Marché européen du diagnostic du cancer du col de l'utérus par type de produit (test d'imagerie, test de dépistage, examen visuel, biopsies cervicales et autres procédures), groupe d'âge (moins de 21 ans, 21-29 ans, 30-65 ans, 65 ans et plus), stades (stade I, stade II, stade III, stade IV), utilisateurs finaux (hôpitaux, laboratoires de diagnostic, cliniques spécialisées, centres de santé communautaires, organisation de recherche sur le cancer, centres de cancérologie et de radiothérapie), canal de distribution (appel d'offres direct, ventes au détail, ventes en ligne) - Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et perspectives du marché européen du diagnostic du cancer du col de l'utérus
Le marché européen du diagnostic du cancer du col de l'utérus connaît une croissance au cours de l'année de prévision en raison de l'augmentation du nombre d'acteurs du marché et de la disponibilité de divers produits et marques de diagnostic du cancer du col de l'utérus. Parallèlement à cela, les acteurs du marché sont engagés dans des diagnostics avancés du cancer du col de l'utérus. La prévalence croissante du cancer du col de l'utérus devrait encore stimuler la croissance du marché. Cependant, les règles et réglementations strictes pourraient freiner la croissance du marché au cours de la période de prévision. Les diverses collaborations gouvernementales et privées, l'augmentation des activités de R&D et les initiatives stratégiques des acteurs du marché offrent des opportunités au marché. Cependant, les faux résultats des tests de dépistage du cancer du col de l'utérus devraient constituer un défi majeur pour la croissance du marché.
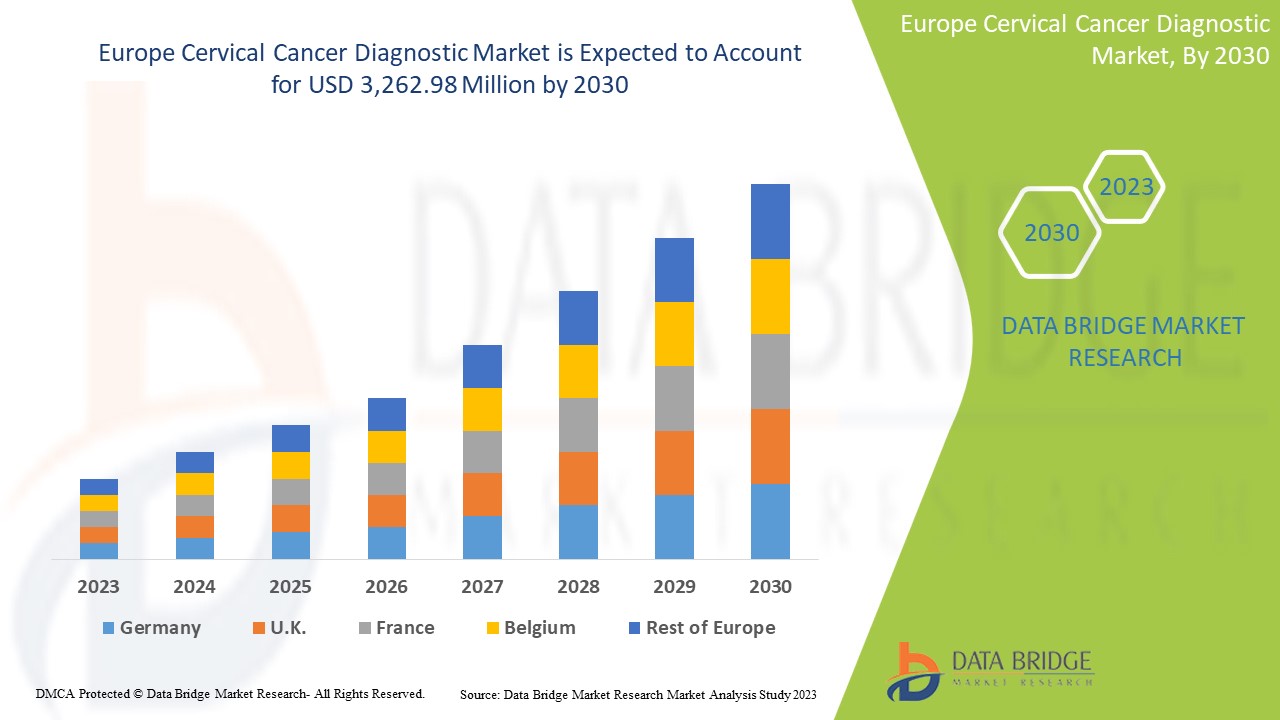
Le marché européen du diagnostic du cancer du col de l'utérus devrait connaître une croissance de marché au cours de la période de prévision de 2023 à 2030. Data Bridge Market Research analyse que le marché croît avec un TCAC de 6,4 % au cours de la période de prévision de 2023 à 2030 et devrait atteindre 3 262,98 millions USD d'ici 2030.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable pour 2020-2015) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD |
|
Segments couverts |
Par type de produit (test d'imagerie, test de dépistage, examen visuel, biopsies cervicales et autres procédures), groupe d'âge (moins de 21 ans, 21-29 ans, 30-65 ans, 65 ans et plus), stades (stade I, stade II, stade III, stade IV), utilisateurs finaux (hôpitaux, laboratoires de diagnostic, cliniques spécialisées, centres de santé communautaires, organismes de recherche sur le cancer, centres de cancérologie et de radiothérapie), canal de distribution (appel d'offres direct, ventes au détail, ventes en ligne) |
|
Pays couverts |
Allemagne, France, Royaume-Uni, Italie, Espagne, Pays-Bas, Russie, Danemark, Suisse, Turquie, Reste de l'Europe |
|
Acteurs du marché couverts |
Siemens Healthcare GmbH, BD, F. Hoffmann-La Roche Ltd, Abbott, Hologic, Inc., Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., QIAGEN, The Cooper Companies Inc., Seegene Inc., Sysmex Corporation, MobileODT, Zilico, Jiangsu Mole Bioscience Co. Ltd., Guided Therapeutics, Inc., GenomeMe Lab Inc., Arbor Vita Corporation et LCM GENECT Srl |
Définition du marché :
Le cancer du col de l’utérus est un type de cancer qui se développe dans le col de l’utérus de l’appareil reproducteur féminin. Sa prévalence augmente à mesure que le nombre de patientes infectées par le VPH augmente et que l’accent est mis sur la détection et le traitement précoces, ce qui devrait accélérer le développement du diagnostic du cancer du col de l’utérus. L’augmentation des investissements publics dans la sensibilisation à la détection précoce du cancer et l’augmentation des dépenses de santé stimuleraient également la croissance des entreprises. La cause la plus courante du cancer du col de l’utérus est l’infection par le VPH (virus du papillome humain). D’autres choix de vie peuvent augmenter le risque, notamment le tabagisme, l’alcool, un régime pauvre en fruits et légumes, la prise de pilules contraceptives et les relations sexuelles à l’adolescence. Étant donné que le cancer du col de l’utérus est traitable une fois diagnostiqué à un stade précoce, les femmes à risque de développer la maladie doivent subir des tests de routine pour identifier la maladie à un stade précoce, ce qui permet au marché de se développer.
Dynamique du marché européen du diagnostic du cancer du col de l'utérus
Cette section traite de la compréhension des moteurs, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
-
Sensibilisation accrue au diagnostic précoce du cancer du col de l'utérus
Il existe une très grande variété de facteurs de risque pour le cancer du col de l'utérus. Par conséquent, la sensibilisation à son diagnostic a augmenté ces dernières années. Différents tests de diagnostic sont disponibles, tels que le test PAP (Papanicolaou), le test du papillomavirus humain , la colposcopie , les biopsies cervicales et la cystoscopie, entre autres. Par conséquent, pour réduire les facteurs de risque, un diagnostic précoce est très important.
-
Augmentation de la prévalence et de l’incidence du cancer du col de l’utérus
Le cancer du col de l'utérus est un type de cancer qui se développe dans le col de l'utérus de l'appareil reproducteur féminin. Le développement irrégulier de cellules cancéreuses dans le tissu du col de l'utérus définit souvent le cancer du col de l'utérus. L'adénocarcinome ou le carcinome épidermoïde peuvent se développer à partir du cancer du col de l'utérus. La cause la plus courante du cancer du col de l'utérus est l'infection par le VPH (virus du papillome humain). Le cancer du col de l'utérus est classé en deux types : l'adénocarcinome et le carcinome épidermoïde. Le cancer du col de l'utérus est diagnostiqué à l'aide d'une variété de tests, d'outils et de procédures de laboratoire avancés qui évaluent les cellules et les souches anormales du virus du papillome humain (VPH).
Opportunités
-
Augmentation des dépenses de santé
Les dépenses de santé ont augmenté dans le monde entier à mesure que le revenu disponible des citoyens de divers pays augmentait. De plus, pour répondre aux besoins de la population, les organismes gouvernementaux et les organisations de santé prennent des initiatives pour accélérer les dépenses de santé. L'augmentation des dépenses de santé aide simultanément les établissements de santé à améliorer leurs installations de traitement pour le diagnostic du cancer du col de l'utérus, car cette maladie est très répandue ces dernières années.
En outre, les initiatives stratégiques prises par les principaux acteurs du marché assureront l’intégrité structurelle et les opportunités futures du marché européen du diagnostic du cancer du col de l’utérus au cours de la période de prévision.
Contraintes/Défis
Cependant, les médicaments de diagnostic du cancer du col de l’utérus peuvent avoir des effets secondaires indésirables ; il est difficile d’équilibrer les risques avec les avantages du traitement en raison des effets secondaires croissants des médicaments contre le cancer qui entravent la demande du marché.
De plus, l’approbation croissante des vaccins contre le VPH par les autorités réglementaires devrait freiner la croissance du marché européen du diagnostic du cancer du col de l’utérus. Ces développements se produisent rapidement dans le monde entier pour réduire les cas de cancer du col de l’utérus, ce qui peut constituer un facteur de restriction pour le marché.
L'utilisation de divers médicaments de traitement à travers le monde augmente rapidement et, avec la prévalence croissante du cancer du col de l'utérus, il est nécessaire de procéder à un diagnostic et à un traitement rapides. Dans le même temps, les fabricants de diagnostics du cancer du col de l'utérus sur le marché doivent suivre certaines réglementations pour obtenir l'approbation des autorités supérieures pour lancer le produit sur le marché. Ces directives strictes doivent être respectées ; c'est l'une des tâches les plus difficiles de toutes les étapes. L'approbation préalable à la mise sur le marché de divers médicaments varie d'un pays à l'autre.
Développement récent
- En août 2020, Siemens Healthcare GmbH a conclu un accord d'acquisition avec Varian Medical Systems, Inc. ; avec cette acquisition, Siemens Healthcare a contribué à développer des solutions avancées pour traiter le cancer et à renforcer sa position dans le secteur de la santé
Portée du marché européen du diagnostic du cancer du col de l'utérus
Le marché européen du diagnostic du cancer du col de l'utérus est segmenté en type de produit, tranche d'âge, stades, utilisateurs finaux et canal de distribution. La croissance parmi ces segments vous aidera à analyser les segments de faible croissance dans les industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour prendre des décisions stratégiques afin d'identifier les principales applications du marché.
Type de produit
- Test d'imagerie
- Test de dépistage
- Examen visuel
- Biopsies cervicales
- Autres procédures
Sur la base du type de produit, le marché européen du diagnostic du cancer du col de l'utérus est segmenté en test d'imagerie, test de dépistage, examen visuel, biopsies cervicales et autres procédures.
Groupe d'âge
- Moins de 21 ans
- 21-29
- 30-65
- 65 ans et plus
Sur la base de la tranche d'âge, le marché européen du diagnostic du cancer du col de l'utérus est segmenté en moins de 21 ans, 21-29 ans, 30-65 ans et 65 ans et plus.
Étapes
- Étape I
- Stade II
- Stade III
- Stade IV
Sur la base du stade, le marché européen du diagnostic du cancer du col de l’utérus est segmenté en stade I, stade II, stade III et stade IV.
Utilisateurs finaux
- Centres de cancérologie et de radiothérapie
- Hôpitaux
- Cliniques spécialisées
- Organisation de recherche sur le cancer
- Laboratoires de diagnostic
- Centres de santé communautaires
Sur la base des utilisateurs finaux, le marché européen du diagnostic du cancer du col de l'utérus est segmenté en hôpitaux, laboratoires de diagnostic, cliniques spécialisées, centres de santé communautaires, organisations de recherche sur le cancer et centres de cancérologie et de radiothérapie.
Canal de distribution
- Appel d'offres direct
- Ventes au détail
- Ventes en ligne

Sur la base du canal de distribution, le marché européen du diagnostic du cancer du col de l’utérus est segmenté en appels d’offres directs, ventes au détail et ventes en ligne.
Analyse/perspectives régionales du marché du diagnostic du cancer du col de l'utérus
Le marché européen du diagnostic du cancer du col de l’utérus est analysé et des informations et tendances sur la taille du marché sont fournies par pays, type de produit, tranche d’âge, étapes, utilisateurs finaux et canal de distribution comme référencé ci-dessus.
Certains pays couverts sur le marché européen du diagnostic du cancer du col de l’utérus sont l’Allemagne, la France, le Royaume-Uni, l’Italie, l’Espagne, les Pays-Bas, la Russie, le Danemark, la Suisse, la Turquie et le reste de l’Europe.
L'Allemagne devrait dominer le marché européen du diagnostic du cancer du col de l'utérus en termes de part de marché et de chiffre d'affaires et continuera à accroître sa domination au cours de la période de prévision. Cela est dû à l'augmentation du nombre d'acteurs du marché et à la disponibilité de divers produits et marques de diagnostic du cancer du col de l'utérus.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données, tels que les ventes de produits neufs et de remplacement, la démographie des pays, l'épidémiologie des maladies et les tarifs d'importation et d'exportation, sont quelques-uns des principaux indicateurs utilisés pour prévoir le scénario du marché pour les différents pays. En outre, la présence et la disponibilité des marques européennes et les défis auxquels elles sont confrontées en raison de la forte concurrence des marques locales et nationales et l'impact des canaux de vente sont pris en compte tout en fournissant une analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché du diagnostic du cancer du col de l'utérus en Europe
Le paysage concurrentiel du marché européen du diagnostic du cancer du col de l'utérus fournit des détails sur les concurrents. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les investissements dans la recherche et le développement, les nouvelles initiatives du marché, la présence en Europe, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que les entreprises se concentrant sur le marché européen du diagnostic du cancer du col de l'utérus.
Certains des principaux acteurs opérant sur le marché européen du diagnostic du cancer du col de l'utérus sont Siemens Healthcare GmbH, BD, F. Hoffmann-La Roche Ltd, Abbott, Hologic, Inc., Quest Diagnostics Incorporated, Bio-Rad Laboratories, Inc., QIAGEN, The Cooper Companies Inc., Seegene Inc., Sysmex Corporation, MobileODT, Zilico, Jiangsu Mole Bioscience Co. Ltd., Guided Therapeutics, Inc., GenomeMe Lab Inc., Arbor Vita Corporation et LCM GENECT Srl, entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE CERVICAL CANCER DIAGNOSTIC MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRODUCT TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
4.3 INDUSTRY INSIGHTS:
4.3.1 CERVICAL CANCER DIAGNOSIS
4.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
5 REGULATORY FRAMEWORK
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF CERVICAL CANCER
6.1.2 RISING AWARENESS OF EARLY DIAGNOSIS OF CERVICAL CANCER
6.1.3 HIGH PREVALENCE OF HPV-INFECTED PATIENTS AND RISING INCIDENCE OF TEENAGE SEXUAL ENCOUNTERS
6.2 RESTRAINTS
6.2.1 DEVELOPMENT IN THE FIELD OF HPV VACCINE
6.2.2 SIDE EFFECTS OF TREATMENT DRUGS
6.3 OPPORTUNITIES
6.3.1 RISING HEALTHCARE EXPENDITURE
6.3.2 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.4 CHALLENGES
6.4.1 STRINGENT RULES AND REGULATIONS
6.4.2 FALSE RESULTS IN SCREENING TESTS AND UNAVAILABILITY OF IMPROVED HEALTHCARE INFRASTRUCTURE
7 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE
7.1 OVERVIEW
7.2 SCREENING TEST
7.2.1 HPV TEST
7.2.1.1 ASSAYS
7.2.1.2 KITS/REAGENTS
7.2.2 PAP TEST
7.2.2.1 ASSAYS
7.2.2.2 KITS/REAGENTS
7.2.3 BLOOD TEST
7.3 IMAGING TEST
7.3.1 PET CT-SCAN
7.3.2 MAGNETIC RESONANCE IMAGING (MRI)
7.3.3 ULTRASOUND
7.3.4 X-RAY
7.3.5 OTHERS
7.4 CERVICAL BIOPSIES
7.4.1 LIQUID BIOPSY
7.4.2 ENDOCERVICAL CURETTAGE
7.4.3 COLPOSCOPIC BIOPSY
7.5 VISUAL EXAMINATION
7.5.1 CYSTOSCOPY
7.5.2 SIGMOIDOSCOPY
7.6 OTHER PROCEDURES
8 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP
8.1 OVERVIEW
8.2 30-65
8.3 65 AND ABOVE
8.4 21-29
8.5 BELOW 21
9 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES
9.1 OVERVIEW
9.2 STAGE I
9.3 STAGE II
9.4 STAGE III
9.5 STAGE IV
10 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER
10.1 OVERVIEW
10.2 CANCER AND RADIATION THERAPY CENTERS
10.3 HOSPITALS
10.4 SPECIALTY CLINICS
10.5 CANCER RESEARCH ORGANIZATION
10.6 DIAGNOSTIC LABORATORIES
10.7 COMMUNITY HEALTH CENTERS
11 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 RETAIL SALES
11.4 ONLINE SALES
12 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION
12.1 EUROPE
12.1.1 GERMANY
12.1.2 U.K.
12.1.3 FRANCE
12.1.4 ITALY
12.1.5 SPAIN
12.1.6 BELGIUM
12.1.7 RUSSIA
12.1.8 NETHERLANDS
12.1.9 SWITZERLAND
12.1.10 TURKEY
12.1.11 REST OF EUROPE
13 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: EUROPE
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 SIEMENS HEALTHCARE GMBH
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUE ANALYSIS
15.1.3 COMPANY SHARE ANALYSIS
15.1.4 PRODUCT PORTFOLIO
15.1.5 RECENT DEVELOPMENT
15.2 BD
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUE ANALYSIS
15.2.3 COMPANY SHARE ANALYSIS
15.2.4 PRODUCT PORTFOLIO
15.2.5 RECENT DEVELOPMENT
15.3 F. HOFFMANN- LA ROCHE LTD
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUE ANALYSIS
15.3.3 COMPANY SHARE ANALYSIS
15.3.4 PRODUCT PORTFOLIO
15.3.5 RECENT DEVELOPMENT
15.4 ABBOTT
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 COMPANY SHARE ANALYSIS
15.4.4 PRODUCT PORTFOLIO
15.4.5 RECENT DEVELOPMENTS
15.5 HOLOGIC, INC.
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 COMPANY SHARE ANALYSIS
15.5.4 PRODUCT PORTFOLIO
15.5.5 RECENT DEVELOPMENTS
15.6 ARBOR VITA CORPORATION
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENTS
15.7 BIO-RAD LABORATORIES, INC.
15.7.1 COMPANY SNAPSHOT
15.7.2 REVENUE ANALYSIS
15.7.3 PRODUCT PORTFOLIO
15.7.4 RECENT DEVELOPMENT
15.8 GENOMEME LAB INC.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 GUIDED THERAPEUTICS, INC
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENT
15.1 JIANGSU MOLE BIOSCIENCE CO., LTD.
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENTS
15.11 LCM GENECT SRL
15.11.1 COMPANY SNAPSHOT
15.11.2 PRODUCT PORTFOLIO
15.11.3 RECENT DEVELOPMENT
15.12 MOBILEODT
15.12.1 COMPANY SNAPSHOT
15.12.2 PRODUCT PORTFOLIO
15.12.3 RECENT DEVELOPMENT
15.13 QIAGEN
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUE ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 QUEST DIAGNOSTICS INCORPORATED
15.14.1 COMPANY SNAPSHOT
15.14.2 REVENUE ANALYSIS
15.14.3 PRODUCT PORTFOLIO
15.14.4 RECENT DEVELOPMENTS
15.15 SEEGENE INC.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENTS
15.16 SYSMEX CORPORATION
15.16.1 COMPANY SNAPSHOT
15.16.2 PRODUCT PORTFOLIO
15.16.3 RECENT DEVELOPMENT
15.17 THE COOPER COMPANIES INC
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUE ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 ZILICO
15.18.1 COMPANY SNAPSHOT
15.18.2 PRODUCT PORTFOLIO
15.18.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Liste des tableaux
TABLE 1 CERVICAL CANCER CAN BE DIAGNOSED BY VARIOUS TESTS AND BIOPSIES, AS LISTED BELOW:
TABLE 2 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 3 EUROPE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 EUROPE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 5 EUROPE HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 6 EUROPE PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 7 EUROPE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 EUROPE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 EUROPE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 11 EUROPE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 EUROPE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE OTHER PROCEDURES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 15 EUROPE 30-65 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 EUROPE 65 AND ABOVE IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 EUROPE 21-29 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 EUROPE BELOW 21 IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 20 EUROPE STAGE I IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 EUROPE STAGE II IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 EUROPE STAGE III IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 EUROPE STAGE IV IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 25 EUROPE CANCER AND RADIATION THERAPY CENTERS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 EUROPE HOSPITALS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 EUROPE SPECIALTY CLINICS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 EUROPE CANCER RESEARCH ORGANIZATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 EUROPE DIAGNOSTIC LABORATORIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 EUROPE COMMUNITY HEALTH CENTERS IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 32 EUROPE DIRECT TENDER IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 EUROPE RETAIL SALES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 EUROPE ONLINE SALES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 36 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 37 EUROPE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 38 EUROPE HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 39 EUROPE PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 40 EUROPE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 41 EUROPE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 42 EUROPE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 43 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 44 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 45 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 47 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 48 GERMANY SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 49 GERMANY HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 50 GERMANY PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 51 GERMANY IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 52 GERMANY CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 53 GERMANY VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 54 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 55 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 56 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 57 GERMANY CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 58 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 59 U.K. SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 60 U.K. HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 61 U.K. PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 62 U.K. IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 63 U.K. CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 64 U.K. VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 65 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 66 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 67 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 68 U.K. CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 69 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 75 FRANCE VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 76 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 77 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 78 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 79 FRANCE CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 80 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 81 ITALY SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 82 ITALY HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 83 ITALY PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 84 ITALY IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 85 ITALY CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 86 ITALY VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 87 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 88 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 89 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 90 ITALY CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 91 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 92 SPAIN SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 93 SPAIN HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 94 SPAIN PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 95 SPAIN IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 96 SPAIN CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 97 SPAIN VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 98 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 99 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 100 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 101 SPAIN CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 102 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 103 BELGIUM SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 104 BELGIUM HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 105 BELGIUM PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 106 BELGIUM IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 107 BELGIUM CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 108 BELGIUM VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 109 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 110 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 111 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 112 BELGIUM CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 113 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 114 RUSSIA SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 115 RUSSIA HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 116 RUSSIA PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 117 RUSSIA IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 118 RUSSIA CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 119 RUSSIA VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 120 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 121 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 122 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 123 RUSSIA CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 124 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 125 NETHERLANDS SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 126 NETHERLANDS HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 127 NETHERLANDS PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 128 NETHERLANDS IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 129 NETHERLANDS CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 130 NETHERLANDS VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 131 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 132 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 133 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 134 NETHERLANDS CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 135 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 136 SWITZERLAND SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 137 SWITZERLAND HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 138 SWITZERLAND PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 139 SWITZERLAND IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 140 SWITZERLAND CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 141 SWITZERLAND VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 142 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 143 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 144 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 145 SWITZERLAND CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 146 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 147 TURKEY SCREENING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 148 TURKEY HPV TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 149 TURKEY PAP TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 150 TURKEY IMAGING TEST IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 151 TURKEY CERVICAL BIOPSIES IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 152 TURKEY VISUAL EXAMINATION IN CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
TABLE 153 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY AGE GROUP, 2021-2030 (USD MILLION)
TABLE 154 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 155 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 156 TURKEY CERVICAL CANCER DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 157 REST OF EUROPE CERVICAL CANCER DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: MARKET END USER COVERAGE GRID
FIGURE 9 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 THE RISING PREVALENCE AND INCIDENCE OF CERVICAL CANCER ARE EXPECTED TO DRIVE THE EUROPE CERVICAL CANCER DIAGNOSTIC MARKET IN THE FORECAST PERIOD
FIGURE 12 SCREENING TEST SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE CERVICAL CANCER DIAGNOSTIC MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE CERVICAL CANCER DIAGNOSTIC MARKET
FIGURE 14 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, 2022
FIGURE 15 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, 2023-2030 (USD MILLION)
FIGURE 16 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, CAGR (2023-2030)
FIGURE 17 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY PRODUCT TYPE, LIFELINE CURVE
FIGURE 18 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP 2022
FIGURE 19 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, CAGR (2023-2030)
FIGURE 21 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY AGE GROUP, LIFELINE CURVE
FIGURE 22 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, 2022
FIGURE 23 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 24 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 25 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY STAGES, LIFELINE CURVE
FIGURE 26 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, 2022
FIGURE 27 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET : BY END USER, LIFELINE CURVE
FIGURE 30 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: SNAPSHOT (2022)
FIGURE 35 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2022)
FIGURE 36 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: PRODUCT TYPE (2023-2030)
FIGURE 39 EUROPE CERVICAL CANCER DIAGNOSTIC MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.













