Asia Pacific Molecular Diagnostics Services Market
Taille du marché en milliards USD
TCAC :
% 
 USD
25.84 Million
USD
49.99 Million
2024
2032
USD
25.84 Million
USD
49.99 Million
2024
2032
| 2025 –2032 | |
| USD 25.84 Million | |
| USD 49.99 Million | |
|
|
|
|
Segmentation du marché des services de diagnostic moléculaire en Asie-Pacifique, par type de service (services de réparation d'instruments, services de formation, services de conformité , services d' étalonnage , services de maintenance, services d'automatisation évolutifs, services clés en main, services de relocalisation d'instruments, personnalisation du matériel, services d'assurance des performances, services de conception et de développement, solutions de chaîne d'approvisionnement, services d'introduction de nouveaux produits, services de fabrication, services environnementaux et réglementaires, certification et audit des systèmes de gestion médicale , services de recherche clinique, services de conseil et autres services), technologie (PCR, PCR en temps réel, séquençage de nouvelle génération et autres technologies), utilisateur final ( hôpitaux , centres de diagnostic, établissements universitaires et de recherche, et autres) Tendances et prévisions de l'industrie jusqu'en 2030.
Taille du marché des services de diagnostic moléculaire en Asie-Pacifique
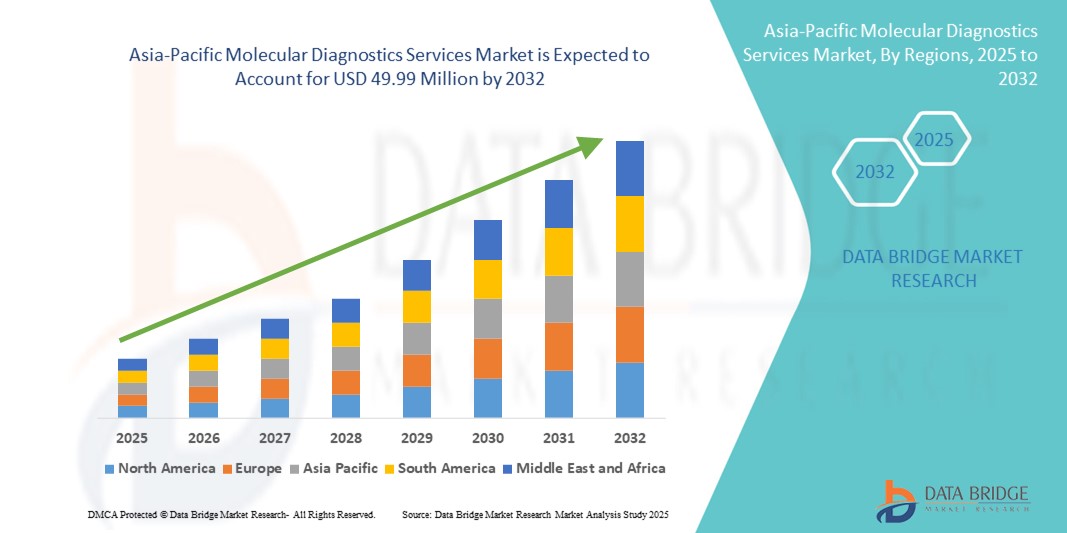
- La taille du marché des services de diagnostic moléculaire en Asie-Pacifique était évaluée à 25,84 millions USD en 2024 et devrait atteindre 49,99 millions USD d'ici 2032 , à un TCAC de 8,60 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par une sensibilisation accrue, un meilleur accès aux soins et les avancées des technologies de diagnostic en Asie-Pacifique, permettant la détection et la prise en charge rapides d'un large éventail de maladies génétiques, infectieuses et chroniques. Des pays comme l'Inde, la Chine et l'Indonésie connaissent un développement rapide des infrastructures de santé, contribuant à l'adoption croissante des services de diagnostic moléculaire.
- Par ailleurs, l'augmentation des investissements dans les laboratoires, l'expansion des services de diagnostic en zones rurales et semi-urbaines et la multiplication des partenariats public-privé stimulent l'innovation et la disponibilité de technologies de tests moléculaires avancés. Les initiatives gouvernementales en matière de santé, associées à la présence croissante d'entreprises internationales de diagnostic et aux capacités de production locales, stimulent considérablement la croissance du marché des services de diagnostic moléculaire en Asie-Pacifique.
Analyse du marché des services de diagnostic moléculaire en Asie-Pacifique
- Le marché des services de diagnostic moléculaire en Asie-Pacifique connaît une croissance significative, portée par l'augmentation des investissements dans les plateformes de diagnostic avancées, la prévalence croissante des maladies infectieuses et génétiques, et l'adoption croissante d'initiatives de médecine de précision. Des pays comme la Chine, l'Inde, le Japon et la Corée du Sud développent leurs infrastructures de laboratoire et leurs capacités de recherche, contribuant ainsi à une demande accrue de services de diagnostic moléculaire de haute qualité.
- L'intérêt croissant pour les soins de santé personnalisés et la recherche clinique dans la région est soutenu par un financement public accru, des investissements privés croissants dans le secteur de la santé et le développement de centres de diagnostic spécialisés. La sensibilisation accrue au dépistage précoce des maladies, conjuguée aux avancées des technologies de tests moléculaires telles que la PCR, la PCR en temps réel et le séquençage de nouvelle génération (NGS), favorise une adoption plus large dans les hôpitaux et les instituts de recherche.
- La Chine a dominé le marché des services de diagnostic moléculaire en Asie-Pacifique, représentant la plus grande part de revenus de 35,1 % en 2024, grâce à son réseau hospitalier bien établi, sa large population de patients et l'intégration rapide de technologies avancées de diagnostic moléculaire dans les flux de travail cliniques et de recherche.
- L'Inde devrait enregistrer le TCAC le plus rapide sur le marché des services de diagnostic moléculaire en Asie-Pacifique, soit 13,6 %, au cours de la période de prévision, grâce à l'élargissement de l'accès aux soins de santé, à la demande croissante de tests moléculaires abordables et à la croissance des investissements dans les infrastructures de laboratoire. Des initiatives telles que les programmes de diagnostic soutenus par les gouvernements et le renforcement des collaborations avec le secteur privé accélèrent l'adoption du diagnostic moléculaire dans les régions urbaines et semi-urbaines.
- Les services basés sur la PCR ont dominé le marché des services de diagnostic moléculaire en Asie-Pacifique avec une part de 36,5 % en 2024, grâce à leur large adoption pour les tests moléculaires dans le domaine des maladies infectieuses, le dépistage génétique et les applications diagnostiques de routine. La PCR demeure une technologie fondamentale en raison de sa fiabilité, de son accessibilité financière et de son adaptabilité à de nombreux flux de travail cliniques.
Portée du rapport et segmentation du marché des services de diagnostic moléculaire en Asie-Pacifique
|
Attributs |
Informations clés sur le marché des services de diagnostic moléculaire en Asie-Pacifique |
|
Segments couverts |
|
|
Pays couverts |
Asie-Pacifique
|
|
Principaux acteurs du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research comprennent également une analyse approfondie des experts, une analyse des prix, une analyse de la part de marque, une enquête auprès des consommateurs, une analyse démographique, une analyse de la chaîne d'approvisionnement, une analyse de la chaîne de valeur, un aperçu des matières premières/consommables, des critères de sélection des fournisseurs, une analyse PESTLE, une analyse Porter et un cadre réglementaire. |
Tendances du marché des services de diagnostic moléculaire en Asie-Pacifique
Faire progresser les thérapies et étendre la recherche clinique en Asie-Pacifique
- Une tendance significative et croissante sur le marché des services de diagnostic moléculaire en Asie-Pacifique est l'importance croissante accordée aux innovations thérapeutiques et à la recherche clinique, notamment dans des domaines tels que l'oncologie, les maladies infectieuses et les troubles génétiques. Les tests moléculaires avancés permettent un diagnostic plus précoce et des approches thérapeutiques plus ciblées.
- Par exemple, plusieurs entreprises de diagnostic et instituts de recherche en Asie-Pacifique investissent dans le séquençage de nouvelle génération (NGS), les tests basés sur la PCR et les panels de biomarqueurs multiplex. Ces développements visent à fournir des résultats diagnostiques plus rapides, plus précis et plus économiques, essentiels à la médecine personnalisée et à la prise en charge des maladies.
- L'adoption croissante de modèles de médecine de précision dans les hôpitaux et les cliniques spécialisées permet des interventions thérapeutiques plus efficaces. Ces modèles s'appuient sur le profilage moléculaire avancé et la bioinformatique pour guider le choix des traitements, suivre la progression de la maladie et prédire la réponse des patients.
- Les partenariats entre les entreprises de technologie de diagnostic, les centres de recherche universitaires et les programmes soutenus par le gouvernement contribuent également à élargir l’accès aux tests moléculaires en améliorant les cadres de remboursement, en normalisant les pratiques de laboratoire et en améliorant la formation des cliniciens.
- Alors que l'Asie-Pacifique continue de privilégier les soins de santé de précision et les résultats basés sur la valeur, le marché des services de diagnostic moléculaire est prêt à connaître une croissance soutenue, tirée par l'innovation, l'amélioration de la précision du diagnostic et la demande croissante de détection précoce et de stratégies thérapeutiques personnalisées.
Dynamique du marché des services de diagnostic moléculaire en Asie-Pacifique
Conducteur
Besoin croissant en raison de l'augmentation des taux de diagnostic et des progrès de la recherche génétique
- La prévalence croissante des maladies complexes et chroniques en Asie-Pacifique, soutenue par une sensibilisation accrue et des capacités de diagnostic améliorées, stimule considérablement la croissance du marché. Des pays comme la Chine, l'Inde, le Japon et la Corée du Sud améliorent leurs infrastructures de santé et leurs programmes de diagnostic, permettant ainsi une détection plus précoce et une intervention rapide pour des affections telles que le cancer, les maladies infectieuses et les troubles génétiques.
- Par exemple, en avril 2024, Anavex Life Sciences a annoncé des progrès positifs dans son essai clinique de phase III pour l'Anavex 2-73 (blarcamésine), ciblant les maladies neurodégénératives grâce à des diagnostics de précision et des biomarqueurs moléculaires. Ces innovations devraient catalyser l'adoption de diagnostics moléculaires avancés, accélérant ainsi le développement du marché des services de diagnostic moléculaire en Asie-Pacifique au cours de la période de prévision.
- L'intérêt croissant pour la médecine personnalisée et la disponibilité des technologies moléculaires de nouvelle génération, notamment la PCR, la PCR en temps réel et le séquençage de nouvelle génération, incitent le marché à abandonner les méthodes de diagnostic conventionnelles pour des solutions de test plus précises et spécifiques au patient.
- Les organismes de réglementation de la région Asie-Pacifique, tels que l'Agence des produits pharmaceutiques et des dispositifs médicaux (PMDA) au Japon et l'Administration nationale des produits médicaux (NMPA) en Chine, soutiennent de plus en plus l'innovation diagnostique par le biais d'approbations accélérées, d'un soutien aux essais cliniques et de directives de conformité simplifiées, favorisant ainsi un accès rapide au marché pour les services de diagnostic moléculaire avancés.
- Les collaborations entre entreprises régionales de biotechnologie, centres de recherche universitaires et associations de santé renforcent l'écosystème d'innovation en Asie-Pacifique. Ces partenariats jouent un rôle essentiel dans l'élargissement de l'accès des patients aux diagnostics moléculaires avancés, le développement des initiatives de recherche clinique et la sensibilisation au dépistage précoce des maladies et aux tests génétiques au sein de populations diverses.
Retenue/Défi
Infrastructure limitée et variabilité dans l'adoption clinique
- Le coût élevé associé aux services de diagnostic moléculaire avancés, notamment l’instrumentation sophistiquée, les réactifs et le séquençage à haut débit, constitue un obstacle important à une adoption généralisée, en particulier dans les zones rurales ou sous-financées.
- Même lorsqu'ils sont soutenus par des incitations gouvernementales, les tests de diagnostic moléculaire impliquent généralement des flux de travail complexes, du personnel spécialisé et un contrôle qualité rigoureux, ce qui les rend moins accessibles aux systèmes de santé aux budgets limités.
- De plus, les laboratoires spécialisés et le personnel qualifié sont souvent concentrés dans les centres urbains, ce qui oblige les patients des zones reculées à parcourir de longues distances ou à subir des retards dans les tests et les rapports.
- Un autre défi réside dans l'absence de protocoles standardisés pour certains tests moléculaires et génétiques. L'expérience clinique limitée et les capacités variables des laboratoires contribuent à une adoption inégale parmi les professionnels de santé.
- Pour surmonter ces défis, des réformes politiques, un financement gouvernemental accru, une collaboration transfrontalière en matière de recherche et la création de pôles de diagnostic moléculaire dédiés dans toute la région Asie-Pacifique seront essentiels pour élargir l'accès et parvenir à une croissance durable sur le marché des services de diagnostic moléculaire en Asie-Pacifique.
Portée du marché des services de diagnostic moléculaire en Asie-Pacifique
Le marché est segmenté en fonction du type de service, de la technologie et de l’utilisateur final.
- Par type de service
En fonction du type de service, le marché des services de diagnostic moléculaire en Asie-Pacifique est segmenté en services de réparation d'instruments, services de formation, services de conformité, services d'étalonnage, services de maintenance, services d'automatisation évolutive, services clés en main, services de relocalisation d'instruments, personnalisation du matériel, services d'assurance de la performance, services de conception et développement, solutions de chaîne d'approvisionnement, services de lancement de nouveaux produits, services de fabrication, services environnementaux et réglementaires, certification et audit des systèmes de gestion médicale, services de recherche clinique, services de conseil, etc. Les services de maintenance ont dominé le marché avec la plus grande part de chiffre d'affaires (23,7 %) en 2024, en raison de leur rôle essentiel pour garantir le fonctionnement continu et précis des instruments de diagnostic moléculaire. Ces services sont essentiels pour minimiser les temps d'arrêt, prolonger la durée de vie des équipements et maintenir une précision diagnostique constante dans les réseaux hospitaliers et de laboratoires.
Les services de recherche clinique devraient connaître leur plus fort TCAC de 9,1 % entre 2025 et 2032, portés par l'importance croissante accordée à la recherche translationnelle et en médecine de précision. Cette croissance est soutenue par des investissements accrus dans la découverte de biomarqueurs, la génomique et les initiatives de soins de santé personnalisés, ainsi que par le développement des collaborations entre les institutions de recherche et les prestataires de services de diagnostic.
- Par technologie
Sur le plan technologique, le marché des services de diagnostic moléculaire en Asie-Pacifique est segmenté en PCR, PCR en temps réel, séquençage de nouvelle génération (NGS) et autres technologies. Les services basés sur la PCR ont dominé le marché avec une part de 36,5 % en 2024, grâce à leur large adoption pour les tests moléculaires dans le domaine des maladies infectieuses, les dépistages génétiques et les applications de diagnostic de routine. La PCR reste une technologie fondamentale en raison de sa fiabilité, de son prix abordable et de son adaptabilité à de multiples flux de travail cliniques.
Le séquençage de nouvelle génération (NGS) devrait connaître une croissance annuelle composée (TCAC) record de 14,2 % entre 2025 et 2032, alimentée par la demande croissante d'analyses génomiques à haut débit, d'oncologie de précision et de profilage complet des maladies. Le NGS permet aux chercheurs et aux cliniciens de réaliser des études génomiques à grande échelle avec une précision accrue, favorisant ainsi le diagnostic précoce, la stratification des traitements et la personnalisation des approches thérapeutiques.
- Par utilisateur final
En fonction de l'utilisateur final, le marché des services de diagnostic moléculaire en Asie-Pacifique est segmenté entre hôpitaux, centres de diagnostic, établissements universitaires et de recherche, et autres. Les hôpitaux détenaient la plus grande part de marché, avec 41,8 % en 2024, grâce à leur volume important de patients, à leur infrastructure de laboratoire intégrée et à l'adoption constante de plateformes de diagnostic moléculaire avancées. Les hôpitaux utilisent ces services non seulement pour les diagnostics de routine, mais aussi pour soutenir des services spécialisés comme l'oncologie, les maladies infectieuses et le conseil génétique.
Les établissements universitaires et de recherche devraient connaître une croissance annuelle composée (TCAC) record de 10,3 % au cours de la période de prévision, portée par l'intensification des initiatives de recherche moléculaire, le financement public et privé des études génomiques et cliniques, et l'adoption de technologies diagnostiques de pointe pour la recherche translationnelle. Ces établissements jouent un rôle crucial dans la promotion de l'innovation et la validation de nouvelles solutions de diagnostic moléculaire avant leur commercialisation.
Analyse régionale du marché des services de diagnostic moléculaire en Asie-Pacifique
- L'Asie-Pacifique a dominé le marché mondial des services de diagnostic moléculaire, avec une part de chiffre d'affaires de 32,5 % en 2024, portée par le développement des infrastructures de santé, la prévalence croissante des maladies chroniques et infectieuses et l'adoption rapide de technologies avancées de diagnostic moléculaire. Les investissements dans les installations médicales, le nombre croissant de centres de diagnostic et les initiatives gouvernementales favorisant le dépistage précoce des maladies stimulent encore la croissance du marché.
- Des cadres réglementaires solides, une couverture d'assurance étendue et une forte sensibilisation des patients favorisent la croissance des secteurs de la santé, tant public que privé. L'augmentation du financement public des programmes de diagnostic, conjuguée aux partenariats public-privé et aux initiatives de santé post-pandémiques, accélère l'adoption de services de diagnostic moléculaire avancés.
- En outre, la région Asie-Pacifique abrite plusieurs fournisseurs de services de diagnostic, institutions universitaires et centres de R&D de premier plan, facilitant l'innovation continue dans le développement de tests, l'évaluation clinique et l'intégration de technologies de nouvelle génération.
Analyse du marché des services de diagnostic moléculaire en Chine et en Asie-Pacifique
En 2024, le marché chinois des services de diagnostic moléculaire détenait la plus grande part de marché de la région Asie-Pacifique, avec 35,1 %, grâce à une population importante, à la prévalence croissante des maladies infectieuses et génétiques et à un accès élargi aux centres de diagnostic spécialisés. Les réformes gouvernementales du système de santé, l'augmentation de la couverture d'assurance et les politiques de remboursement favorables encouragent les prestataires de services nationaux et internationaux à élargir leur offre de diagnostic moléculaire. Les entreprises locales investissent également massivement dans la R&D pour répondre à la demande croissante de solutions de diagnostic avancées.
Analyse du marché des services de diagnostic moléculaire au Japon et en Asie-Pacifique
Le marché japonais des services de diagnostic moléculaire représentait 21,5 % du marché de la région Asie-Pacifique en 2024, grâce à son infrastructure de santé hautement développée, sa solide couverture d'assurance et son environnement de laboratoire technologiquement avancé. L'adoption croissante du séquençage de nouvelle génération (NGS), des tests basés sur la PCR et d'autres technologies de diagnostic moléculaire, notamment dans les hôpitaux et les centres de recherche, stimule la demande. L'accent mis par le Japon sur la détection précoce des maladies et la médecine de précision renforce sa position de leader dans la région.
Analyse du marché des services de diagnostic moléculaire en Inde et en Asie-Pacifique
Le marché indien des services de diagnostic moléculaire devrait connaître la croissance la plus rapide en Asie-Pacifique, avec un TCAC de 13,6 % entre 2025 et 2032, porté par une sensibilisation accrue aux soins de santé, une accessibilité accrue aux services de diagnostic et une hausse des revenus disponibles. Les programmes nationaux de dépistage des maladies, le développement des infrastructures de diagnostic dans les villes de niveaux 2 et 3 et la participation croissante du secteur privé accélèrent l'adoption de ces services. L'Inde s'impose également comme une plaque tournante du diagnostic moléculaire rentable, renforçant ainsi sa compétitivité régionale.
Part de marché des services de diagnostic moléculaire en Asie-Pacifique
L'industrie des services de diagnostic moléculaire en Asie-Pacifique est principalement dirigée par des entreprises bien établies, notamment :
- F. Hoffmann-La Roche SA (Suisse)
- Danaher Corporation (États-Unis)
- BIOMÉRIEUX (France)
- QIAGEN NV (Pays-Bas)
- Thermo Fisher Scientific Inc. (États-Unis)
- Bio-Rad Laboratories, Inc. (États-Unis)
- Abbott (États-Unis)
- DiaSorin SpA (Italie)
- Hologic, Inc. (États-Unis)
Derniers développements sur le marché des services de diagnostic moléculaire en Asie-Pacifique
- En août 2025 , les entreprises chinoises de R&D pharmaceutique se sont de plus en plus approvisionnées en réactifs de laboratoire auprès de fournisseurs nationaux, tels que Shanghai Titan Scientific et Nanjing Vazyme Biotech, afin de réduire les coûts et les délais de livraison. Cette évolution répondait à la hausse des droits de douane et aux inquiétudes concernant la fiabilité de la chaîne d'approvisionnement dans un contexte de tensions commerciales persistantes avec les États-Unis. Auparavant dominé par des entreprises occidentales comme Thermo Fisher et Merck, le marché chinois des réactifs, estimé à 5,76 milliards de dollars, a connu une forte évolution vers les entreprises locales.
- En mars 2025 , Qiagen a annoncé l'arrêt de ses systèmes de test PCR intégrés NeuMoDx 96 et 288 en raison de l'évolution du marché et des besoins des clients après la pandémie de COVID-19. L'entreprise a entamé des discussions avec ses clients afin de comprendre l'impact sur ses ventes en 2024 et a confirmé son intention de soutenir ses clients existants jusqu'en 2025.
- En février 2024 , PlexBio a présenté sa technologie avancée de détection du cancer du poumon au Medlab Dubaï, soulignant son engagement à étendre les capacités de diagnostic moléculaire en oncologie.
- En janvier 2024 , Revvity, anciennement filiale de PerkinElmer, a considérablement augmenté ses investissements dans la recherche, les logiciels et les opérations internes suite à sa scission et à son changement de marque. L'entreprise s'est concentrée sur le développement de ses secteurs des sciences de la vie et du diagnostic, en visant à accroître ses dépenses de R&D et à investir dans l'efficacité opérationnelle, notamment grâce à une nouvelle plateforme e-commerce et à l'optimisation de sa chaîne d'approvisionnement.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.