Marché européen du diagnostic du cancer de la vessie, par type de test (cystoscopie, test de laboratoire d'urine, biopsie, test d'imagerie et autres), stades (stade I, stade II, stade III et stade IV), type de cancer (cancer de la vessie à cellules transitionnelles, cancer de la vessie à cellules squameuses et autres types de cancer), utilisateur final (hôpital, centres d'imagerie diagnostique, instituts de recherche sur le cancer, laboratoires de diagnostic indépendants et laboratoires associés), canal de distribution (appel d'offres direct et ventes au détail) - Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et taille du marché européen du diagnostic du cancer de la vessie
Le marché européen du diagnostic du cancer de la vessie est fragmenté par nature, car il se compose de nombreux acteurs européens tels que F. Hoffmann-La Roche Ltd, Merck KGaA, Thermo Fisher Scientific Inc., Koninklijke Philips NV et Bio-Rad Laboratories, Inc., entre autres. La présence de ces entreprises génère des prix compétitifs pour le diagnostic du cancer de la vessie dans toute la région. En raison de la présence de ces acteurs aux niveaux régional et international, les fournisseurs et les fabricants proposent des produits avec des spécifications et des caractéristiques différentes pour tous les budgets. La prévalence croissante du cancer de la vessie stimule la croissance du marché. En outre, la sensibilisation croissante à la détection précoce du cancer de la vessie devrait stimuler la croissance du marché.
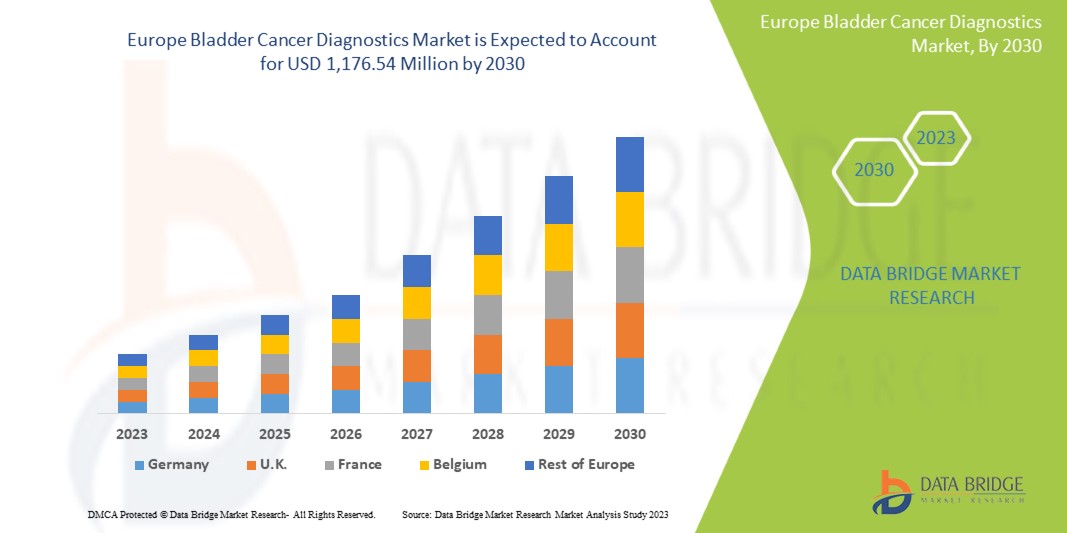
Data Bridge Market Research estime que le marché européen du diagnostic du cancer de la vessie devrait atteindre la valeur de 1 176,54 millions USD d'ici 2030, à un TCAC de 8,0 % au cours de la période de prévision. Ce rapport de marché couvre également en profondeur l'analyse des prix et les avancées technologiques.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable de 2015 à 2020) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD |
|
Segments couverts |
Type de test (cystoscopie, test de laboratoire d'urine, biopsie, test d'imagerie et autres), stades (stade I, stade II, stade III et stade IV), type de cancer (cancer de la vessie à cellules transitionnelles, cancer de la vessie à cellules squameuses et autres types de cancer), utilisateur final (hôpital, centres d'imagerie diagnostique, instituts de recherche sur le cancer, laboratoires de diagnostic indépendants et laboratoires associés), canal de distribution (appel d'offres direct et vente au détail) |
|
Pays couverts |
Allemagne, France, Royaume-Uni, Italie, Espagne, Pays-Bas, Russie, Suisse, Turquie, Belgique, Reste de l'Europe |
|
Acteurs du marché couverts |
F. Hoffmann-La Roche Ltd, Merck KGaA, Thermo Fisher Scientific Inc., Koninklijke Philips NV, Bio-Rad Laboratories, Inc., Agilent Technologies, Inc., FUJIFILM Corporation, CANON MEDICAL SYSTEMS CORPORATION, Siemens Healthcare GmbH, BD, Illumina, Inc. Neusoft Corporation, Abbott, General Electric Company, Hologic Inc., QIAGEN, Cepheid, Ambu A/S, Time Medical Holding et MinFound Medical Systems Co., entre autres |
Définition du marché
Le cancer de la vessie débute lorsque des cellules saines de la paroi de la vessie, le plus souvent des cellules urothéliales, se modifient et se développent de manière incontrôlable, formant une masse appelée tumeur. Les cellules urothéliales tapissent également le bassinet du rein, les uretères et l’urètre. Le cancer qui se développe dans le bassinet du rein et les uretères est également considéré comme un type de cancer urothélial et est souvent appelé cancer urothélial des voies supérieures. Dans la plupart des cas, il est traité de la même manière que le cancer de la vessie, qui est décrit dans ce guide. Une tumeur peut être cancéreuse ou bénigne. Une tumeur cancéreuse est maligne, ce qui signifie qu’elle peut se développer et se propager à d’autres parties du corps. Une tumeur bénigne signifie que la tumeur peut se développer mais ne se propage pas. Les tumeurs bénignes de la vessie sont très rares.
Dynamique du marché européen du diagnostic du cancer de la vessie
Cette section traite de la compréhension des moteurs, des avantages, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
CONDUCTEURS
Augmentation de l'incidence du cancer de la vessie à l'échelle mondiale
La plupart des mutations génétiques liées au cancer de la vessie se développent au cours de la vie d'une personne plutôt que d'être héritées avant la naissance. Certaines de ces mutations génétiques acquises résultent de l'exposition à des substances chimiques ou à des radiations cancérigènes. Par exemple, les substances chimiques contenues dans la fumée de tabac peuvent être absorbées dans le sang, filtrées par les reins et se retrouver dans l'urine, où elles peuvent affecter les cellules de la vessie. D'autres substances chimiques peuvent atteindre la vessie de la même manière. Cependant, il arrive parfois que les modifications génétiques soient simplement des événements aléatoires qui se produisent parfois à l'intérieur d'une cellule sans cause extérieure.
Le cancer de la vessie touche principalement les personnes âgées. Environ 9 personnes sur 10 atteintes de ce cancer ont plus de 55 ans. L'âge moyen des personnes au moment du diagnostic est de 73 ans. L'augmentation des cas de cancer de la vessie au sein de la population favorise l'utilisation de produits de diagnostic du cancer de la vessie et stimule ainsi la croissance du marché du diagnostic du cancer de la vessie.
Sensibilisation accrue au diagnostic précoce du cancer de la vessie
Le mois de sensibilisation au cancer de la vessie est célébré à l'échelle nationale aux États-Unis au mois de mai. Il vise à rassembler la communauté des personnes atteintes de tumeurs de la vessie pour contribuer à sensibiliser et mettre en lumière une population de patients souvent négligée. Bien que le cancer de la vessie ne soit pas aussi courant que le cancer du sein ou du poumon, le besoin de méthodes nouvelles et innovantes pour traiter les patients atteints de tumeurs de la vessie n'a jamais été aussi crucial. Les recherches suggèrent que plus de 720 000 patients aux États-Unis sont aux prises avec un cancer de la vessie.
Alors que la crise sanitaire actuelle en Europe continue de se transformer en raison de l’impact du COVID-19, l’importance de comprendre les impacts des soins aux patients, de l’éducation et de la recherche sur le cancer devient plus évidente que jamais. En regardant vers l’avenir, il reste encore beaucoup à faire pour découvrir de nouveaux médicaments efficaces pour les patients atteints de tumeurs de la vessie, ainsi qu’un besoin urgent de formation continue sur une multitude de défis auxquels nous sommes confrontés dans le traitement du cancer de la vessie.
Le mois de sensibilisation au cancer de la vessie est un mouvement entièrement dédié à ces efforts, et même en pleine pandémie, la nécessité d'aider cette population de patients reste une priorité absolue. Par conséquent, la sensibilisation croissante au diagnostic précoce du cancer de la vessie stimule la croissance du marché.
OPPORTUNITÉ
Augmentation des procédures de diagnostic du cancer de la vessie
Le cancer de la vessie est l'un des dix cancers les plus fréquents dans le monde. Il est responsable d'une morbidité et d'une mortalité considérables et représente donc un fardeau considérable pour les systèmes de santé. Plusieurs techniques sont utilisées pour diagnostiquer le cancer de la vessie, notamment l'échographie, la tomodensitométrie (TDM), l'imagerie par résonance magnétique (IRM) et, parfois, la tomographie par émission de positons (TEP).
Le traitement du cancer de la vessie à croissance lente peut nécessiter une surveillance. La chimiothérapie pour les tumeurs malignes est parfois associée à une radiothérapie et à une greffe de cellules souches. Les personnes préfèrent davantage ce traitement et obtenir un diagnostic. En outre, l'augmentation des taux de cancer a été un facteur favorisant l'approbation croissante de produits de diagnostic.
RETENUE / DÉFI
Effets secondaires des médicaments et thérapies pour le traitement du cancer de la vessie
La chimiothérapie (chimio) utilise des médicaments anticancéreux qui sont généralement administrés par voie intraveineuse (IV) ou par voie orale. Ces médicaments pénètrent dans la circulation sanguine et atteignent presque toutes les zones du corps. Cependant, de nombreux médicaments de chimiothérapie ne parviennent pas à pénétrer dans la vessie et à atteindre les cellules tumorales. Le bacille de Calmette-Guérin (BCG) est l'immunothérapie intravésicale la plus courante pour traiter le cancer de la vessie à un stade précoce. Le BCG est un germe apparenté à celui qui cause la tuberculose (TB), mais il ne provoque généralement pas de maladies graves. Lorsque le BCG est introduit dans la vessie sous forme liquide par un cathéter, il aide à « activer » les cellules du système immunitaire qui s'y trouvent, qui attaquent ensuite les cellules cancéreuses de la vessie.
Les médicaments de chimiothérapie peuvent provoquer des effets secondaires. Ceux-ci dépendent du type et de la dose des médicaments et de la durée du traitement. Les effets secondaires courants peuvent inclure la perte de cheveux, des aphtes, une perte d'appétit, des nausées et des vomissements, une diarrhée, un risque accru d'infections (en raison d'un manque de globules blancs), des ecchymoses ou des saignements faciles (en raison d'un manque de plaquettes sanguines), de la fatigue (en raison d'un manque de globules rouges, de changements dans le métabolisme ou d'autres facteurs). Certains médicaments de chimiothérapie peuvent également provoquer d'autres effets secondaires moins courants. Par exemple, le cisplatine et le carboplatine peuvent également provoquer des lésions rénales et une perte auditive. Parfois, il peut être nécessaire de réduire les doses de médicaments ou de retarder ou d'arrêter le traitement pour éviter que les effets ne s'aggravent. Par conséquent, les effets secondaires des médicaments et des thérapies de traitement du cancer de la vessie peuvent freiner la croissance du marché européen du diagnostic du cancer de la vessie.
Impact post-COVID-19 sur le marché européen du diagnostic du cancer de la vessie
La COVID-19 a eu un impact négatif sur le marché européen du diagnostic du cancer de la vessie. Les mesures de confinement ont entraîné l'émergence de divers défis, tels que la diminution des visites à l'hôpital et les retards dans les procédures de traitement.
La pandémie de COVID-19 a eu un impact négatif sur le marché. Cependant, l'augmentation du soutien gouvernemental et les progrès des techniques d'imagerie devraient offrir des opportunités lucratives de croissance du marché. De plus, l'augmentation des partenariats, des acquisitions et de la collaboration entre les acteurs du marché devrait alimenter davantage la croissance du marché. En outre, la croissance est élevée depuis l'ouverture du marché après la COVID-19, et on s'attend à ce qu'il y ait une croissance considérable dans le secteur. Les acteurs du marché mènent de multiples activités pour améliorer les techniques d'imagerie pour la détection précoce du cancer de la vessie. Grâce à cela, les entreprises apporteront des progrès et de l'innovation au marché.
Développements récents
- En mai 2020, F. Hoffmann-La Roche Ltd a officiellement acquis Stratos Genomics. Avec cette acquisition, l'entreprise se consacrera également au développement du séquençage basé sur l'ADN à des fins de diagnostic. Cela a renforcé le segment du diagnostic médical de l'entreprise, ce qui lui a permis de générer davantage de revenus.
- En janvier 2023, Thermo Fisher Scientific Inc. a annoncé avoir conclu un accord pour acquérir The Binding Site Group, un leader européen du diagnostic spécialisé. Cette acquisition contribuera au développement du segment du diagnostic spécialisé.
Portée du marché européen du diagnostic du cancer de la vessie
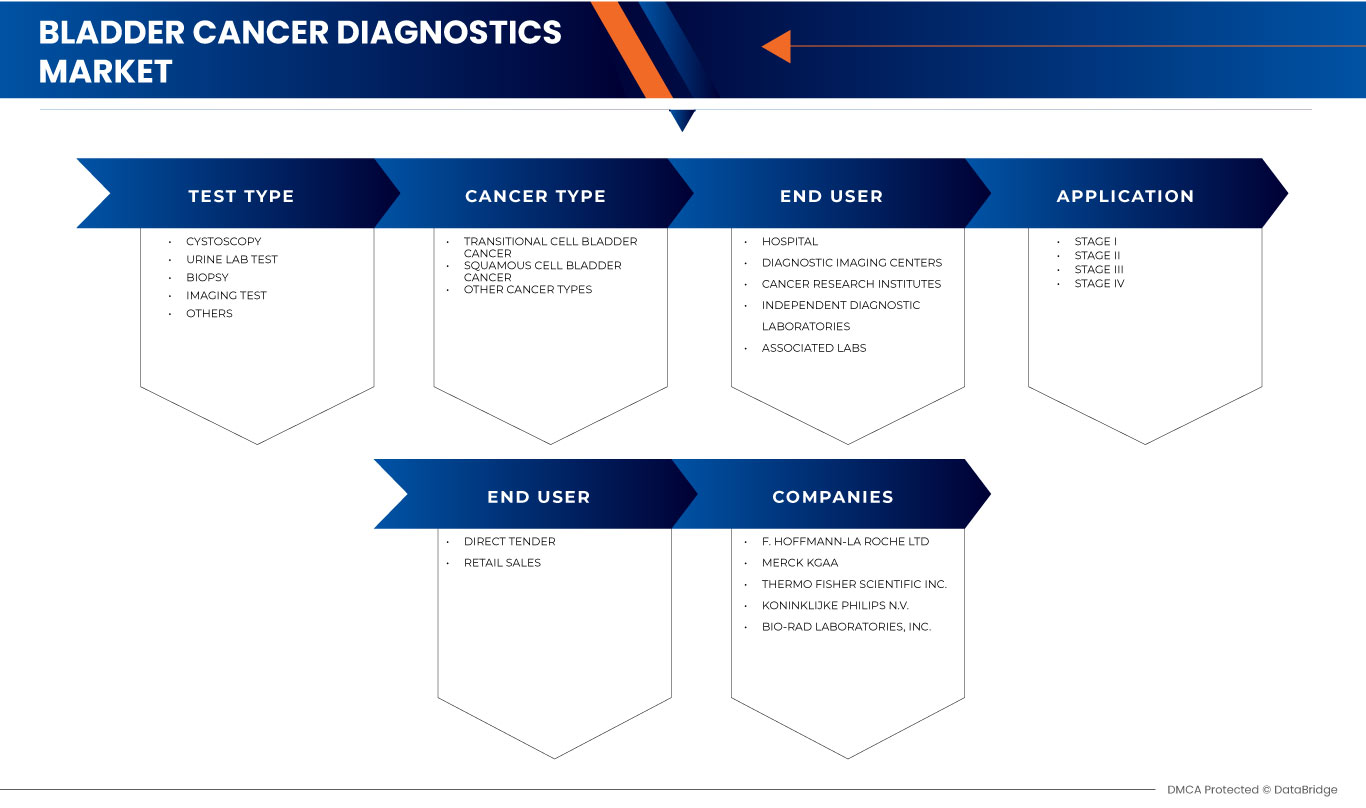
Le marché européen du diagnostic du cancer de la vessie est segmenté en cinq segments notables basés sur le type de test, les stades, le type de cancer, l'utilisateur final et le canal de distribution. La croissance parmi ces segments vous aidera à analyser les segments de croissance faibles dans les industries et à fournir aux utilisateurs un aperçu précieux du marché et des informations sur le marché pour les aider à prendre des décisions stratégiques pour identifier les principales applications du marché.
Type de test
- Cystoscopie
- Analyse d'urine en laboratoire
- Biopsie
- Test d'imagerie
- Autres
En fonction du type de test, le marché est segmenté en cystoscopie, test de laboratoire d'urine, biopsie, test d'imagerie et autres.
Étapes
- Étape I
- Stade II
- Stade III
- Stade IV
En fonction des étapes, le marché est segmenté en étape I, étape II, étape III et étape IV.
Type de cancer
- Cancer de la vessie à cellules transitionnelles
- Cancer épidermoïde de la vessie
- Autres types de cancer
En fonction du type de cancer, le marché est segmenté en cancer de la vessie à cellules transitionnelles, cancer de la vessie à cellules squameuses et autres types de cancer.
Utilisateur final
- Hôpital
- Centres d'imagerie diagnostique
- Instituts de recherche sur le cancer
- Laboratoires Dignostic Indépendants
- Laboratoires associés
En fonction de l’utilisateur final, le marché est segmenté en hôpitaux, centres d’imagerie diagnostique, instituts de recherche sur le cancer, laboratoires de diagnostic indépendants et laboratoires associés.
Canal de distribution
- Appel d'offres direct
- Ventes au détail
En fonction du canal de distribution, le marché est segmenté en ventes directes et ventes au détail.
Analyse/perspectives régionales du marché européen du diagnostic du cancer de la vessie
Le marché européen du diagnostic du cancer de la vessie est analysé et des informations et tendances sur la taille du marché sont fournies par pays, type de test, stades, type de cancer, utilisateur final et canal de distribution comme référencé ci-dessus.
Les pays couverts dans ce rapport de marché sont l'Allemagne, la France, le Royaume-Uni, l'Italie, l'Espagne, les Pays-Bas, la Russie, la Suisse, la Turquie, la Belgique et le reste de l'Europe.
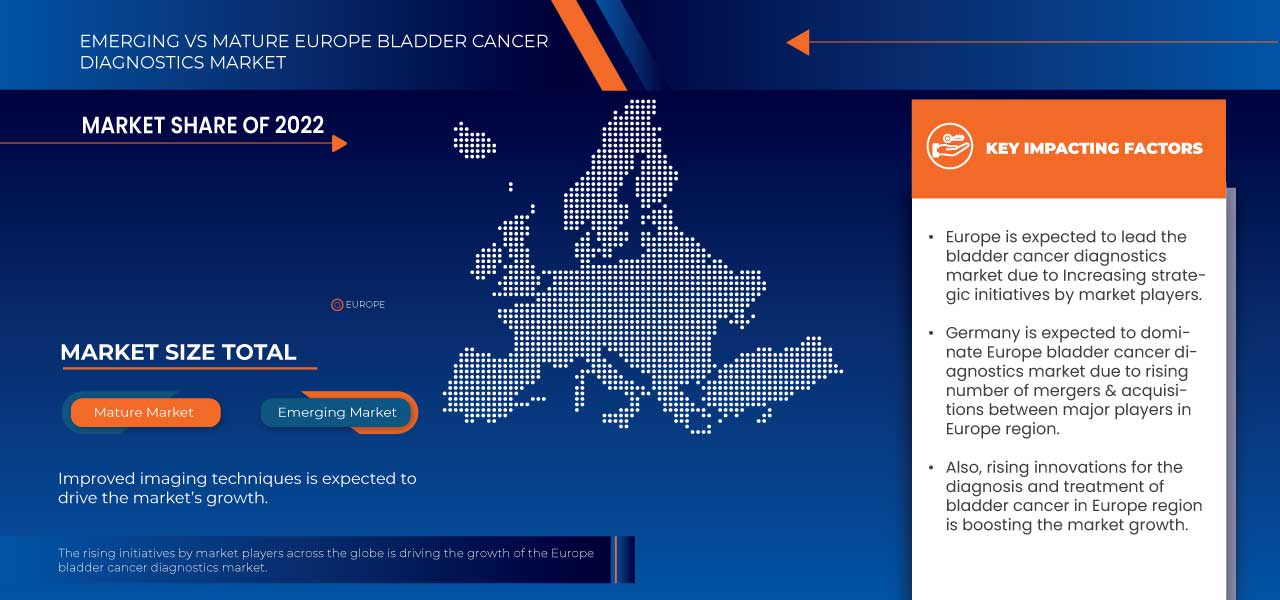
L'Allemagne devrait dominer le marché avec la plus grande part de marché. Cette part est attribuable à la présence d'acteurs clés du marché tels que F. Hoffmann-La Roche Ltd, Merck KGaA, Thermo Fisher Scientific Inc., Koninklijke Philips NV et Bio-Rad Laboratories, Inc., entre autres. La prévalence croissante du cancer de la vessie et l'amélioration des techniques d'imagerie devraient alimenter la croissance du marché au cours de la période de prévision dans cette région.
La section par pays du rapport fournit également des facteurs individuels ayant un impact sur le marché et des changements dans la réglementation du marché qui ont un impact sur les tendances actuelles et futures du marché. Des points de données tels que l'analyse de la chaîne de valeur en aval et en amont, les tendances techniques, l'analyse des cinq forces du porteur et les études de cas sont quelques-uns des indicateurs utilisés pour prévoir le scénario du marché pour chaque pays. En outre, la présence et la disponibilité des marques européennes et les défis auxquels elles sont confrontées en raison de la concurrence importante ou rare des marques locales et nationales, l'impact des tarifs nationaux et les routes commerciales sont pris en compte lors de l'analyse prévisionnelle des données nationales.
Analyse du paysage concurrentiel et des parts de marché du diagnostic du cancer de la vessie en Europe
Le paysage concurrentiel du marché européen du diagnostic du cancer de la vessie fournit des détails sur le concurrent. Les détails inclus sont la présentation de l'entreprise, les finances de l'entreprise, les revenus générés, le potentiel du marché, les nouvelles initiatives du marché, la présence en Europe, les sites et installations de production, les capacités de production, les forces et les faiblesses de l'entreprise, le lancement du produit, la largeur et l'étendue du produit et la domination des applications. Les points de données ci-dessus fournis ne concernent que l'orientation des entreprises par rapport au marché.
Français Certains des principaux acteurs du marché opérant sur le marché européen du diagnostic du cancer de la vessie sont F. Hoffmann-La Roche Ltd, Merck KGaA, Thermo Fisher Scientific Inc., Koninklijke Philips NV, Bio-Rad Laboratories, Inc., Agilent Technologies, Inc., FUJIFILM Corporation, CANON MEDICAL SYSTEMS CORPORATION, Siemens Healthcare GmbH, BD, Illumina, Inc. Neusoft Corporation, Abbott, General Electric Company, Hologic Inc., QIAGEN, Cepheid, Ambu A/S, Time Medical Holding et MinFound Medical Systems Co., entre autres.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE EUROPE BLADDER CANCER DIAGNOSTICS MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 TEST TYPE SEGMENT LIFELINE CURVE
2.8 MARKET END USER COVERAGE GRID
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTLE ANALYSIS
4.2 PORTER'S FIVE FORCES MODEL
5 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, INDUSTRY INSIGHT
6 REGULATORY FRAMEWORK
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 RISING INCIDENCE OF BLADDER CANCER EUROPELY
7.1.2 RISING AWARENESS FOR THE EARLY DIAGNOSIS OF BLADDER CANCER
7.1.3 IMPROVED IMAGING TECHNIQUES AND TECHNOLOGY
7.1.4 INNOVATIONS IN DRUG DELIVERY TO BLADDER CANCER CELLS
7.2 RESTRAINTS
7.2.1 HIGH COST ASSOCIATED WITH DIAGNOSIS AND TREATMENT OF BLADDER CANCER
7.2.2 SIDE EFFECTS OF BLADDER CANCER TREATMENT DRUGS & THERAPIES
7.2.3 LATE DIAGNOSIS OF BLADDER CANCER RESULTING IN POOR PROGNOSIS
7.3 OPPORTUNITIES
7.3.1 INCREASE IN DIAGNOSTIC PROCEDURES FOR BLADDER CANCER
7.3.2 RISE IN HEALTHCARE EXPENDITURE FOR CANCER DIAGNOSIS
7.3.3 INCREASE IN THE GERIATRIC POPULATION
7.4 CHALLENGES
7.4.1 ETHICAL CHALLENGES FACED DURING BLADDER CANCER TESTING
7.4.2 STRINGENT REGULATIONS AMONG BLADDER CANCER DIAGNOSTICS TESTS AND PROCEDURES
8 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE
8.1 OVERVIEW
8.2 CYSTOSCOPY
8.3 URINE LAB TEST
8.3.1 URINE CYTOLOGY
8.3.2 URINALYSIS
8.3.3 URINE TUMOR MARKER TEST
8.3.4 URINE CULTURE
8.3.5 OTHERS
8.4 BIOPSY
8.5 IMAGING TEST
8.5.1 MAGNETIC RESONANCE IMAGING (MRI) SCAN
8.5.2 COMPUTED TOMOGRAPHY (CT) SCAN
8.5.3 ULTRASOUND
8.5.4 INTRAVENOUS PYELOGRAM (IVP)
8.6 OTHERS
9 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES
9.1 OVERVIEW
9.2 STAGE IV
9.3 STAGE III
9.4 STAGE II
9.5 STAGE I
10 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE
10.1 OVERVIEW
10.2 TRANSITIONAL CELL BLADDER CANCER
10.3 SQUAMOUS CELL BLADDER CANCER
10.4 OTHER CANCER TYPES
11 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITAL
11.3 DIAGNOSTIC IMAGING CENTERS
11.4 CANCER RESEARCH INSTITUTES
11.5 INDEPENDENT DIAGNOSTIC LABORATORIES
11.6 ASSOCIATED LABS
12 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
13 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY REGION
13.1 EUROPE
13.1.1 GERMANY
13.1.2 FRANCE
13.1.3 U.K.
13.1.4 ITALY
13.1.5 SPAIN
13.1.6 RUSSIA
13.1.7 TURKEY
13.1.8 NETHERLANDS
13.1.9 BELGIUM
13.1.10 SWITZERLAND
13.1.11 REST OF EUROPE
14 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: EUROPE
15 SWOT ANALYSIS
16 EUROPE BLADDER CANCER DIAGNOSTICS MARKET
16.1 F. HOFFMANN- LA ROCHE LTD
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 MERCK KGAA
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENT
16.3 THERMO FISHER SCIENTIFIC INC.
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENT
16.4 KONINLIJKE PHILIPS N.V.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENT
16.5 BIO-RAD LABORATORIES, INC.
16.5.1 COMPANY SNAPSHOT
16.5.2 COMPANY SNAPSHOT
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENT
16.6 ABBOTT
16.6.1 COMPANY SNAPSHOT
16.6.2 REVENUE ANALYSIS
16.6.3 PRODUCT PORTFOLIO
16.6.4 RECENT DEVELOPMENT
16.7 AGILENT TECHNOLOGIES, INC.
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 PRODUCT PORTFOLIO
16.7.4 RECENT DEVELOPMENT
16.8 AMBU A/S
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENT
16.9 BD
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENT
16.1 CANON MEDICAL SYSTEMS CORPORATION
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 CEPHEID
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENTS
16.12 FUJIFILM CORPORATION
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 PRODUCT PORTFOLIO
16.12.4 RECENT DEVELOPMENTS
16.13 GENERAL ELECTRIC COMPANY
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENTS
16.14 HOLOGIC INC.
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENT
16.15 ILLUMINA INC
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 MINFOUND MEDICAL SYSTEMS CO.,
16.16.1 COMPANY SNAPSHOT
16.16.2 PRODUCT PORTFOLIO
16.16.3 RECENT DEVELOPMENTS
16.17 NEUSOFT CORPORATION
16.17.1 COMPANY SNAPSHOT
16.17.2 COMPANY SNAPSHOT
16.17.3 PRODUCT PORTFOLIO
16.17.4 RECENT DEVELOPMENT
16.18 QIAGEN
16.18.1 COMPANY SNAPSHOT
16.18.2 REVENUE ANALYSIS
16.18.3 PRODUCT PORTFOLIO
16.18.4 RECENT DEVELOPMENT
16.19 SIEMENS HEALTHCARE GMBH
16.19.1 COMPANY SNAPSHOT
16.19.2 REVENUE ANALYSIS
16.19.3 PRODUCT PORTFOLIO
16.19.4 RECENT DEVELOPMENT
16.2 TIME MEDICAL HOLDING.
16.20.1 COMPANY SNAPSHOT
16.20.2 PRODUCT PORTFOLIO
16.20.3 RECENT DEVELOPMENTS
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 2 EUROPE CYSTOSCOPY IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 3 EUROPE URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 4 EUROPE URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 EUROPE BIOPSY IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 EUROPE IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 EUROPE IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 EUROPE OTHERS IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 10 EUROPE STAGE IV IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 EUROPE STAGE III IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 EUROPE STAGE II IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 EUROPE STAGE I IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 15 EUROPE TRANSITIONAL CELL BLADDER CANCER IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 16 EUROPE SQUAMOUS CELL BLADDER CANCER IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 EUROPE OTHER CANCER TYPES IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 19 EUROPE HOSPITAL IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 EUROPE DIAGNOSTIC IMAGING CENTERS IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 EUROPE CANCER RESEARCH INSTITUTES IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 EUROPE INDEPENDENT DIAGNOSTIC LABORATORIES IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 EUROPE ASSOCIATED LABS IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 25 EUROPE DIRECT TENDER IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 EUROPE RETAIL SALES IN BLADDER CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 28 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 29 EUROPE URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 30 EUROPE IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 31 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 32 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 33 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 35 GERMANY BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 36 GERMANY URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 37 GERMANY IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 38 GERMANY BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 39 GERMANY BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 40 GERMANY BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 41 GERMANY BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 42 FRANCE BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 43 FRANCE URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 44 FRANCE IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 45 FRANCE BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 46 FRANCE BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 47 FRANCE BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 48 FRANCE BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 49 U.K. BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 50 U.K. URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 51 U.K. IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 52 U.K. BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 53 U.K. BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 54 U.K. BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 55 U.K. BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 56 ITALY BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 57 ITALY URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 58 ITALY IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 59 ITALY BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 60 ITALY BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 61 ITALY BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 62 ITALY BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 63 SPAIN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 64 SPAIN URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 65 SPAIN IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 66 SPAIN BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 67 SPAIN BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 68 SPAIN BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 69 SPAIN BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 70 RUSSIA BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 71 RUSSIA URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 72 RUSSIA IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 RUSSIA BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 74 RUSSIA BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 75 RUSSIA BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 76 RUSSIA BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 77 TURKEY BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 78 TURKEY URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 79 TURKEY IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 80 TURKEY BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 81 TURKEY BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 82 TURKEY BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 83 TURKEY BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 84 NETHERLANDS BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 85 NETHERLANDS URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 86 NETHERLANDS IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 NETHERLANDS BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 88 NETHERLANDS BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 89 NETHERLANDS BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 90 NETHERLANDS BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 91 BELGIUM BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 92 BELGIUM URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 93 BELGIUM IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 94 BELGIUM BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 95 BELGIUM BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 96 BELGIUM BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 97 BELGIUM BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 98 SWITZERLAND BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 99 SWITZERLAND URINE LAB TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 100 SWITZERLAND IMAGING TEST IN BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 SWITZERLAND BLADDER CANCER DIAGNOSTICS MARKET, BY STAGES, 2021-2030 (USD MILLION)
TABLE 102 SWITZERLAND BLADDER CANCER DIAGNOSTICS MARKET, BY CANCER TYPE, 2021-2030 (USD MILLION)
TABLE 103 SWITZERLAND BLADDER CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 104 SWITZERLAND BLADDER CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 105 REST OF EUROPE BLADDER CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 RISING INCIDENCE OF BLADDER CANCER EUROPELY IS EXPECTED TO DRIVE THE GROWTH OF THE EUROPE BLADDER CANCER DIAGNOSTICS MARKET FROM 2023 TO 2030
FIGURE 12 CYSTOSCOPY SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE BLADDER CANCER DIAGNOSTICS MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE EUROPE BLADDER CANCER DIAGNOSTICS MARKET
FIGURE 14 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2022
FIGURE 15 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY TEST TYPE, LIFELINE CURVE
FIGURE 18 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY STAGES, 2022
FIGURE 19 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY STAGES, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY STAGES, CAGR (2023-2030)
FIGURE 21 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY STAGES, LIFELINE CURVE
FIGURE 22 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2022
FIGURE 23 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, 2023-2030 (USD MILLION)
FIGURE 24 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, CAGR (2023-2030)
FIGURE 25 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY CANCER TYPE, LIFELINE CURVE
FIGURE 26 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY END USER, 2022
FIGURE 27 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 28 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 29 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 30 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 31 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 32 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 33 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 34 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 35 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 36 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 37 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 38 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 39 EUROPE BLADDER CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.