Marché européen des dispositifs de neurostimulation interne, par type de produit ( stimulation de la moelle épinière (SCS), stimulation cérébrale profonde , stimulation du nerf vague, stimulation du nerf sacré et stimulation électrique gastrique ), canal de distribution (appel d'offres direct et fournisseur de services tiers) - Tendances et prévisions de l'industrie jusqu'en 2029.
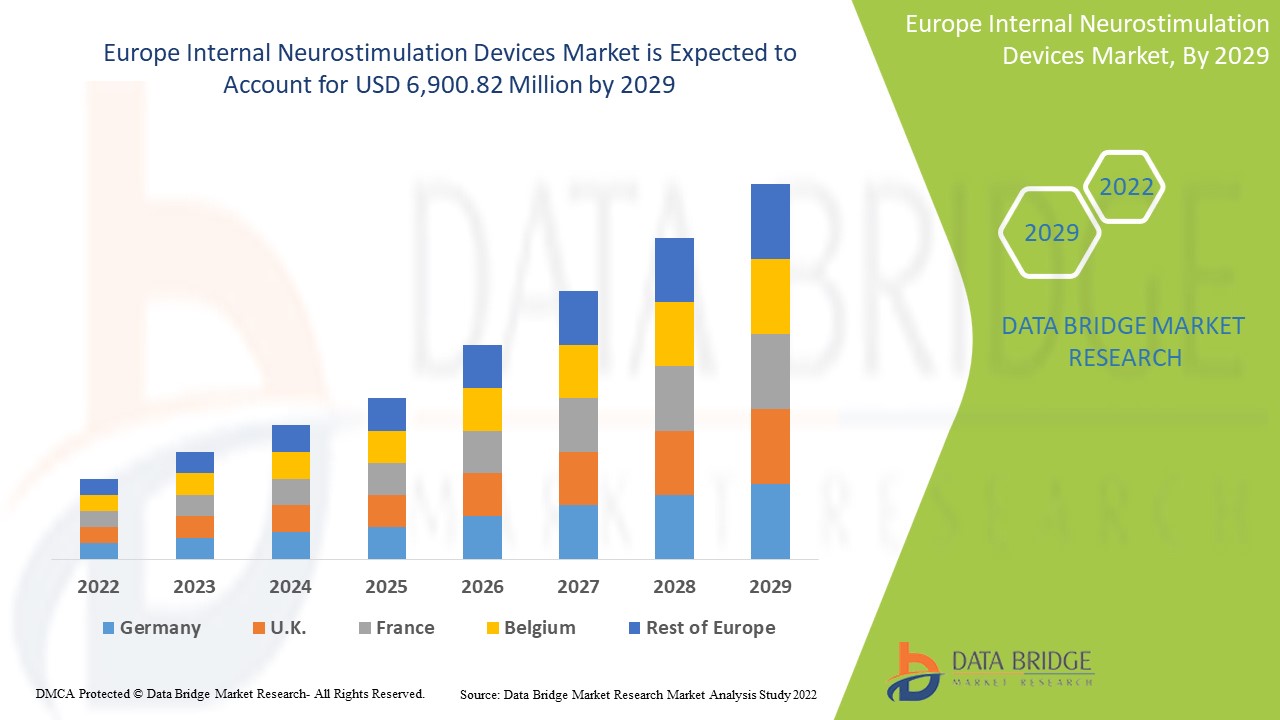
Analyse et perspectives du marché des dispositifs de neurostimulation interne en Europe
L'augmentation de la demande d'appareils de neurostimulation interne en tant que thérapie complémentaire, l'augmentation de la prévalence et de l'incidence des maladies neurologiques, l'augmentation du financement des appareils de neurostimulation, les avancées technologiques dans les appareils de neurostimulation interne et l'augmentation des approbations de produits devraient stimuler la croissance du marché.


Les initiatives stratégiques des acteurs du marché et l'augmentation du financement des acteurs publics et privés pour les dispositifs de neurostimulation interne devraient créer des opportunités de croissance du marché. Cependant, le manque d'experts qualifiés et formés dans les dispositifs de neurostimulation interne devrait freiner la croissance du segment. La disponibilité d'autres dispositifs d'imagerie devrait remettre en cause la croissance du marché.
Data Bridge Market Research analyse que le marché européen des dispositifs de neurostimulation interne croîtra à un TCAC de 18,0 % et 6 900,82 millions USD au cours de la période de prévision de 2022 à 2029.
|
Rapport métrique |
Détails |
|
Période de prévision |
2022 à 2029 |
|
Année de base |
2021 |
|
Années historiques |
2020 (personnalisable de 2019 à 2024) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD, prix en USD |
|
Segments couverts |
Par type de produit ( stimulation de la moelle épinière (SCS), stimulation cérébrale profonde, stimulation du nerf vague, stimulation du nerf sacré et stimulation électrique gastrique ), canal de distribution (appel d'offres direct et fournisseur de services tiers) |
|
Pays couverts |
Allemagne, Royaume-Uni, Italie, Espagne, France, Suisse, Belgique, Pays-Bas, Russie, Turquie et le reste de l'Europe |
|
Acteurs du marché couverts |
Medtronic, LivaNova PLC, Abbott, Microsemi (une filiale de Microchip Technology Inc.), Boston Scientific Corporation, Inspire Medical Systems, Inc., Integer Holdings Corporation, Finetech Medical, ALEVA NEUROTHERAPEUTICS, OptoGenTech GmbH., INBRAIN NEUROELECTRONICS, Neuronano AB, NEURIMPULSE srl, Newronika SpA, NEVRO CORP., Nalu Medical, Inc., Valencia Technologies, CIRTEC, Sequana Medical NV, BIOINDUCTION, ONWARD, Axonics, Inc., Mainstay Medical, entre autres |
Définition du marché
Un dispositif de neurostimulation interne est un dispositif placé chirurgicalement. Il délivre de légers signaux électriques à l'espace épidural près de votre colonne vertébrale via un ou plusieurs fils fins appelés sondes. La neurostimulation soulage la douleur en perturbant les signaux de douleur circulant entre la moelle épinière et le cerveau.
Les dispositifs de neurostimulation comprennent des approches invasives et non invasives impliquant une stimulation électrique pour stimuler la fonction neuronale dans un circuit. La demande accrue de dispositifs de neurostimulation interne est due aux progrès technologiques de nouvelle génération dans les dispositifs de neurostimulation, procurant un soulagement thérapeutique indispensable à un nombre sans précédent de personnes touchées par des troubles neurologiques et psychiatriques débilitants dans le monde entier. L'essor des thérapies de neuromodulation modernes a augmenté au cours d'un demi-siècle, riche en découvertes fortuites et en avancées technologiques qui ont conduit à différentes stratégies de neurostimulation. Au cours des deux dernières décennies, l'innovation dans la technologie des dispositifs médicaux a commencé à accélérer l'évolution de ces systèmes de neurostimulation
Le dispositif de neurostimulation interne le plus pratique utilisé par les patients est la stimulation du nerf vague. Le dispositif de neurostimulation du nerf vague utilise un dispositif pour stimuler le nerf vague avec des impulsions électriques. Un stimulateur du nerf vague implantable est actuellement approuvé par la FDA pour traiter l'épilepsie et la dépression. Il y a un nerf vague de chaque côté du corps, allant du tronc cérébral à travers le cou jusqu'à la poitrine et l'abdomen. À l'avenir, les avancées logicielles telles que la stimulation en boucle fermée et la programmation à distance permettront aux dispositifs de neurostimulation interne d'être une technologie plus personnalisée et plus accessible. L'avenir des dispositifs de neurostimulation interne devrait encore améliorer la qualité de vie.
Dynamique du marché des dispositifs de neurostimulation interne en Europe
Cette section traite de la compréhension des moteurs, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
- Augmentation de la demande d'implants de neurostimulation interne dans les cliniques de neurologie en Europe
La diminution de la demande de ressources médicales par les patients bénéficiant d'une neurostimulation suggère que le traitement par stimulation des nerfs périphériques et de la moelle épinière, bien qu'associé à des coûts initiaux relativement élevés, présente des avantages économiques substantiels à long terme en Europe. La neurostimulation devrait être considérée comme une option viable pour traiter précocement les patients souffrant de douleurs neuropathiques chroniques intraitables.
Par exemple,
- Le centre médical universitaire de Fribourg en Allemagne fournit des implants neurologiques aux patients
La demande croissante de dispositifs de neurostimulation interne implantables en neurologie signifie l’augmentation des investissements liés à la recherche et au développement pour la découverte et le développement de dispositifs de neurostimulation interne avancés et sans douleur, ce qui devrait stimuler la croissance du marché.
- Augmentation du financement des appareils de neurostimulation
L'objectif du financement des dispositifs de neurostimulation interne est d'encourager les applications visant à développer la prochaine génération de dispositifs de stimulation cérébrale pour le traitement des troubles de santé mentale. Les nouveaux dispositifs devraient aller au-delà de la stimulation électrique ou magnétique existante et développer de nouvelles techniques de stimulation capables d'une précision spatiotemporelle accrue et d'approches multifocales en boucle fermée.
Par exemple,
- En février 2022, Nalu Medical a levé environ 104 millions de dollars de financement pour la technologie de neurostimulation. Ce financement a été accordé pour faire progresser ses solutions mini-invasives pour les patients souffrant de douleurs neuropathiques chroniques
Le financement par le gouvernement permettrait d'assurer la sécurité des patients et de réaliser des économies. De plus, les hôpitaux et les organismes de santé pourraient administrer ce traitement à un prix inférieur grâce à la collaboration avec les organismes gouvernementaux. Par conséquent, les progrès dans les activités de recherche et développement et le financement par le gouvernement devraient stimuler la croissance du marché.
Opportunités
- Initiatives stratégiques des acteurs du marché
La demande d'appareils de neurostimulation interne augmente sur le marché en raison de la recherche et du développement et de la croissance du marché des appareils de neurostimulation interne favorisée par le désir de médicaments innovants. Ainsi, les principaux acteurs du marché ont mis en œuvre une nouvelle stratégie en développant de nouveaux appareils et équipements, en collaborant avec d'autres acteurs du marché et en améliorant les opérations commerciales et la rentabilité.
Par exemple,
- En janvier 2021, Boston Scientific a commencé à expédier ses cadres de déclenchement de ligne vertébrale Wave Writer Alpha aux États-Unis
- En mai 2019, les laboratoires Abbott se sont associés au NIH dans le cadre de l'initiative BRAIN (Brain Research through Advancing Innovative Neurotechnologies) pour accélérer les avancées en sciences neurologiques.
Ainsi, les entreprises opérant à l'échelle mondiale sur le marché des dispositifs de neurostimulation adoptent la collaboration pour élargir leur portefeuille de produits avec des produits riches en technologie de pointe afin de stimuler leurs activités dans diverses dimensions. Ainsi, les initiatives stratégiques des principaux acteurs du marché devraient offrir des opportunités significatives aux acteurs du marché opérant sur le marché interne européen des dispositifs de neurostimulation.
- Progrès technologiques dans les dispositifs de neurostimulation interne
Les avancées technologiques dans le domaine des dispositifs de neurostimulation interne utilisent la technologie de neuromodulation, qui délivre directement les agents électriques ou pharmaceutiques à une zone ciblée. Les dispositifs et traitements de neuromodulation et de neurostimulation interne changent la vie. Les avancées technologiques modulent les fonctions neuronales de manière programmable et régulent l'activité neuronale désordonnée. Les résultats peuvent être obtenus avec un minimum d'erreurs.
- En mai 2022, ONWARD a présenté le générateur d'impulsions implantable ARC (IPG) pour stimuler la moelle épinière afin de restaurer le mouvement et la fonction autonome des personnes atteintes de LME et d'autres affections ayant un impact sur la mobilité.
L'essor des applications technologiques dans les appareils d'IRM cérébrale entraînera une réduction des ressources humaines et un diagnostic et une guérison rapides des maladies neurologiques chroniques. À l'avenir, la technologie de l'intelligence artificielle remplacera les appareils d'IRM manuels ouverts et fermés. Cet aspect deviendra une force motrice pour le marché intérieur européen des appareils de neurostimulation.
Contraintes/Défis
- Risques associés à l’implantation de ces dispositifs
Les implants médicaux comportent plusieurs risques, notamment ceux liés à la chirurgie lors de la pose ou du retrait, à l'infection et à la défaillance de l'implant. Les matériaux utilisés dans les implants peuvent potentiellement provoquer des réactions chez certaines personnes. Chaque intervention chirurgicale comporte certains risques. Ceux-ci comprennent des ecchymoses, une gêne, un gonflement et une rougeur au niveau du site chirurgical. Cela entrave proportionnellement la croissance des dispositifs implantaires. La prise de conscience croissante des risques croissants liés aux implants de neurostimulation peut donc freiner la croissance du marché.
Par exemple,
- Échec de l'implant
- Risques chirurgicaux lors de la mise en place ou du retrait
- Risque de détournement de dispositifs implantables
- Hausse des infections
- Les matériaux utilisés dans les implants peuvent provoquer des réactions indésirables chez les patients
Les risques mentionnés ci-dessus peuvent entraver la croissance du marché européen des dispositifs de neurostimulation interne, car les risques pour les patients sont d'une importance capitale. Ainsi, la prise de conscience des risques potentiels des dispositifs implantables constitue un défi pour le marché européen des dispositifs de neurostimulation interne.
- Manque de professionnels de santé qualifiés
Les dispositifs de neurostimulation sont des dispositifs médicaux implantables et programmables qui délivrent une stimulation électrique à des parties spécifiques du cerveau, de la moelle épinière ou du système nerveux périphérique du patient pour aider à traiter diverses pathologies, notamment la douleur chronique, les troubles du mouvement, l'épilepsie et la maladie de Parkinson. L'application de ces technologies puissantes nécessite une procédure de développement coûteuse et des professionnels qualifiés pour manipuler des appareils sensibles. Le remplacement doit être effectué tous les 3 à 6 ans après l'implantation, ce qui représente un fardeau financier et est généralement à la charge de la plupart des patients.
Dans les pays pauvres, seul un nombre relativement restreint de patients peuvent se permettre une thérapie neurologique en raison des prix élevés, d’un système de remboursement peu efficace et d’un manque de personnel médical qualifié. En conséquence, les établissements de santé hésitent à investir dans des technologies nouvelles ou de pointe, ce qui limite l’expansion du marché de la neurostimulation interne. Ces défis freinent donc la croissance du marché.
Impact du COVID-19 sur le marché européen des dispositifs de neurostimulation interne
Pendant la pandémie, le marché européen des dispositifs de neurostimulation interne se concentre sur l'utilisation d'une combinaison de biologie et de technologie de l'information. Pendant la pandémie de COVID-19, de nouveaux défis thérapeutiques se sont ajoutés à ceux habituels des dispositifs de neurostimulation interne. Les patients porteurs d'un dispositif implantable pour perfusion intrathécale ont besoin d'une recharge de la pompe pour éviter le syndrome de sevrage. Les patients porteurs d'implants de neurostimulation peuvent avoir besoin de contrôles en cas d'infection, de déhiscence de plaie ou de migration de sonde.
Développements récents
- En janvier 2022, Medtronic a reçu l'approbation de la Food and Drug Administration (FDA) pour le neurostimulateur rechargeable Intellis, une thérapie de stimulation de la moelle épinière destinée au traitement de la douleur chronique résultant de la neuropathie diabétique périphérique. L'approbation du produit reçue a donné lieu à l'ajout d'un nouveau produit dans la catégorie des produits de stimulation de la moelle épinière. L'approbation devrait être approuvée après la mise sur le marché aux États-Unis
- En juillet 2022, Abbott a reçu l'approbation de la Food and Drug Administration (FDA) pour le système de stimulation cérébrale profonde Infinity pour le traitement de la dépression. L'approbation reçue a entraîné une augmentation du nombre d'approbations avant et après commercialisation et l'ajout d'un nouveau produit au portefeuille de produits
Portée du marché européen des dispositifs de neurostimulation interne
Le marché européen des dispositifs de neurostimulation interne est classé en type de produit et en canal de distribution. La croissance de ces segments vous aidera à analyser les segments de croissance faibles dans les industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour les aider à prendre des décisions stratégiques pour identifier les principales applications du marché.
Type de produit
- Stimulation de la moelle épinière (SCS)
- Stimulation cérébrale profonde
- Stimulation du nerf vague
- Stimulation du nerf sacré
- Stimulation électrique gastrique
Sur la base du type de produit, le marché européen des dispositifs de neurostimulation interne est segmenté en stimulation de la moelle épinière (SCS), stimulation cérébrale profonde, stimulation du nerf vague , stimulation du nerf sacré et stimulation électrique gastrique.
Canal de distribution
- Appel d'offres direct
- Fournisseur de services tiers
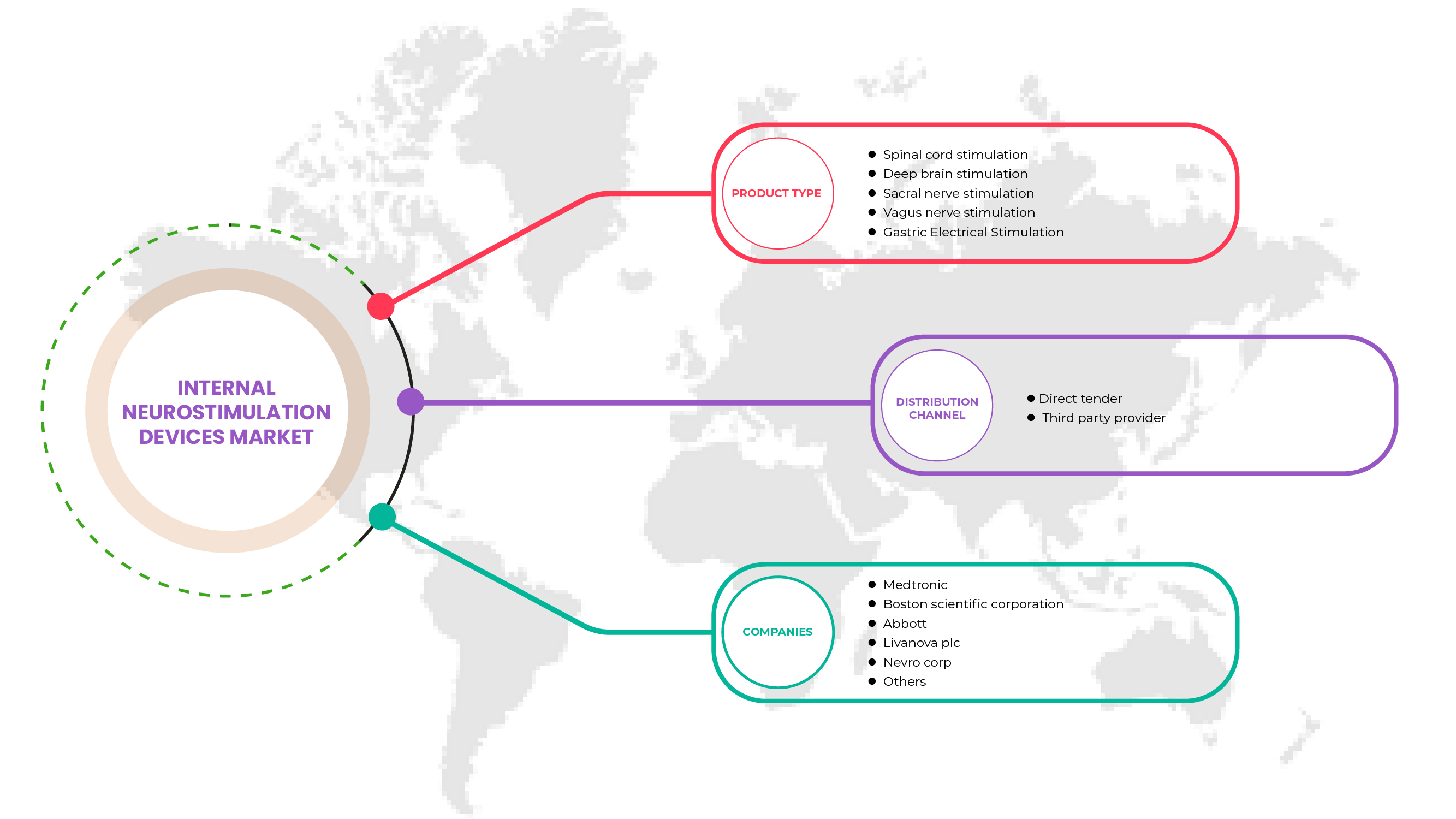
Sur la base du canal de distribution, le marché européen des dispositifs de neurostimulation interne est segmenté en appels d'offres directs et fournisseurs de services tiers.
Analyse/perspectives régionales du marché des dispositifs de neurostimulation interne en Europe
Le marché européen des dispositifs de neurostimulation interne est analysé et les régions fournissent des informations sur la taille du marché et les tendances par pays, type de produit et canal de distribution, comme référencé ci-dessus.
Certains pays couverts par le marché européen des dispositifs de neurostimulation interne sont l'Allemagne, le Royaume-Uni, l'Italie, l'Espagne, la France, la Suisse, la Belgique, les Pays-Bas, la Russie, la Turquie et le reste de l'Europe. L'Allemagne devrait dominer le marché en raison de l'augmentation des activités de recherche et développement, des besoins intégrés en dispositifs de neurostimulation interne et de la demande croissante de dispositifs de neurostimulation avancés. Initiatives stratégiques des acteurs du marché en Allemagne dans le développement de dispositifs de neurostimulation interne et demande accrue de dispositifs de neurostimulation de la moelle épinière.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché national qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que les nouvelles ventes, les ventes de remplacement, la démographie du pays, l'épidémiologie des maladies et les tarifs d'importation et d'exportation sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario du marché pour les différents pays. En outre, la présence et la disponibilité des marques européennes et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales ont un impact sur les canaux de vente, sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché des dispositifs de neurostimulation internes en Europe
Le paysage concurrentiel du marché européen des dispositifs de neurostimulation interne fournit des détails sur le concurrent. Les détails comprennent un aperçu de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, la présence en Europe, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises liées au marché européen des dispositifs de neurostimulation interne.
Français Certains des principaux acteurs opérant sur le marché européen des dispositifs de neurostimulation interne sont Medtronic, LivaNova PLC, Abbott, Microsemi (une filiale de Microchip Technology Inc.), Boston Scientific Corporation, Inspire Medical Systems, Inc., Integer Holdings Corporation, Finetech Medical, ALEVA NEUROTHERAPEUTICS, OptoGenTech GmbH., INBRAIN NEUROELECTRONICS, Neuronano AB, NEURIMPULSE srl, Newronika SpA, NEVRO CORP., Nalu Medical, Inc., Valencia Technologies, CIRTEC, Sequana Medical NV, BIOINDUCTION, ONWARD, Axonics, Inc., Mainstay Medical, entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATION
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHIC SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 RESEARCH METHODOLOGY
2.6 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.7 DBMR MARKET POSITION GRID
2.8 THE CATEGORY VS TIME GRID
2.9 SECONDARY SOURCES
2.1 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
4.3 PIPELINE ANALYSIS FOR INTERNAL NEUROSTIMULATION DEVICES MARKET
5 REGULATIONS OF EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET
6 EPIDEMIOLOGY OF DISEASES
6.1 INCIDENCE OF ISCHEMIA
6.2 INCIDENCE OF PARKINSON'S DISEASE
6.3 INCIDENCE OF FAILED BACK SYNDROME
6.4 INCIDENCE OF TREMOR
6.5 INCIDENCE OF DEPRESSION
6.6 INCIDENCE OF URINE INCONTINENCE
6.7 INCIDENCE OF FECAL INCONTINENCE
6.8 INCIDENCE RATE OF EPILEPSY
6.9 INCIDENCE OF GASTROPARESIS
6.1 PREVALENCE OF OBESITY
7 EPIDEMIOLOGY OF NEUROSTIMULATION PROCEDURES
7.1 NUMBER OF SPINAL CORD STIMULATION (SCS) PROCEDURES
7.1.1 NUMBER OF TEST PROCEDURES
7.1.2 NUMBER OF IMPLANTATION PROCEDURES
7.2 NUMBER OF DEEP BRAIN STIMULATION PROCEDURES
7.3 NUMBER OF VAGUS NERVE STIMULATION PROCEDURES
7.4 NUMBER OF SACRAL NEVER STIMULATION PROCEDURES
7.5 NUMBER OF TRANSCRANIAL MAGNETIC STIMULATION (TMS) PROCEDURES
7.6 NUMBER OF INTERMITTENT THETA BURST STIMULATION (ITBS) PROCEDURES
7.7 NUMBER OF TRANSCRANIAL DIRECT ELECTRICAL STIMULATION (TDCS) PROCEDURES
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISE IN PREVALENCE AND INCIDENCE OF NEUROLOGICAL DISORDERS
8.1.2 DEMAND FOR INTERNAL NEUROSTIMULATION DEVICES AS A ADD ON THERAPY
8.1.3 INCREASED FUNDING FOR THE NEUROSTIMULATION DEVICES
8.1.4 TECHNOLOGICAL ADVANCEMENTS IN THE INTERNAL NEUROSTIMULATION DEVICES
8.1.5 RISE IN PRODUCT APPROVALS
8.2 RESTRAINTS
8.2.1 RISE IN COST OF THE DEEP BRAIN STIMULATION DEVICES
8.2.2 RISKS NOTICED WHILE USING THE INTERNAL NEUROSTIMULATION DEVICES
8.2.3 RISE IN PRODUCT RECALL
8.2.4 AVAILABILITY OF ALTERNATE IMAGING DIAGNOSTIC DEVICES
8.3 OPPORTUNITIES
8.3.1 STRATEGIC INITIATIVES BY THE KEY MARKET PLAYER
8.3.2 RECENT PRODUCT DEVELOPMENTS IN THE INTERNAL NEUROSTIMULATION DEVICES
8.3.3 DEMAND FOR MINIMALLY INVASIVE SURGERY
8.4 CHALLENGES
8.4.1 RISKS ASSOCIATED WITH THE IMPLANTATION OF THESE DEVICES
8.4.2 LACK OF SKILLED HEALTHCARE PROFESSIONALS
9 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE
9.1 OVERVIEW
9.2 SPINAL CORD STIMULATION
9.2.1 SPINAL CORD STIMULATION, BY TYPE
9.2.1.1 BATTERY
9.2.1.1.1 RECHARGEABLE
9.2.1.1.2 NON-RECHARGEABLE
9.2.1.2 LEAD
9.2.1.2.1 PERCUTANEOUS
9.2.1.2.2 PADDLE
9.2.2 SPINAL CORD STIMULATION, BY APPLICATION
9.2.2.1 ISCHEMIA
9.2.2.2 CHRONIC LOW BACK PAIN (CLBP)
9.2.2.3 DIABETIC NEUROPATHY
9.2.2.4 FAILED BACK SYNDROME
9.3 DEEP BRAIN STIMULATION
9.3.1 DEEP BRAIN STIMULATION, BY TYPE
9.3.1.1 SINGLE CHANNEL DEEP BRAIN STIMULATOR
9.3.1.2 DOUBLE CHANNEL DEEP BRAIN STIMULATOR
9.3.1.3 BATTERY
9.3.1.3.1 RECHARGEABLE
9.3.1.3.2 NON-RECHARGEABLE
9.3.1.4 LEAD
9.3.2 DEEP BRAIN STIMULATION, BY APPLICATION
9.3.2.1 PARKINSON’S DISEASE
9.3.2.2 TREMOR
9.3.2.3 DEPRESSION
9.4 SACRAL NERVE STIMULATION
9.4.1 SACRAL NERVE STIMULATION, BY TYPE
9.4.1.1 BATTERY
9.4.1.2 LEAD
9.5 SACRAL NERVE STIMULATION, BY APPLICATION
9.5.1 URINE INCONTINENCE
9.5.2 FECAL INCONTINENCE
9.6 VAGUS NERVE STIMULATION
9.6.1 VAGUS NERVE STIMULATION, BY TYPE
9.6.1.1 BATTERY
9.6.1.2 LEAD
9.6.2 VAGUS NERVE STIMULATION, BY APPLICATION
9.6.2.1 EPILEPSY
9.6.2.2 OTHERS
9.7 GASTRIC ELECTRICAL STIMULATION
9.7.1 GASTRIC ELECTRICAL STIMULATION, BY TYPE
9.7.1.1 BATTERY
9.7.1.2 LEAD
9.7.2 GASTRIC ELECTRICAL STIMULATION, BY APPLICATION
9.7.2.1 GASTROPARESIS
9.7.2.2 OTHERS
10 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL
10.1 OVERVIEW
10.2 DIRECT TENDER
10.3 THIRD PARTY PROVIDER
11 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION
11.1 EUROPE
11.1.1 GERMANY
11.1.2 U.K.
11.1.3 ITALY
11.1.4 SPAIN
11.1.5 FRANCE
11.1.6 SWITZERLAND
11.1.7 BELGIUM
11.1.8 NETHERLANDS
11.1.9 RUSSIA
11.1.10 TURKEY
11.1.11 REST OF EUROPE
12 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: EUROPE
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 MEDTRONIC (2021)
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENTS
14.2 BOSTON SCIENTIFIC CORPORATION (2021)
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENTS
14.3 ABBOTT (2021)
14.3.1 COMPANY SNAPSHOT
14.3.2 REVENUE ANALYSIS
14.3.3 COMPANY SHARE ANALYSIS
14.3.4 PRODUCT PORTFOLIO
14.3.5 RECENT DEVELOPMENTS
14.4 LIVANOVA PLC (2021)
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENTS
14.5 NEVRO CORP. (2021)
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENTS
14.6 AXONICS, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENTS
14.7 ALEVA NEUROTHERAPEUTICS
14.7.1 COMPANY SNAPSHOT
14.7.2 PRODUCT PORTFOLIO
14.7.3 RECENT DEVELOPMENTS
14.8 BIONIC VISION TECHNOLOGIES.
14.8.1 COMPANY SNAPSHOT
14.8.2 PRODUCT PORTFOLIO
14.8.3 RECENT DEVELOPMENTS
14.9 BLUEWIND MEDICAL
14.9.1 COMPANY SNAPSHOT
14.9.2 PRODUCT PORTFOLIO
14.9.3 RECENT DEVELOPMENTS
14.1 BIOINDUCTION
14.10.1 COMPANY SNAPSHOT
14.10.2 PRODUCT PORTFOLIO
14.10.3 RECENT DEVELOPMENT
14.11 CIRTEC
14.11.1 COMPANY SNAPSHOT
14.11.2 PRODUCT PORTFOLIO
14.11.3 RECENT DEVELOPMENTS
14.12 FINETECH MEDICAL
14.12.1 COMPANY SNAPSHOT
14.12.2 PRODUCT PORTFOLIO
14.12.3 RECENT DEVELOPMENTS
14.13 GIMER MEDICAL
14.13.1 COMPANY SNAPSHOT
14.13.2 PRODUCT PORTFOLIO
14.13.3 RECENT DEVELOPMENTS
14.14 INSPIRE MEDICAL SYSTEMS, INC.
14.14.1 COMPANY SNAPSHOT
14.14.2 REVENUE ANALYSIS
14.14.3 PRODUCT PORTFOLIO
14.14.4 RECENT DEVELOPMENTS
14.15 INTEGER HOLDINGS CORPORATION
14.15.1 COMPANY SNAPSHOT
14.15.2 REVENUE ANALYSIS
14.15.3 PRODUCT PORTFOLIO
14.15.4 RECENT DEVELOPMENT
14.16 INBRAIN NEUROELECTRONICS
14.16.1 COMPANY SNAPSHOT
14.16.2 PRODUCT PORTFOLIO
14.16.3 RECENT DEVELOPMENT
14.17 MAINSTAY MEDICAL
14.17.1 COMPANY SNAPSHOT
14.17.2 REVENUE ANALYSIS
14.17.3 PRODUCT PORTFOLIO
14.17.4 RECENT DEVELOPMENTS
14.18 MICROSEMI (A WHOLLY OWNDED SUBSIDIARY OF MICROCHIP TECHNOLOGY INC.)
14.18.1 COMPANY SNAPSHOT
14.18.2 REVENUE ANALYSIS
14.18.3 PRODUCT PORTFOLIO
14.18.4 RECENT DEVELOPMENTS
14.19 MICROTRANSPONDER INC
14.19.1 COMPANY SNAPSHOT
14.19.2 PRODUCT PORTFOLIO
14.19.3 RECENT DEVELOPMENT
14.2 MICRO-LEADS
14.20.1 COMPANY SNAPSHOT
14.20.2 PRODUCT PORTFOLIO
14.20.3 RECENT DEVELOPMENT
14.21 NALU MEDICAL, INC
14.21.1 COMPANY SNAPSHOT
14.21.2 PRODUCT PORTFOLIO
14.21.3 RECENT DEVELOPMENT
14.22 NEURONANO AB
14.22.1 COMPANY SNAPSHOT
14.22.2 PRODUCT PORTFOLIO
14.22.3 RECENT DEVELOPMENTS
14.23 NEURIMPULSE S.R.L.
14.23.1 COMPANY SNAPSHOT
14.23.2 PRODUCT PORTFOLIO
14.23.3 RECENT DEVELOPMENTS
14.24 NEWRONIKA S.P.A.
14.24.1 COMPANY SNAPSHOT
14.24.2 PRODUCT PORTFOLIO
14.24.3 RECENT DEVELOPMENTS
14.25 OPTOGENTECH GMBH.
14.25.1 COMPANY SNAPSHOT
14.25.2 PRODUCT PORTFOLIO
14.25.3 RECENT DEVELOPMENTS
14.26 ONWARD
14.26.1 COMPANY SNAPSHOT
14.26.2 PRODUCT PORTFOLIO
14.26.3 RECENT DEVELOPMENT
14.27 STIMWAVE LLC (2021)
14.27.1 COMPANY SNAPSHOT
14.27.2 PRODUCT PORTFOLIO
14.27.3 RECENT DEVELOPMENTS
14.28 SEQUANA MEDICAL NV (2021)
14.28.1 COMPANY SNAPSHOT
14.28.2 REVENUE ANALYSIS
14.28.3 PRODUCT PORTFOLIO
14.28.4 RECENT DEVELOPMENTS
14.29 VALENCIA TECHNOLOGIES
14.29.1 COMPANY SNAPSHOT
14.29.2 PRODUCT PORTFOLIO
14.29.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
Liste des tableaux
TABLE 1 EUROPE PREVALENCE OF OBESITY
TABLE 2 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 3 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 6 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 7 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 8 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 9 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 10 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 11 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 13 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 15 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 16 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 17 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 18 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 19 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 21 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 22 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 23 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 24 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 26 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 27 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 28 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 29 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 31 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 32 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 33 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 34 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 35 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 36 EUROPE DIRECT TENDER IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 37 EUROPE THIRD PARTY PROVIDER IN INTERNAL NEUROSTIMULATION DEVICES MARKET, 2020-2029 (USD MILLION)
TABLE 38 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 39 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 40 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 41 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 42 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 43 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 44 EUROPE LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 EUROPE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 46 EUROPE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 47 EUROPE SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 48 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 EUROPE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 50 EUROPE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 51 EUROPE BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 52 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 53 EUROPE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 54 EUROPE DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 55 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 56 EUROPE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 57 EUROPE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 58 EUROPE SACRAL NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 59 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 EUROPE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 61 EUROPE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 62 EUROPE VAGUS NERVE STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 EUROPE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 65 EUROPE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 66 EUROPE GASTRIC ELECTRICAL STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 68 GERMANY INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 69 GERMANY SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 70 GERMANY BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 71 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 72 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 73 GERMANY LEAD IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 74 GERMANY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 75 GERMANY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 76 GERMANY SPINAL CORD STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 77 GERMANY DEEP BRAIN STIMULATION IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 78 GERMANY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 79 GERMANY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 80 GERMANY BATTERY IN INTERNAL NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 81 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 82 GERMANY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 83 GERMANY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 84 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 85 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 86 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 87 GERMANY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 88 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 89 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 90 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 91 GERMANY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 92 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 93 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 94 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 95 GERMANY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 96 GERMANY NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 97 U.K. NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 98 U.K. SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 99 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 100 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 101 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 102 U.K. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 103 U.K. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 104 U.K. LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 105 U.K. SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 106 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 107 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 108 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 109 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 110 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 111 U.K. BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 112 U.K. DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 114 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 115 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 116 U.K. SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 117 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 118 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 119 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 120 U.K. VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 121 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 122 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 123 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 124 U.K. GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 125 ITALY NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 126 ITALY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 127 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 128 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 129 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 130 ITALY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 131 ITALY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 132 ITALY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 133 ITALY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 134 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 135 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 136 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 137 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 138 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 139 ITALY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 140 ITALY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 141 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 142 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 143 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 144 ITALY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 145 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 146 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 147 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 148 ITALY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 149 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 150 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 151 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 152 ITALY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 153 ITALY NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 154 SPAIN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 155 SPAIN SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 156 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 157 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 158 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 159 SPAIN LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 160 SPAIN LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 161 SPAIN LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 162 SPAIN SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 163 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 164 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 165 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 166 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 167 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 168 SPAIN BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 169 SPAIN DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 170 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 171 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 172 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 173 SPAIN SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 174 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 175 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 176 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 177 SPAIN VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 178 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 179 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 180 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 181 SPAIN GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 182 SPAIN NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 183 FRANCE NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 184 FRANCE SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 185 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 186 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 187 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 188 FRANCE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 189 FRANCE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 190 FRANCE LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 191 FRANCE SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 192 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 193 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 194 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 195 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 196 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 197 FRANCE BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 198 FRANCE DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 199 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 200 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 201 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 202 FRANCE SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 203 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 204 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 205 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 206 FRANCE VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 207 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 208 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 209 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 210 FRANCE GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 211 FRANCE NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 212 SWITZERLAND NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 213 SWITZERLAND SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 214 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 215 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 216 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 217 SWITZERLAND LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 218 SWITZERLAND LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 219 SWITZERLAND LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 220 SWITZERLAND SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 221 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 222 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 223 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 224 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 225 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 226 SWITZERLAND BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 227 SWITZERLAND DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 228 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 229 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 230 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 231 SWITZERLAND SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 232 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 233 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 234 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 235 SWITZERLAND VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 236 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 237 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 238 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 239 SWITZERLAND GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 240 SWITZERLAND NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 241 BELGIUM NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 242 BELGIUM SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 243 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 244 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 245 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 246 BELGIUM LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 247 BELGIUM LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 248 BELGIUM LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 249 BELGIUM SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 250 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 251 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 252 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 253 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 254 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 255 BELGIUM BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 256 BELGIUM DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 257 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 258 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 259 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 260 BELGIUM SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 261 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 262 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 263 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 264 BELGIUM VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 265 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 266 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 267 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 268 BELGIUM GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 269 BELGIUM NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 270 NETHERLANDS NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 271 NETHERLANDS SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 272 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 273 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 274 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 275 NETHERLANDS LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 276 NETHERLANDS LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 277 NETHERLANDS LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 278 NETHERLANDS SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 279 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 280 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 281 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 282 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 283 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 284 NETHERLANDS BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 285 NETHERLANDS DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 286 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 287 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 288 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 289 NETHERLANDS SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 290 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 291 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 292 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 293 NETHERLANDS VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 294 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 295 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 296 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 297 NETHERLANDS GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 298 NETHERLANDS NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 299 RUSSIA NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 300 RUSSIA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 301 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 302 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 303 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 304 RUSSIA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 305 RUSSIA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 306 RUSSIA LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 307 RUSSIA SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 308 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 309 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)-
TABLE 310 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 311 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 312 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 313 RUSSIA BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 314 RUSSIA DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 315 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 316 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 317 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 318 RUSSIA SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 319 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 320 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 321 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 322 RUSSIA VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 323 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 324 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 325 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 326 RUSSIA GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 327 RUSSIA NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 328 TURKEY NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 329 TURKEY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 330 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 331 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 332 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 333 TURKEY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 334 TURKEY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 335 TURKEY LEAD IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 336 TURKEY SPINAL CORD STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 337 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 338 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 339 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 340 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 341 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (ASP)
TABLE 342 TURKEY BATTERY IN NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (UNITS)
TABLE 343 TURKEY DEEP BRAIN STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 344 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 345 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 346 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 347 TURKEY SACRAL NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 348 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 349 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 350 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 351 TURKEY VAGUS NERVE STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 352 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 353 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (ASP)
TABLE 354 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY TYPE, 2020-2029 (UNITS)
TABLE 355 TURKEY GASTRIC ELECTRICAL STIMULATION IN NEUROSTIMULATION DEVICES MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 356 TURKEY NEUROSTIMULATION DEVICES MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 357 REST OF EUROPE NEUROSTIMULATION DEVICES MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: SEGMENTATION
FIGURE 2 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: GEOGRAPHIC SCOPE
FIGURE 3 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: DATA TRIANGULATION
FIGURE 4 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: SNAPSHOT
FIGURE 5 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BOTTOM UP APPROACH
FIGURE 6 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: TOP DOWN APPROACH
FIGURE 7 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: INTERVIEWS BY REGION AND DESIGNATION
FIGURE 8 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: THE CATEGORY VS TIME GRID
FIGURE 10 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET SEGMENTATION
FIGURE 11 INCREASE IN PREVALENCE AND INCIDENCE OF NEUROLOGICAL DISEASES AND DEMAND FOR INTERNAL NEUROSTIMULATION DEVICES AS A ADD ON THERAPY ARE EXPECTED TO DRIVE THE EUROPE INTERNAL NEUROSTIMULATIOJ DEVICES MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SPINAL CORD STIMULATION IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET IN 2022 TO 2029
FIGURE 13 EUROPE INCIDENCE OF ISCHEMIA
FIGURE 14 EUROPE INCIDENCE OF PARKINSON'S DISEASES
FIGURE 15 EUROPE INCIDENCE OF TREMOR
FIGURE 16 EUROPE INCIDENCE RATE OF EPILEPSY
FIGURE 17 EUROPE INCIDENCE RATE OF GASTROPARESIS
FIGURE 18 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET
FIGURE 19 INCIDENCE OF ADULT ONSET BRAIN DISORDERS IN THE U.S. IN 2021
FIGURE 20 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, 2021
FIGURE 21 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, 2022-2029 (USD MILLION)
FIGURE 22 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, CAGR (2022-2029)
FIGURE 23 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 24 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 25 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 26 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 27 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 28 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: SNAPSHOT (2021)
FIGURE 29 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2021)
FIGURE 30 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2022 & 2029)
FIGURE 31 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY COUNTRY (2021 & 2029)
FIGURE 32 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: BY PRODUCT TYPE (2022-2029)
FIGURE 33 EUROPE INTERNAL NEUROSTIMULATION DEVICES MARKET: COMPANY SHARE 2021 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.













