladies rares au Royaume-Uni et en France mettent en œuvre des protocoles de diagnostic standardisés pour la LAM, facilitant ainsi un dépistage plus précoce et une meilleure prise en charge de l'évolution de la maladie.
- Les progrès réalisés dans le domaine du diagnostic moléculaire et de l'identification des biomarqueurs permettent aux cliniciens de différencier la LAM sporadique de la LAM associée à la sclérose tubéreuse de Bourneville (STB), améliorant ainsi la planification personnalisée des traitements.
- Des associations de patients spécialisées et des campagnes de sensibilisation à travers l'Europe informent les professionnels de santé et les patients sur les symptômes de la LAM, améliorant ainsi les taux de diagnostic et réduisant les délais de mise en place du traitement.
- Cette tendance vers un dépistage plus proactif, une détection précoce et une meilleure sensibilisation aux maladies redéfinit les attentes en matière de soins aux patients, les entreprises et les institutions de recherche se concentrant de plus en plus sur l'innovation diagnostique.
- La demande de solutions de diagnostic précoce augmente rapidement dans les hôpitaux et les cliniques spécialisées, car les cliniciens et les patients privilégient l'identification rapide de la LAM afin d'améliorer la prise en charge à long terme et la qualité de vie.
- La collaboration croissante entre les entreprises pharmaceutiques et les institutions de recherche favorise le développement de kits de diagnostic avancés et d'outils de prédiction pour la LAM. L'intégration des solutions de santé numérique et de la télémédecine dans la prise en charge des patientes atteintes de LAM permet un suivi à distance et des consultations diagnostiques plus rapides, accélérant ainsi le dépistage précoce.
Dynamique du marché européen de la lymphangioléiomyomatose (LAM)
Conducteur
Adoption croissante des thérapies ciblées et des médicaments orphelins
- La disponibilité et l'adoption croissantes des thérapies ciblées, notamment les traitements à base de sirolimus , constituent un facteur important de croissance du marché européen de la LAM, améliorant les résultats pour les patients et stabilisant la fonction pulmonaire.
- Par exemple, en mars 2024, le Service national de santé britannique (NHS) a élargi l'accès au traitement par sirolimus pour les patientes atteintes de LAM sporadique, favorisant ainsi une adoption plus large des options de traitement efficaces.
- L'augmentation des investissements dans la recherche sur les maladies rares et les programmes de médicaments orphelins, tant par les institutions publiques que par les entreprises pharmaceutiques, accélère le développement de nouvelles thérapies contre la LAM.
- La sensibilisation croissante des cliniciens aux inhibiteurs de mTOR et autres traitements ciblés conduit à une augmentation du nombre de patients recevant les traitements recommandés par les directives, stimulant ainsi la croissance du marché.
- Le renforcement du suivi des patients grâce à des cliniques spécialisées, des registres et un soutien en télémédecine facilite l'observance du traitement et la prise en charge à long terme de la maladie.
- La reconnaissance croissante des avantages des thérapies ciblées et l'expansion des réseaux de soins spécialisés à travers l'Europe favorisent une adoption accrue des traitements LAM dans les hôpitaux et les cliniques spécialisées.
- L'expansion des essais cliniques et des études en vie réelle en Allemagne, en France et en Italie favorise l'approbation de nouvelles thérapies et renforce la confiance dans leur efficacité. Les partenariats entre les entreprises pharmaceutiques et les associations de patients atteints de maladies rares améliorent l'accès aux soins et l'information des patients, contribuant ainsi à une meilleure adoption des traitements.
Retenue/Défi
Sensibilisation limitée et coûts de traitement élevés
- La méconnaissance de la LAM chez les médecins généralistes et certains pneumologues demeure un défi majeur, entraînant souvent un retard dans le diagnostic et la mise en place du traitement, ce qui peut freiner la croissance du marché.
- Par exemple, des enquêtes menées en Allemagne et en Italie indiquent que de nombreux patients subissent un retard de diagnostic de plusieurs années en raison du manque de connaissances des cliniciens de première ligne sur les maladies pulmonaires rares.
- Le coût élevé du sirolimus et d'autres traitements orphelins peut en limiter l'accès, notamment dans les pays où les budgets sont restreints ou la couverture d'assurance pour les maladies rares limitée.
- Bien que les programmes de soutien aux patients et les subventions gouvernementales améliorent l'accessibilité aux soins, le fardeau financier du traitement demeure un obstacle pour certains patients et prestataires de soins.
- Il est crucial de sensibiliser davantage le public par le biais de campagnes éducatives, de recommandations cliniques et de formations médicales afin d'améliorer le diagnostic précoce et l'accès au traitement.
- Il est nécessaire de surmonter les coûts élevés des traitements grâce à des initiatives de remboursement, des programmes d'aide aux patients et un soutien politique afin de faciliter un accès plus large aux soins et de renforcer la croissance du marché.
- La variabilité des infrastructures de santé entre les pays européens peut engendrer un accès inégal aux soins et aux diagnostics spécialisés en LAM, limitant ainsi la pénétration du marché. Les difficultés réglementaires liées aux autorisations de mise sur le marché des médicaments orphelins et aux procédures de remboursement peuvent retarder la disponibilité des traitements, freinant l'expansion du marché.
Portée du marché européen de la lymphangioleiomyomatose (LAM)
Le marché est segmenté en fonction du type de maladie, du type de traitement, des complications, de la voie d'administration, de l'utilisateur final et du canal de distribution.
- Par type de maladie
Selon le type de maladie, le marché européen de la LAM est segmenté en LAM associée à la sclérose tubéreuse de Bourneville (TSC-LAM), LAM sporadique et autres. Le segment de la LAM sporadique dominait le marché en 2024, représentant 58,4 % des revenus, en raison de sa prévalence plus élevée chez les femmes en âge de procréer. Les patientes atteintes de LAM sporadique nécessitent un suivi continu et un traitement ciblé, ce qui alimente la demande. Des recherches cliniques approfondies, des recommandations thérapeutiques établies et des registres de patientes actifs à travers l'Europe facilitent le diagnostic et la prise en charge précoces. Les hôpitaux et les cliniques spécialisées se concentrent de plus en plus sur la LAM sporadique en raison de son caractère progressif et de la nécessité d'un traitement par inhibiteurs de mTOR. Des pays européens comme le Royaume-Uni, l'Allemagne et la France sont à la pointe de l'adoption des traitements et de la prise en charge des patientes. La prise en charge multidisciplinaire, impliquant pneumologues, néphrologues et généticiens, renforce encore la position dominante de ce segment.
Le segment de la LAM associée à la sclérose tubéreuse de Bourneville (TSC-LAM) devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide, soit 7,9 %, entre 2025 et 2032, grâce à une meilleure sensibilisation à la TSC parmi les conseillers en génétique et les pneumologues. Les patients atteints de TSC-LAM nécessitent une prise en charge multidisciplinaire incluant des consultations en néphrologie et en pneumologie. L'amélioration des tests génétiques et les programmes de dépistage précoce facilitent une prise en charge thérapeutique rapide. L'élaboration de recommandations cliniques spécifiques à la TSC et de réseaux européens de maladies rares soutient cette croissance. Les actions de sensibilisation des patients et les campagnes d'information contribuent à l'augmentation des taux de diagnostic et, par conséquent, à l'expansion du marché. Les collaborations de recherche en cours sur les traitements de la TSC-LAM attirent des investissements dans des thérapies spécialisées.
- Par type
Le marché est segmenté, selon le type de traitement, en diagnostic et traitement. Le segment du traitement a dominé le marché en 2024, représentant 62,3 % des revenus, grâce à l'adoption croissante des inhibiteurs de mTOR, tels que le sirolimus, pour stabiliser la fonction pulmonaire. Les hôpitaux et les cliniques spécialisées constituent les principaux canaux d'administration des traitements, et le développement des essais cliniques favorise leur adoption. La sensibilisation accrue à l'évolution de la maladie et la disponibilité de médicaments orphelins approuvés renforcent la demande. Les traitements de la LAM sont de plus en plus intégrés aux programmes de suivi des patients afin d'assurer l'observance thérapeutique. L'infrastructure de santé européenne, notamment en Europe occidentale, facilite l'accès aux thérapies de pointe. Les traitements au long cours pour la prise en charge des maladies chroniques contribuent également à la croissance des revenus de ce segment.
Le segment du diagnostic devrait connaître la croissance la plus rapide, avec un TCAC de 8,1 % entre 2025 et 2032, grâce aux progrès de l'imagerie CT haute résolution, des explorations fonctionnelles respiratoires et de l'identification des biomarqueurs. Un diagnostic précoce est crucial dans la LAM pour prévenir les lésions pulmonaires irréversibles. Les hôpitaux et les centres de diagnostic adoptent les tests génétiques pour différencier la sclérose tubéreuse de Bourneville (TSC) de la LAM. Les initiatives européennes en faveur des maladies rares promeuvent la sensibilisation au diagnostic et les programmes de formation des médecins. La télémédecine et les comptes rendus numériques améliorent l'accessibilité au diagnostic de la LAM. L'essor du plaidoyer des patients et des campagnes de sensibilisation à la maladie favorise une adoption plus rapide. L'augmentation des financements alloués aux programmes de diagnostic des maladies rares contribue également à la croissance de ce segment.
- En raison de complications
En fonction des complications, le marché est segmenté en pneumothorax, chylothorax, tumeur rénale, épanchements pleuraux, œdème et accumulation de liquide, et autres. Le segment du pneumothorax dominait le marché en 2024 avec une part de 41,7 %, car le collapsus pulmonaire récurrent est une complication grave nécessitant une hospitalisation et une intervention. La prise en charge du pneumothorax implique souvent une approche multidisciplinaire, ce qui contribue à l'augmentation des coûts de traitement. Les cliniques et hôpitaux spécialisés assurent la surveillance et les interventions. La sensibilisation des patients et le dépistage précoce du risque de pneumothorax chez les patients atteints de LAM progressent en Europe. Des recommandations cliniques standardisées pour la prise en charge des complications confortent la position dominante de ce segment. Les hôpitaux d'Allemagne, de France et du Royaume-Uni sont à la pointe des soins et interventions avancés pour le pneumothorax.
Le segment du chylothorax devrait connaître la croissance la plus rapide, avec un TCAC de 9,2 % entre 2025 et 2032, alimentée par la prévalence croissante de l'atteinte lymphatique chez les patientes atteintes de LAM. Les centres d'excellence européens pour les maladies pulmonaires rares développent leurs services de prise en charge du chylothorax. Le diagnostic précoce et les interventions mini-invasives favorisent l'adoption de ces traitements. Les campagnes de sensibilisation à cette complication contribuent à une meilleure implication des patientes. Les cliniques et hôpitaux spécialisés investissent dans des techniques de traitement avancées. La recherche clinique axée sur les complications lymphatiques soutient la croissance de ce segment.
- Par voie d'administration
Selon la voie d'administration, le marché est segmenté en voie orale, parentérale et autres. Le segment oral dominait le marché en 2024, avec une part de revenus de 69,1 %, grâce à l'utilisation généralisée du sirolimus par voie orale pour la prise en charge au long cours. L'administration orale est privilégiée pour sa commodité, l'observance du traitement par le patient et son adéquation aux soins ambulatoires. Les hôpitaux, les cliniques spécialisées et les services de soins à domicile facilitent la gestion du traitement oral. Les politiques de remboursement européennes et les programmes d'aide aux patients encouragent son adoption. L'administration orale s'intègre facilement aux programmes de suivi pour l'ajustement des doses. Les médecins privilégient souvent le traitement oral pour sa facilité d'utilisation dans les plans de soins chroniques.
Le segment des traitements parentéraux devrait connaître la croissance la plus rapide, avec un TCAC de 8,5 % entre 2025 et 2032, portée par les thérapies injectables destinées aux cas avancés ou aux patients intolérants à la voie orale. L'administration parentérale est généralement pratiquée en milieu hospitalier sous la supervision d'un spécialiste. Les innovations dans le domaine des produits biologiques et des thérapies ciblées contribuent à cette croissance. Des essais cliniques menés en Allemagne, en France et en Italie soutiennent l'expansion du marché. Les pharmacies hospitalières et les cliniques spécialisées proposent de plus en plus l'administration parentérale. La télémédecine et les programmes d'accompagnement des patients facilitent l'observance thérapeutique.
- Par l'utilisateur final
En fonction de l'utilisateur final, le marché est segmenté en hôpitaux, cliniques spécialisées, centres de diagnostic, soins à domicile et autres. Le segment des hôpitaux dominait le marché en 2024 avec une part de 53,4 %, grâce à la concentration d'établissements spécialisés dans le traitement et le suivi de la LAM. Les hôpitaux offrent une prise en charge intégrée incluant le diagnostic, le traitement et la gestion des complications. Ils constituent des centres de référence pour les essais cliniques et la recherche sur les maladies rares. Les hôpitaux européens collaborent de plus en plus avec les associations de patients pour mettre en place des programmes d'éducation et d'accompagnement thérapeutique. Des infrastructures de pointe et des modèles de soins multidisciplinaires expliquent la position dominante de ce segment. Les hôpitaux demeurent le choix privilégié pour les cas de LAM sévères ou complexes.
Le segment des cliniques spécialisées devrait connaître la croissance la plus rapide, avec un TCAC de 9,0 % entre 2025 et 2032, grâce à l'augmentation du nombre de centres spécialisés dans les maladies pulmonaires rares. Ces cliniques offrent une prise en charge ciblée aux patients atteints de LAM, assurant souvent un suivi à long terme et des thérapies personnalisées. L'adoption de solutions de santé numérique et de la télémédecine élargit la portée des services. Les cliniques intègrent des approches multidisciplinaires, notamment des consultations en néphrologie et en génétique pour les cas de LAM associée à la sclérose tubéreuse de Bourneville (TSC). Les initiatives de sensibilisation des patients stimulent la demande pour les services des cliniques spécialisées. La collaboration avec les hôpitaux et les réseaux de maladies rares renforce les capacités des cliniques et améliore les résultats pour les patients.
- Par canal de distribution
En fonction du canal de distribution, le marché est segmenté en appels d'offres directs, pharmacies hospitalières, pharmacies de détail, pharmacies en ligne et autres. Le segment des pharmacies hospitalières a dominé le marché en 2024, représentant 47,8 % des revenus, les hôpitaux demeurant le principal point de dispensation des inhibiteurs de mTOR et autres traitements LAM. Les pharmacies hospitalières assurent le stockage adéquat des médicaments, le suivi de l'observance thérapeutique et le conseil aux patients. Elles sont étroitement liées aux essais cliniques et aux programmes de traitement. Les pharmacies hospitalières européennes augmentent leurs capacités pour répondre à la demande croissante des patients. Elles dispensent également une formation sur l'administration des médicaments et la gestion des effets indésirables. L'intégration avec les cliniques spécialisées améliore l'efficacité de la distribution et l'observance thérapeutique.
Le segment des pharmacies en ligne devrait connaître la croissance la plus rapide, avec un TCAC de 10,3 % entre 2025 et 2032, portée par l'adoption croissante du numérique, les services de livraison à domicile et un meilleur accès aux médicaments pour les maladies rares. Les plateformes en ligne offrent un confort accru aux patients vivant dans des régions éloignées. L'intégration de la télémédecine aux pharmacies en ligne améliore l'observance thérapeutique et le suivi. Les initiatives d'éducation des patients et les modèles de livraison directe au patient favorisent également l'adoption de ces plateformes. La sensibilisation croissante aux traitements des maladies rares encourage les achats en ligne. Les systèmes numériques de gestion des ordonnances garantissent une livraison sûre et rapide des médicaments.
Analyse régionale du marché européen de la lymphangioléiomyomatose (LAM)
- Le Royaume-Uni a dominé le marché européen du LAM avec la plus grande part de revenus (35,6 %) en 2024, grâce à une infrastructure clinique solide, des organisations de patients actives telles que LAM Action et un soutien gouvernemental important au développement des médicaments orphelins et aux registres des maladies rares.
- Au Royaume-Uni, les patients et les cliniciens bénéficient d'infrastructures de diagnostic de pointe, de centres de traitement spécialisés et d'un accès aux médicaments orphelins, ce qui, ensemble, améliore la prise en charge et le pronostic des patientes atteintes de LAM.
- Cette adoption généralisée est également favorisée par une forte sensibilisation des pneumologues, une activité croissante d'essais cliniques et des initiatives nationales en matière de maladies rares, faisant du Royaume-Uni un centre névralgique pour le diagnostic et le traitement de la LAM en Europe.
Analyse du marché britannique de la lymphangioleiomyomatose (LAM)
Le Royaume-Uni a dominé le marché européen de la LAM en 2024, avec une part de marché de 35,6 %, grâce à une infrastructure de santé performante, un soutien gouvernemental important aux programmes de lutte contre les maladies rares et des réseaux de défense des droits des patients actifs, tels que LAM Action. Les hôpitaux et les cliniques spécialisées britanniques offrent des services de diagnostic de pointe, notamment l'imagerie à haute résolution et les tests génétiques, permettant un dépistage précoce et une prise en charge efficace de la maladie. La large disponibilité du sirolimus et d'autres traitements ciblés contribue à leur fort taux d'adoption. Les initiatives nationales et les registres des maladies rares facilitent un diagnostic rapide et un suivi à long terme des patients.
Analyse du marché allemand de la lymphangioleiomyomatose (LAM)
Le marché allemand de la LAM devrait connaître une croissance annuelle composée importante, portée par une meilleure sensibilisation aux maladies pulmonaires rares, des infrastructures de santé de pointe et une activité accrue d'essais cliniques. Les hôpitaux et cliniques spécialisées allemands offrent des services complets de diagnostic et de traitement, assurant une prise en charge optimale des patients atteints de LAM sporadique et de LAM associée à la sclérose tubéreuse de Bourneville (TSC-LAM). L'adoption des inhibiteurs de mTOR et une prise en charge multidisciplinaire impliquant la pneumologie, la néphrologie et la génétique stimulent la croissance du marché. Les initiatives de recherche et le soutien gouvernemental aux programmes de maladies rares améliorent l'accès aux traitements. L'éducation des patients et le suivi par registre facilitent le diagnostic précoce et l'observance thérapeutique. Le marché bénéficie de politiques de remboursement avantageuses et de réseaux de maladies rares bien établis.
Analyse du marché français de la lymphangioleiomyomatose (LAM)
Le marché français de la LAM est en pleine croissance grâce à la présence de centres spécialisés, à l'accès à des thérapies de pointe et à une meilleure sensibilisation des patients. Les hôpitaux et les centres de diagnostic intègrent l'imagerie à haute résolution et les tests génétiques pour le dépistage précoce de la LAM. La recherche collaborative et les réseaux européens de référence pour les maladies pulmonaires rares améliorent la prise en charge des patients. Le soutien gouvernemental aux politiques relatives aux maladies rares et au remboursement facilite l'accès aux médicaments orphelins. Les essais cliniques et les programmes de soutien aux patients favorisent l'adoption des traitements. Les campagnes de sensibilisation destinées aux cliniciens et aux patients améliorent les taux d'intervention précoce et contribuent à l'expansion globale du marché.
Analyse du marché italien de la lymphangioleiomyomatose (LAM)
Le marché italien de la LAM devrait connaître une croissance régulière, soutenue par le développement des infrastructures de santé, des registres de patients et un accès accru aux médicaments orphelins. Les hôpitaux et les cliniques spécialisées privilégient le diagnostic précoce et la prise en charge à long terme des patients atteints de LAM. Les thérapies ciblées, notamment le sirolimus, sont de plus en plus utilisées en raison de leur efficacité prouvée pour stabiliser la fonction pulmonaire. Les centres italiens participent activement aux réseaux de recherche sur les maladies rares afin de promouvoir l'accès aux traitements. Des campagnes de sensibilisation et des formations destinées aux pneumologues permettent de réduire les délais de diagnostic. La télémédecine et le suivi numérique des patients améliorent l'observance thérapeutique et le suivi des soins.
Part de marché en Europe de la lymphangioleiomyomatose (LAM)
L’industrie européenne de la lymphangioleiomyomatose (LAM) est principalement dominée par des entreprises bien établies, notamment :
- Pfizer Inc. (États-Unis)
- Novartis AG (Suisse)
- Société pharmaceutique Takeda Limitée (Japon)
- Services Johnson & Johnson, Inc. (États-Unis)
- GSK plc (Royaume-Uni)
- AstraZeneca (Royaume-Uni)
- Laboratoires Dr. Reddy's Ltd (Inde)
- Zydus Pharmaceuticals, Inc. (Inde)
- Intas Pharmaceuticals Ltd (Inde)
- Apotex Inc. (Canada)
- Amneal Pharmaceuticals LLC (États-Unis)
- Terumo Corporation (Japon)
- Hikma Pharmaceuticals plc (Royaume-Uni)
- TransMedics, Inc. (États-Unis)
- XVIVO Perfusion AB (Suède)
- Taj Pharmaceuticals Limited (Inde)
- Morgan Scientific Inc. (États-Unis)
- Sun Pharmaceutical Industries Ltd (Inde)
- Sanofi (France)
- Boehringer Ingelheim International GmbH (Allemagne)
Quels sont les développements récents sur le marché européen de la lymphangioleiomyomatose (LAM) ?
- En mars 2025, la Fondation LAM a mis en avant un nouveau projet de recherche : « Ciblage des interactions fibroblastes-endothélium dans la pathogenèse de la LAM à l’aide de modèles sphéroïdes 3D et de la transcriptomique spatiale », témoignant d’une collaboration européenne croissante dans la recherche moléculaire avancée et les modèles translationnels visant à développer de nouvelles approches thérapeutiques pour la LAM.
- En janvier 2025, une étude publiée dans Frontiers in Medicine et intitulée « Caractéristiques et devenir des patientes atteintes de LAM » a analysé une cohorte de patientes européennes et fourni des données actualisées en situation réelle sur la progression de la maladie, le recours au traitement et les résultats, renforçant ainsi la nécessité d'un suivi amélioré et de centres spécialisés en Europe.
- En avril 2024, une étude publiée dans European Radiology a démontré qu'un protocole de tomodensitométrie thoracique à très faible dose, utilisant un filtre argent et une reconstruction par apprentissage profond, permet de réduire la dose de rayonnement d'environ 85 % tout en maintenant la précision diagnostique du score des kystes LAM, une avancée importante pour une surveillance de routine plus sûre en Europe.
- En février 2024, la Lymphangiomatosis & Gorham's Disease Alliance a publié un rapport intitulé « Naviguer dans le monde complexe des anomalies lymphatiques : avancées de la recherche sur les maladies pulmonaires », qui mettait en lumière de nouvelles perspectives sur le lien entre la LAM et les troubles lymphatiques, cartographiait les mutations génétiques du gène TSC2 et expliquait comment la recherche sur la LAM en Europe a désormais des implications plus larges pour les anomalies lymphatiques complexes.
- En mai 2021, une étude de suivi à long terme menée en Espagne et publiée dans Scientific Reports a révélé que plus de la moitié des patientes atteintes de LAM traitées par sirolimus ont maintenu une réponse positive pendant cinq ans. Cette étude représente l'un des plus importants suivis en vie réelle en Europe et soutient le maintien de l'utilisation des inhibiteurs de mTOR dans la prise en charge de la LAM.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.