Marché européen des dispositifs d'assistance ventriculaire gauche (DAVG), par type de produit (pompe cardiaque, contrôleur, batteries, fils), thérapie (thérapie de pont vers la transplantation (BTT), thérapie de destination, thérapie de pont vers la candidature (BTC), thérapie de pont vers la récupération (BTR)), groupe d'âge (adulte, pédiatrique), indication (insuffisance cardiaque congestive, cardiopathie congénitale , myocardite, arrêt cardiaque, arythmies familiales et arythmiques, cardiomyopathies, insuffisance cardiaque avancée, autres), génération (dispositifs de deuxième génération, dispositifs de troisième génération, dispositifs de première génération), durabilité (long terme, moyen terme, court terme), conception (axiale, centrifuge), type d'impulsion (non pulsatile, pulsatile), utilisateur final (hôpitaux, laboratoires de cathétérisme cardiaque, cliniques spécialisées, autres), canal de distribution (appel d'offres direct, vente au détail, autres), pays (Allemagne, (France, Royaume-Uni, Italie, Russie, Espagne, Turquie, Pays-Bas, Suisse, Belgique, Irlande, Reste de l'Europe) Tendances et prévisions du secteur jusqu'en 2029.
Analyse et perspectives du marché : Marché européen des dispositifs d'assistance ventriculaire gauche (DAVG)
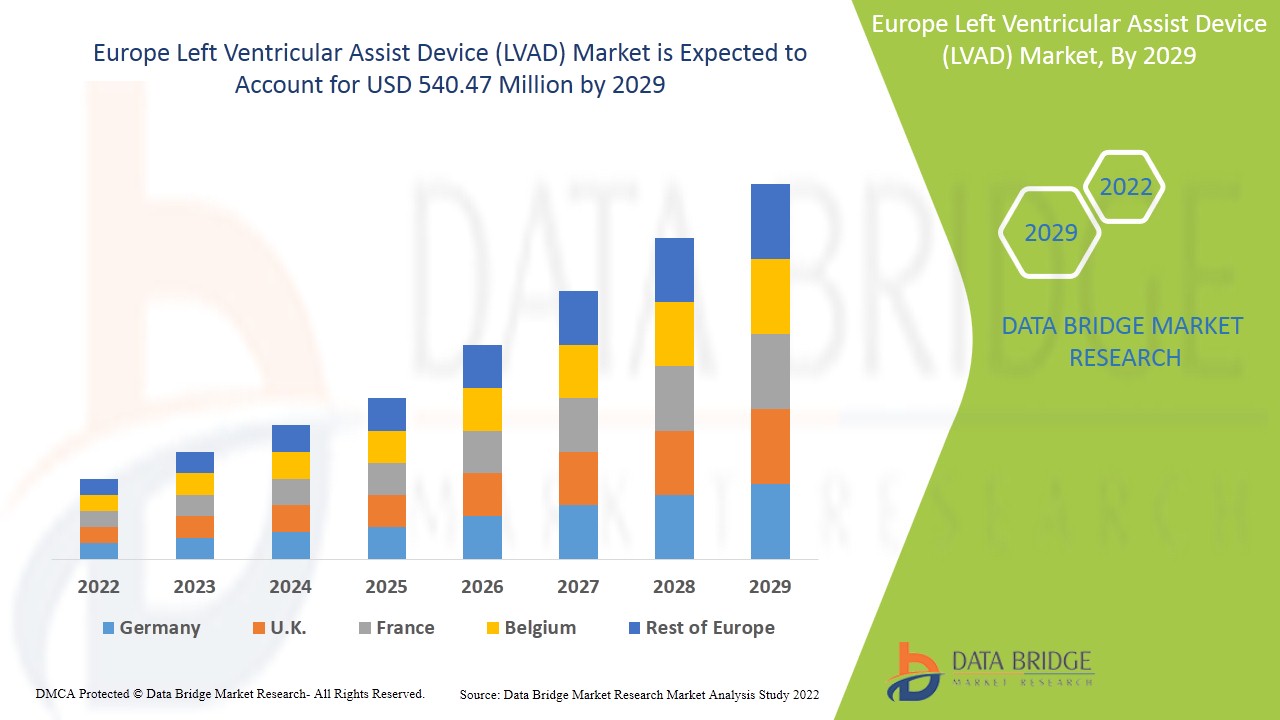
Le marché européen des dispositifs d’assistance ventriculaire gauche (LVAD) devrait connaître une croissance de marché au cours de la période de prévision de 2022 à 2029. Data Bridge Market Research analyse que le marché croît avec un TCAC de 9,2 % au cours de la période de prévision de 2022 à 2029 et devrait atteindre 540,47 millions USD d’ici 2029. Les avancées technologiques croissantes dans le dispositif d’assistance ventriculaire gauche agissent comme moteur de la croissance du marché des dispositifs d’assistance ventriculaire gauche (LVAD).
Un dispositif d'assistance ventriculaire gauche (DAVG) est une pompe mécanique implantée chez les patients souffrant d'insuffisance cardiaque. Il aide la cavité inférieure gauche du cœur (ventricule gauche) à pomper le sang hors du ventricule vers l'aorte et le reste du corps.
Il est utilisé chez les patients qui ont atteint un stade terminal d'insuffisance cardiaque. Le LVAD est une pompe mécanique à batterie implantée chirurgicalement qui aide le ventricule gauche (principale chambre de pompage du cœur) à pomper le sang vers le reste du corps.
Les principales raisons qui stimulent la croissance du marché européen des dispositifs d'assistance ventriculaire gauche sont l'augmentation du nombre de patients souffrant d'insuffisance cardiaque et la pénurie de donneurs de cœur. En outre, les dispositifs d'assistance ventriculaire gauche (DAVG) techniquement sophistiqués (par exemple, HeartMate III) contribuent à la croissance du marché. De plus, les avancées en cours dans cette discipline, ainsi que les nouvelles applications de thérapies innovantes, devraient ouvrir de nouvelles perspectives pour le marché des dispositifs d'assistance ventriculaire gauche. Néanmoins, ces dispositifs sont coûteux et présentent des risques tels que des caillots sanguins et des saignements, qui devraient considérablement entraver l'expansion du marché. En outre, l'augmentation des rappels de produits constitue un défi important pour le marché.
Le rapport sur le marché des dispositifs d'assistance ventriculaire gauche (DAVG) fournit des détails sur la part de marché, les nouveaux développements et l'analyse du pipeline de produits, l'impact des acteurs du marché national et local, analyse les opportunités en termes de poches de revenus émergentes, les changements dans la réglementation du marché, les approbations de produits, les décisions stratégiques, les lancements de produits, les expansions géographiques et les innovations technologiques sur le marché. Pour comprendre l'analyse et le scénario du marché des dispositifs d'assistance ventriculaire gauche (DAVG), contactez Data Bridge Market Research pour un briefing d'analyste, notre équipe vous aidera à créer une solution d'impact sur les revenus pour atteindre votre objectif souhaité.
Portée et taille du marché des dispositifs d'assistance ventriculaire gauche (DAVG)
Le marché des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en fonction du type de produit, de la thérapie, de la tranche d'âge, de l'indication, de la génération, de la durabilité, de la conception, du type d'impulsion, de l'utilisateur final et du canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
Le marché européen des dispositifs d’assistance ventriculaire gauche (LVAD) est classé en dix segments notables tels que le type de produit, la thérapie, la tranche d’âge, l’indication, la génération, la durabilité, la conception, le type d’impulsion, l’utilisateur final et le canal de distribution.
- En fonction du type de produit, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en pompe cardiaque, contrôleur, batteries et câbles. Les batteries sont ensuite segmentées en batteries rechargeables et non rechargeables. En 2022, la pompe cardiaque devrait dominer le marché car elle remplit les fonctions du cœur sans le remplacer et permet aux patients de vivre plus longtemps que ceux qui ne sont traités que par thérapie médicale.
- En fonction des traitements, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en thérapies de pont vers la transplantation (BTT), thérapies de destination, thérapies de pont vers la candidature (BTC) et thérapies de pont vers la récupération (BTR). En 2022, le secteur des thérapies de pont vers la transplantation (BTT) devrait dominer le marché car il aide les patients à attendre une transplantation et prévient d'autres dommages au cœur et à d'autres organes jusqu'à ce qu'un donneur soit disponible.
- En fonction de la tranche d'âge, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en adultes et en enfants. Les adultes sont eux-mêmes segmentés en 19-39 ans, 40-59 ans, 60-79 ans et plus de 80 ans. En 2022, le segment des adultes devrait dominer le marché, car la majorité des cas d'insuffisance cardiaque surviennent chez les adultes. La prévalence est de 1 à 2 % de la population européenne, atteignant plus de 10 % chez les personnes de plus de 70 ans. Cela pourrait signifier que plus de 10 millions de personnes souffrent d'insuffisance cardiaque.
- En fonction des indications, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en insuffisance cardiaque congestive, cardiopathie congénitale, myocardite, arrêt cardiaque, arythmies familiales et cardiomyopathies arythmiques, insuffisance cardiaque avancée et autres. En 2022, l'insuffisance cardiaque congestive devrait dominer le marché car il s'agit du diagnostic le plus courant chez les patients hospitalisés de plus de 65 ans. En Europe, plus de 16 millions de personnes ont reçu un diagnostic d'ICC.
- En fonction de la génération, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en dispositifs de deuxième génération, de troisième génération et de première génération. En 2022, le marché est dominé par les pompes rotatives de deuxième génération, qui présentent l'avantage d'une conception plus petite et le potentiel d'une plus grande fiabilité mécanique à long terme en éliminant la chambre de réservoir et les valves nécessaires.
- En fonction de leur durabilité, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en long terme, moyen terme et court terme. En 2022, le segment à long terme devrait dominer le marché en raison de la nécessité de continuer à soutenir les pompes cardiaques pendant une durée plus longue, ce qui stimule la croissance du marché.
- En fonction de la conception, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en deux segments : axial et centrifuge. En 2022, le segment axial devrait dominer car il fonctionne efficacement à des vitesses de rotation élevées et sa précharge est réduite.
- En fonction du type d'impulsion, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en non pulsatile et pulsatile. En 2022, le segment de la catégorie non pulsatile devrait dominer le marché car les DAVG sont utilisés plus fréquemment dans les thérapies de destination. Les effets indésirables d'une pulsatilité artérielle inadéquate sur les structures périphériques pourraient être considérablement amplifiés par les longues périodes d'implantation associées à ce type de thérapie.
- En fonction de l'utilisateur final, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en hôpitaux, cliniques spécialisées, laboratoires de cathétérisme cardiaque et autres. En 2022, le secteur hospitalier devrait dominer en raison d'un bassin croissant de patients souffrant de maladies cardiovasculaires résultant de l'évolution des modes de vie et du vieillissement de la population. En 2020, il y avait plus de 19 000 hôpitaux dans 23 pays européens.
- En fonction du canal de distribution, le marché européen des dispositifs d'assistance ventriculaire gauche (DAVG) est segmenté en appels d'offres directs, ventes au détail et autres. En 2022, le secteur des appels d'offres directs devrait dominer car il offre une responsabilité et des produits de meilleure qualité en raison de la concurrence, ce qui augmente la demande pour ces articles.
Analyse du marché des dispositifs d'assistance ventriculaire gauche (DAVG) au niveau des pays
Le marché des dispositifs d'assistance ventriculaire gauche (LVAD) est analysé et des informations sur la taille du marché sont fournies par pays, dix segments notables tels que le type de produit, la thérapie, la tranche d'âge, l'indication, la génération, la durabilité, la conception, le type d'impulsion, l'utilisateur final et le canal de distribution comme référencé ci-dessus.
Les pays couverts dans le rapport sur le marché européen des dispositifs d'assistance ventriculaire gauche (LVAD) sont l'Allemagne, la France, le Royaume-Uni, l'Italie, la Russie, l'Espagne, la Turquie, les Pays-Bas, la Suisse, la Belgique, l'Irlande et le reste de l'Europe.
Le segment adulte en Allemagne devrait connaître le taux de croissance le plus élevé au cours de la période de prévision de 2022 à 2029 en raison de la prévalence croissante des maladies cardiovasculaires, notamment l'insuffisance cardiaque avancée, l'arrêt cardiaque et l'insuffisance cardiaque congestive chez les adultes.
La section par pays du rapport fournit également des facteurs d'impact sur les marchés individuels et des changements de réglementation sur le marché national qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie du pays, les actes réglementaires et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario de marché pour les différents pays. En outre, la présence et la disponibilité des marques européennes et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des canaux de vente sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Les activités stratégiques croissantes des principaux acteurs du marché pour accroître la notoriété du dispositif d'assistance ventriculaire gauche stimulent la croissance du marché des dispositifs d'assistance ventriculaire gauche (LVAD)
Le marché des dispositifs d'assistance ventriculaire gauche (DAVG) vous fournit également une analyse de marché détaillée pour la croissance de chaque pays sur un marché particulier. En outre, il fournit des informations détaillées sur la stratégie des acteurs du marché et leur présence géographique. Les données sont disponibles pour la période historique de 2011 à 2020.
Analyse du paysage concurrentiel et des parts de marché des dispositifs d'assistance ventriculaire gauche (DAVG)
Le paysage concurrentiel du marché des dispositifs d'assistance ventriculaire gauche (DAVG) fournit des détails par concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, les sites et installations de production, les forces et les faiblesses de l'entreprise, le lancement du produit, les pipelines d'essais de produits, les approbations de produits, les brevets, la largeur et l'étendue du produit, la domination des applications, la courbe de survie technologique. Les points de données ci-dessus fournis ne concernent que l'orientation de l'entreprise liée au marché des dispositifs d'assistance ventriculaire gauche (DAVG).
Les principales entreprises qui commercialisent des dispositifs d'assistance ventriculaire gauche (DAVG) sont ABIOMED, Abbott, Evaheart, Inc, Saft, Berlin Heart, CorWave SA, Jarvik Heart, Inc. et d'autres acteurs nationaux. Les analystes de DBMR comprennent les atouts de la concurrence et fournissent une analyse concurrentielle pour chaque concurrent séparément.
De nombreux contrats et accords sont également initiés par des entreprises du monde entier qui accélèrent également le marché des dispositifs d'assistance ventriculaire gauche (DAVG).
Par exemple,
- En janvier 2021, CorWave SA a reçu un financement de 40 millions USD de la part de trois investisseurs pour développer une pompe cardiaque à membrane à ondes de pointe. Le financement reçu a permis à l'entreprise d'accélérer le développement de produits et de renforcer sa présence sur le marché mondial des dispositifs d'assistance ventriculaire gauche (DAVG).
La collaboration, le lancement de produits, l'expansion commerciale, les récompenses et la reconnaissance, les coentreprises et d'autres stratégies des acteurs du marché renforcent l'empreinte de l'entreprise sur le marché des dispositifs d'assistance ventriculaire gauche (LVAD), ce qui offre également l'avantage de la croissance des bénéfices de l'organisation.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S
4.3 INDUSTRIAL INSIGHTS:
5 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.1.1 GUIDELINES-
5.2 REGULATION IN EUROPE
5.2.1 GUIDELINES FOR THE MANUFACTURERS:
5.3 REGULATION IN CANADA
5.3.1 GUIDELINES FOR THE MANUFACTURERS:
5.4 REGULATION IN MEXICO
5.4.1 GUIDELINES FOR THE MANUFACTURERS:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING GERIATRIC POPULATION ALONG WITH RISING PREVALENCE OF CARDIAC DISEASES
6.1.2 PRESENCE OF FAVOURABLE REIMBURSEMENT POLICIES
6.1.3 CHANGING LIFESTYLE TRIGGERS THE DEVELOPMENT OF CARDIOVASCULAR DISEASES
6.2 RESTRAINTS
6.2.1 HIGH COST OF LVAD IMPLANTATION AND TREATMENT
6.2.2 COMPLICATIONS AND RISKS ASSOCIATED WITH LVAD
6.3 OPPORTUNITIES
6.3.1 INCREASE IN HEALTHCARE EXPENDITURE
6.3.2 INCREASE IN MINIMALLY INVASIVE PROCEDURE
6.3.3 INCREASING SHORTAGE OF ORGAN DONORS
6.3.4 TECHNOLOGICAL ADVANCEMENTS IN LEFT VENTRICULAR ASSIST DEVICES
6.4 CHALLENGES
6.4.1 ONGOING COVID-19
6.4.2 INCREASE IN PRODUCT RECALL
7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 HEART PUMP
7.3 CONTROLLER
7.4 BATTERIES
7.4.1 RECHARGEABLE
7.4.2 NON-RECHARGEABLE
7.5 WIRES
8 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY
8.1 OVERVIEW
8.2 BRIDGE-TO-TRANSPLANT (BTT) THERAPY
8.2.1 HEART PUMP
8.2.2 CONTROLLER
8.2.3 BATTERIES
8.2.4 WIRES
8.3 DESTINATION THERAPY
8.3.1 HEART PUMP
8.3.2 CONTROLLER
8.3.3 BATTERIES
8.3.4 WIRES
8.4 BRIDGE-TO-CANDIDACY (BTC) THERAPY
8.4.1 HEART PUMP
8.4.2 CONTROLLER
8.4.3 BATTERIES
8.4.4 WIRES
8.5 BRIDGE-TO-RECOVERY (BTR) THERAPY
8.5.1 HEART PUMP
8.5.2 CONTROLLER
8.5.3 BATTERIES
8.5.4 WIRES
9 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP
9.1 OVERVIEW
9.2 ADULT
9.2.1 19-39 YEARS
9.2.2 40-59 YEARS
9.2.3 60-79 YEARS
9.2.4 ABOVE 80 YEARS
9.3 PEDIATRIC
10 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION
10.1 OVERVIEW
10.2 SECOND GENERATION DEVICES
10.3 THIRD GENERATION DEVICES
10.4 FIRST GENERATION DEVICES
11 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN
11.1 OVERVIEW
11.2 AXIAL
11.3 CENTRIFUGAL
12 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION
12.1 OVERVIEW
12.2 CONGESTIVE HEART FAILURE
12.2.1 HEART PUMP
12.2.2 CONTROLLER
12.2.3 BATTERIES
12.2.4 WIRES
12.3 CONGENITAL HEART DISEASE
12.3.1 HEART PUMP
12.3.2 CONTROLLER
12.3.3 BATTERIES
12.3.4 WIRES
12.4 MYOCARDITIS
12.4.1 HEART PUMP
12.4.2 CONTROLLER
12.4.3 BATTERIES
12.4.4 WIRES
12.5 CARDIAC ARREST
12.5.1 HEART PUMP
12.5.2 CONTROLLER
12.5.3 BATTERIES
12.5.4 WIRES
12.6 FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES
12.6.1 HEART PUMP
12.6.2 CONTROLLER
12.6.3 BATTERIES
12.6.4 WIRES
12.7 ADVANCED HEART FAILURE
12.7.1 HEART PUMP
12.7.2 CONTROLLER
12.7.3 BATTERIES
12.7.4 WIRES
12.8 OTHERS
13 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DURABILITY
13.1 OVERVIEW
13.2 LONG-TERM
13.3 INTERMEDIATE-TERM
13.4 SHORT-TERM
14 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY PULSE TYPE
14.1 OVERVIEW
14.2 NONPULSATILE
14.3 PULSATILE
15 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITAL
15.3 CARDIAC CATH LABORATORIES
15.4 SPECIALTY CLINICS
15.5 OTHERS
16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL
16.1 OVERVIEW
16.2 DIRECT TENDER
16.3 RETAIL SALES
16.4 OTHERS
17 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION
17.1 EUROPE
17.1.1 GERMANY
17.1.2 FRANCE
17.1.3 U.K.
17.1.4 ITALY
17.1.5 RUSSIA
17.1.6 SPAIN
17.1.7 TURKEY
17.1.8 NETHERLANDS
17.1.9 SWITZERLAND
17.1.10 BELGIUM
17.1.11 IRELAND
17.1.12 REST OF EUROPE
18 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY LANDSCAPE
18.1 COMPANY SHARE ANALYSIS: EUROPE
19 SWOT ANALYSIS
20 COMPANY PROFILE
20.1 ABIOMED
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 COMPANY SHARE ANALYSIS
20.1.4 PRODUCT PORTFOLIO
20.1.5 RECENT DEVELOPMENTS
20.1.5.1 FDA APPROVAL
20.1.5.2 FDA APPROVAL
20.2 ABBOTT
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 COMPANY SHARE ANALYSIS
20.2.4 PRODUCT PORTFOLIO
20.2.5 RECENT DEVELOPMENTS
20.2.5.1 SUPPLY INCREMENT
20.2.5.2 BREAK THROUGH DEVICE DESIGNATION
20.3 BERLIN HEART
20.3.1 COMPANY SNAPSHOT
20.3.2 COMPANY SHARE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVELOPMENT
20.3.4.1 PRODUCT APPROVAL
20.4 SAFT (A SUBSIDIARY OF TOTALENERGIES)
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 COMPANY SHARE ANALYSIS
20.4.4 PRODUCT PORTFOLIO
20.4.5 RECENT DEVELOPMENT
20.4.5.1 PARTNERSHIP
20.5 JARVIK HEART, INC.
20.5.1 COMPANY SNAPSHOT
20.5.2 COMPANY SHARE ANALYSIS
20.5.3 PRODUCT PORTFOLIO
20.5.4 RECENT DEVELOPMENT
20.5.4.1 FDA APPROVAL
20.6 CORWAVE SA
20.6.1 COMPANY SNAPSHOT
20.6.2 PRODUCT PORTFOLIO
20.6.3 RECENT DEVELOPMENT
20.6.3.1 EXPANSION
20.7 EVAHEART, INC
20.7.1 COMPANY SNAPSHOT
20.7.2 PRODUCT PORTFOLIO
20.7.3 RECENT DEVELOPMENT
20.7.3.1 PRODUCT TRIAL
21 QUESTIONNAIRE
22 RELATED REPORTS
Liste des tableaux
TABLE 1 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 2 EUROPE HEART PUMP IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 EUROPE CONTROLLER IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 EUROPE WIRES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 8 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 10 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 12 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 14 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 17 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 19 EUROPE PEDIATRIC IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE SECOND GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE THIRD GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE FIRST GENERATION DEVICES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 25 EUROPE AXIAL IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE CENTRIFUGAL IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 27 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 32 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 36 EUROPE FAMILIAL ARRHYTHYMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 38 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 40 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 42 EUROPE LONG TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE INTERMEDIATE-TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 44 EUROPE SHORT-TERM IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION))
TABLE 45 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 46 EUROPE NONPULSATILE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 47 EUROPE PULSATILE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 48 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 49 EUROPE HOSPITALS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 50 EUROPE CARDIAC CATH LABORATORIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 51 EUROPE SPECIALTY CLINICS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 52 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 53 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 54 EUROPE DIRECT TENDER IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 55 EUROPE RETAIL SALES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 56 EUROPE OTHERS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 57 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY COUNTRY, 2022-2029 (USD MILLION)
TABLE 58 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 59 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 60 EUROPE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 62 EUROPE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 63 EUROPE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 64 EUROPE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 65 EUROPE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 66 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 67 EUROPE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 68 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 69 EUROPE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 70 EUROPE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 71 EUROPE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 72 EUROPE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 73 EUROPE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 74 EUROPE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 75 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 76 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 77 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 78 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 79 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 80 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 81 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 82 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 83 GERMANY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 84 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 85 GERMANY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 86 GERMANY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 87 GERMANY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 88 GERMANY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 89 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 90 GERMANY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 91 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 92 GERMANY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 93 GERMANY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 94 GERMANY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 95 GERMANY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 96 GERMANY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 97 GERMANY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 98 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 99 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 100 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 101 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 102 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 GERMANY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 104 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 105 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 106 FRANCE BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 107 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 108 FRANCE BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 109 FRANCE DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 110 FRANCE BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 111 FRANCE BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 112 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 113 FRANCE ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 114 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 115 FRANCE CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 116 FRANCE CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 117 FRANCE MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 118 FRANCE CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 119 FRANCE FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 120 FRANCE ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 121 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 122 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 123 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 124 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 125 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 126 FRANCE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 127 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 128 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 129 U.K. BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 130 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 131 U.K. BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 132 U.K. DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 133 U.K. BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 134 U.K. BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 135 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 136 U.K. ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 137 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 138 U.K. CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 139 U.K. CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 140 U.K. MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 141 U.K. CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 142 U.K. FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 143 U.K. ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 144 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 145 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 146 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 147 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 148 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 149 U.K. LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 150 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 151 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 152 ITALY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 153 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 154 ITALY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 155 ITALY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 156 ITALY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 157 ITALY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 158 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 159 ITALY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 160 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 161 ITALY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 162 ITALY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 163 ITALY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 164 ITALY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 165 ITALY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 166 ITALY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 167 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 168 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 169 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 170 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 171 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 172 ITALY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 173 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 174 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 175 RUSSIA BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 176 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 177 RUSSIA BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 178 RUSSIA DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 179 RUSSIA BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 180 RUSSIA BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 181 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 182 RUSSIA ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 183 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 184 RUSSIA CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 185 RUSSIA CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 186 RUSSIA MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 187 RUSSIA CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 188 RUSSIA FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 189 RUSSIA ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 190 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 191 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 192 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 193 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 194 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 195 RUSSIA LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 196 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 197 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 198 SPAIN BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 199 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 200 SPAIN BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 201 SPAIN DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 202 SPAIN BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 203 SPAIN BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 204 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 205 SPAIN ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 206 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 207 SPAIN CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 208 SPAIN CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 209 SPAIN MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 210 SPAIN CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 211 SPAIN FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 212 SPAIN ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 213 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 214 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 215 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 216 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 217 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 218 SPAIN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 219 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 220 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 221 TURKEY BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 222 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 223 TURKEY BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 224 TURKEY DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 225 TURKEY BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 226 TURKEY BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 227 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 228 TURKEY ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 229 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 230 TURKEY CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 231 TURKEY CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 232 TURKEY MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 233 TURKEY CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 234 TURKEY FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 235 TURKEY ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 236 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 237 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 238 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 239 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 240 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 241 TURKEY LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 242 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 243 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 244 NETHERLANDS BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 245 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 246 NETHERLANDS BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 247 NETHERLANDS DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 248 NETHERLANDS BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 249 NETHERLANDS BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 250 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 251 NETHERLANDS ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 252 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 253 NETHERLANDS CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 254 NETHERLANDS CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 255 NETHERLANDS MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 256 NETHERLANDS CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 257 NETHERLANDS FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 258 NETHERLANDS ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 259 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 260 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 261 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 262 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 263 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 264 NETHERLANDS LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 265 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 266 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 267 SWITZERLAND BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 268 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 269 SWITZERLAND BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 270 SWITZERLAND DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 271 SWITZERLAND BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 272 SWITZERLAND BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 273 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 274 SWITZERLAND ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 275 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 276 SWITZERLAND CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 277 SWITZERLAND CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 278 SWITZERLAND MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 279 SWITZERLAND CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 280 SWITZERLAND FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 281 SWITZERLAND ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 282 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 283 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 284 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 285 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 286 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 287 SWITZERLAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 288 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 289 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 290 BELGIUM BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 291 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 292 BELGIUM BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 293 BELGIUM DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 294 BELGIUM BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 295 BELGIUM BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 296 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 297 BELGIUM ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 298 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 299 BELGIUM CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 300 BELGIUM CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 301 BELGIUM MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 302 BELGIUM CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 303 BELGIUM FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 304 BELGIUM ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 305 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 306 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 307 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 308 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 309 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 310 BELGIUM LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 311 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 312 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 313 IRELAND BATTERIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 314 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 315 IRELAND BRIDGE-TO-TRANSPLANT (BTT) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 316 IRELAND DESTINATION THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 317 IRELAND BRIDGE-TO-CANDIDACY (BTC) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 318 IRELAND BRIDGE-TO-RECOVERY (BTR) THERAPY IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY THERAPY, 2020-2029 (USD MILLION)
TABLE 319 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 320 IRELAND ADULT IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY AGE GROUP, 2020-2029 (USD MILLION)
TABLE 321 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 322 IRELAND CONGESTIVE HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 323 IRELAND CONGENITAL HEART DISEASE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 324 IRELAND MYOCARDITIS IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 325 IRELAND CARDIAC ARREST IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 326 IRELAND FAMILIAL ARRHYTHMIAS AND ARRHYTHMIC CARDIOMYOPATHIES IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 327 IRELAND ADVANCED HEART FAILURE IN LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY INDICATION, 2020-2029 (USD MILLION)
TABLE 328 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY GENERATION, 2020-2029 (USD MILLION)
TABLE 329 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DURABILITY, 2020-2029 (USD MILLION)
TABLE 330 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DESIGN, 2020-2029 (USD MILLION)
TABLE 331 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PULSE TYPE, 2020-2029 (USD MILLION)
TABLE 332 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 333 IRELAND LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 334 REST OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 2 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: EUROPE VS REGIONAL ANALYSIS
FIGURE 5 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET:VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SEGMENTATION
FIGURE 11 INCREASING PREVALENCE OF CARDIOVASCULAR DISEASE AND RISING HEALTHCARE EXPENDITURE ARE EXPECTED TO DRIVE THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 HEART PUMP SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET AND ASIA-PACIFIC TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET
FIGURE 15 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2021
FIGURE 16 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, 2020-2029 (USD MILLION)
FIGURE 17 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 18 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 19 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2021
FIGURE 20 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, 2020-2029 (USD MILLION)
FIGURE 21 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, CAGR (2022-2029)
FIGURE 22 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY THERAPY, LIFELINE CURVE
FIGURE 23 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2021
FIGURE 24 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, 2020-2029 (USD MILLION)
FIGURE 25 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, CAGR (2022-2029)
FIGURE 26 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 27 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2021
FIGURE 28 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, 2020-2029 (USD MILLION)
FIGURE 29 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, CAGR (2022-2029)
FIGURE 30 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY GENERATION, LIFELINE CURVE
FIGURE 31 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2021
FIGURE 32 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, 2020-2029 (USD MILLION)
FIGURE 33 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, CAGR (2022-2029)
FIGURE 34 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY DESIGN, LIFELINE CURVE
FIGURE 35 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2021
FIGURE 36 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, 2020-2029 (USD MILLION)
FIGURE 37 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, CAGR (2022-2029)
FIGURE 38 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY INDICATION, LIFELINE CURVE
FIGURE 39 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2021
FIGURE 40 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, 2020-2029 (USD MILLION)
FIGURE 41 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, CAGR (2022-2029)
FIGURE 42 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DURABILITY, LIFELINE CURVE
FIGURE 43 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2021
FIGURE 44 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, 2020-2029 (USD MILLION)
FIGURE 45 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, CAGR (2022-2029)
FIGURE 46 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY PULSE TYPE, LIFELINE CURVE
FIGURE 47 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2021
FIGURE 48 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 49 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, CAGR (2022-2029)
FIGURE 50 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY END USER, LIFELINE CURVE
FIGURE 51 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 52 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 53 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 54 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) DISEASE MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 55 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: SNAPSHOT (2021)
FIGURE 56 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021)
FIGURE 57 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 58 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 59 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 60 EUROPE LEFT VENTRICULAR ASSIST DEVICE (LVAD) MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.