Asia Pacific Lymphangioleiomyomatosis Lam Market
Taille du marché en milliards USD
TCAC :
% 
 USD
16.14 Million
USD
22.78 Million
2024
2032
USD
16.14 Million
USD
22.78 Million
2024
2032
| 2025 –2032 | |
| USD 16.14 Million | |
| USD 22.78 Million | |
|
|
|
|
Segmentation du marché de la lymphangioleiomyomatose (LAM) en Asie-Pacifique, par type de maladie (LAM associée à la sclérose tubéreuse et LAM sporadique), type (diagnostic et traitement), complications (pneumothorax, chylothorax, tumeur rénale, épanchements pleuraux, œdème et accumulation de liquide, et autres), voie d'administration (orale, parentérale et autres), utilisateur final (hôpitaux, cliniques spécialisées, centres de diagnostic, soins à domicile et autres), canal de distribution (appel d'offres direct, pharmacies hospitalières, pharmacies de détail, pharmacies en ligne et autres) - Tendances du secteur et prévisions jusqu'en 2032
Taille du marché de la lymphangioleiomyomatose (LAM) en Asie-Pacifique
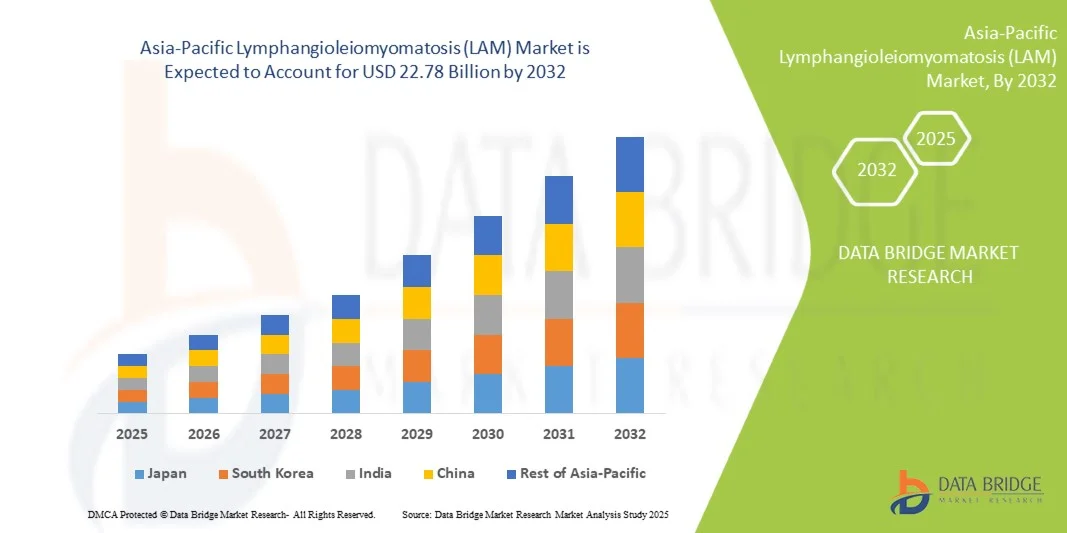
- Le marché de la lymphangioléiomyomatose (LAM) en Asie-Pacifique était évalué à 16,14 millions de dollars américains en 2024 et devrait atteindre 22,78 millions de dollars américains d'ici 2032 , avec un TCAC de 4,40 % au cours de la période de prévision.
- La croissance du marché est largement alimentée par une sensibilisation accrue aux maladies, une meilleure capacité de diagnostic et un accès élargi aux options de traitement dans les économies émergentes d'Asie-Pacifique.
- De plus, la demande croissante de thérapies ciblées et accessibles, conjuguée à l'augmentation des investissements dans les soins de santé dans des pays comme la Chine et le Japon, fait des solutions de traitement de la LAM une option plus viable dans la région. Ces facteurs convergents accélèrent l'adoption des interventions de LAM, stimulant ainsi considérablement la croissance du secteur.
Analyse du marché de la lymphangioléiomyomatose (LAM) en Asie-Pacifique
- Les thérapies et les diagnostics de la lymphangioleiomyomatose (LAM), une maladie pulmonaire progressive rare, constituent des éléments de plus en plus essentiels des soins respiratoires, tant en milieu clinique qu'en recherche, dans les pays d'Asie-Pacifique, en raison de leur potentiel à améliorer le pronostic des patients, à permettre un dépistage précoce et à s'intégrer aux services de santé spécialisés.
- La demande croissante de solutions LAM est principalement alimentée par une meilleure sensibilisation à la maladie, des améliorations des technologies de diagnostic et un accès accru aux thérapies ciblées dans les pays émergents d'Asie-Pacifique.
- Le Japon a dominé le marché de la LAM en Asie-Pacifique avec la plus grande part de revenus (38 %) en 2024, grâce à une infrastructure de santé avancée, une forte adoption des diagnostics de maladies rares et la présence d'acteurs clés du secteur. Le pays a connu une croissance substantielle de la disponibilité des diagnostics et des traitements de la LAM, portée par les innovations des entreprises pharmaceutiques établies et des jeunes entreprises de biotechnologie spécialisées.
- La Chine devrait être le pays connaissant la croissance la plus rapide sur le marché de la LAM au cours de la période de prévision, en raison de l'augmentation des investissements dans les soins de santé, de la sensibilisation accrue des patients et de l'accès élargi aux options de traitement avancées.
- Le segment des traitements a dominé le marché de la LAM en Asie-Pacifique avec une part de marché de 62,9 % en 2024, grâce à l'adoption croissante des thérapies pour la prise en charge de la sclérose tubéreuse de Bourneville (TSC-LAM) et de la LAM sporadique, ainsi qu'à une meilleure connaissance des complications telles que le pneumothorax et le chylothorax, qui nécessitent une intervention clinique.
Portée du rapport et segmentation du marché de la lymphangioléiomyomatose (LAM) en Asie-Pacifique
|
Attributs |
Lymphangioleiomyomatose (LAM) en Asie-Pacifique : Principaux enseignements du marché |
|
Segments couverts |
|
|
Pays couverts |
Asie-Pacifique
|
|
Acteurs clés du marché |
|
|
Opportunités de marché |
|
|
Ensembles d'informations de données à valeur ajoutée |
Outre les informations sur les scénarios de marché tels que la valeur du marché, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché élaborés par Data Bridge Market Research incluent également une analyse approfondie par des experts, des données épidémiologiques sur les patients, une analyse des projets en développement, une analyse des prix et un aperçu du cadre réglementaire. |
Tendances du marché de la lymphangioléiomyomatose (LAM) en Asie-Pacifique
« Amélioration de la prise en charge des maladies grâce à l’intégration du diagnostic et du traitement »
- Une tendance majeure et croissante sur le marché de la LAM en Asie-Pacifique est l'intégration des diagnostics avancés et des thérapies ciblées, améliorant ainsi le dépistage précoce et la personnalisation des traitements pour les patients atteints de sclérose tubéreuse de Bourneville (TSC-LAM) et de LAM sporadique.
- Par exemple, le traitement oral par sirolimus peut être associé à une imagerie CT à haute résolution pour un suivi continu de la progression de la maladie, permettant aux médecins d'adapter les schémas thérapeutiques de manière proactive.
- L'intégration du diagnostic et du traitement permet une meilleure prise en charge des complications telles que le pneumothorax et le chylothorax, tout en optimisant le suivi des patients et les hospitalisations. Par exemple, au Japon, les centres spécialisés dans la LAM utilisent des protocoles combinant diagnostic et traitement pour améliorer la qualité de vie des patients.
- Cette intégration fluide facilite la gestion centralisée des patients, permettant aux hôpitaux, aux cliniques spécialisées et aux centres de diagnostic de coordonner efficacement les soins et de réduire les délais d'initiation du traitement.
- La tendance vers des soins plus complets et centrés sur le patient redéfinit fondamentalement les attentes en matière de prise en charge des maladies pulmonaires rares, avec des entreprises telles que Chiesi et GlaxoSmithKline développant des parcours de soins intégrés combinant thérapies orales et parentérales avec surveillance continue.
- La demande de solutions intégrées de diagnostic et de traitement de la LAM croît rapidement dans les pays d'Asie-Pacifique tels que le Japon, la Chine et l'Inde, car les prestataires de soins de santé privilégient de plus en plus des soins personnalisés et efficaces pour les patients.
Dynamique du marché de la lymphangioléiomyomatose (LAM) en Asie-Pacifique
Conducteur
« Besoins croissants dus à une meilleure sensibilisation aux maladies et à un accès accru aux soins de santé »
- La sensibilisation croissante aux maladies pulmonaires rares, associée au développement des infrastructures de santé dans les pays d'Asie-Pacifique, est un facteur important de la demande accrue de diagnostics et de traitements pour la LAM.
- Par exemple, en mars 2024, Chiesi Pharmaceuticals a lancé un programme de sensibilisation ciblé au Japon afin de mieux informer les pneumologues sur le traitement de la LAM et les stratégies de dépistage précoce.
- À mesure que les patients et les cliniciens prennent davantage conscience des complications de la LAM, telles que le chylothorax et le pneumothorax, la demande en outils de diagnostic avancés, en imagerie et en solutions de traitement augmente considérablement.
- De plus, la disponibilité croissante de cliniques spécialisées en LAM et de centres de recherche rend les thérapies et les diagnostics avancés plus accessibles, garantissant ainsi une prise en charge complète des patientes.
- La facilité d'accès aux traitements oraux et parentéraux, associée à un suivi en milieu hospitalier et en clinique, est essentielle pour améliorer l'observance thérapeutique et les résultats, stimulant ainsi la croissance du marché dans des pays comme la Chine, le Japon et l'Inde.
- Par exemple, en Corée du Sud, les programmes gouvernementaux de lutte contre les maladies rares ont amélioré l'accès des patients aux thérapies LAM, stimulant ainsi l'adoption et la sensibilisation du marché.
- La multiplication des collaborations entre les entreprises pharmaceutiques et les hôpitaux dans la région Asie-Pacifique facilite les essais cliniques et les programmes de formation, favorisant ainsi l'adoption des traitements et des diagnostics de la LAM.
Retenue/Défi
« Sensibilisation limitée et coûts de traitement élevés »
- Les inquiétudes concernant la connaissance limitée de la LAM parmi les professionnels de la santé et les patients constituent un défi important pour une plus large pénétration du marché dans les pays de la région Asie-Pacifique.
- Par exemple, les retards de diagnostic dus à des erreurs d'identification avec d'autres affections pulmonaires ont réduit les possibilités de traitement précoce en Inde et en Corée du Sud.
- Combler ces lacunes en matière de sensibilisation par la formation des médecins, des programmes de sensibilisation des patients et des politiques nationales relatives aux maladies rares est crucial pour favoriser l'adoption des traitements contre la LAM. De plus, le coût relativement élevé des protocoles de traitement, notamment du sirolimus et de l'imagerie diagnostique, peut constituer un obstacle pour les patients sensibles au prix, en particulier dans les pays en développement de la région Asie-Pacifique.
- Bien que l'accès aux traitements s'améliore, le coût élevé des thérapies de pointe peut encore freiner leur adoption par les patients sans couverture d'assurance ou sans soutien gouvernemental.
- Surmonter ces défis grâce à des campagnes de sensibilisation, des programmes de soutien aux patients et des options thérapeutiques subventionnées sera essentiel pour une croissance durable du marché LAM en Asie-Pacifique.
- Par exemple, l'absence de couverture d'assurance pour le traitement des maladies rares dans certaines régions continue de limiter l'accès des patients aux thérapies LAM coûteuses, ralentissant ainsi l'expansion du marché.
- Les obstacles réglementaires et les retards d'approbation des nouvelles thérapies dans des pays comme l'Inde et la Chine constituent des défis supplémentaires, affectant la disponibilité et l'adoption rapides des traitements LAM innovants.
Portée du marché de la lymphangioleiomyomatose (LAM) en Asie-Pacifique
Le marché est segmenté en fonction du type de maladie, du type de traitement, des complications, de la voie d'administration, de l'utilisateur final et du canal de distribution.
- Par type de maladie
Selon le type de maladie, le marché de la LAM en Asie-Pacifique est segmenté en sclérose tubéreuse de Bourneville (TSC-LAM) et LAM sporadique. Le segment TSC-LAM a dominé le marché en 2024, générant la plus grande part de revenus, grâce à sa prévalence plus élevée chez les patients diagnostiqués et à la nécessité d'un suivi et d'une prise en charge continus. Les patients atteints de TSC-LAM nécessitent souvent des examens d'imagerie et des traitements réguliers pour contrôler la progression de la maladie et prévenir les complications telles que le pneumothorax et le chylothorax. Ce segment bénéficie de programmes de sensibilisation accrus, notamment dans des pays comme le Japon et la Corée du Sud, où des initiatives de dépistage précoce sont actives. Les entreprises pharmaceutiques concentrent leurs efforts sur la TSC-LAM pour les essais cliniques et les autorisations de mise sur le marché, contribuant ainsi à une meilleure adoption des thérapies et des services de diagnostic. Les hôpitaux et les cliniques spécialisées privilégient le traitement de la TSC-LAM en raison de la complexité des soins requis. Les programmes gouvernementaux et les programmes pour les maladies rares dans des pays comme la Chine soutiennent également ce segment, renforçant ainsi sa position dominante sur le marché.
Le segment de la LAM sporadique devrait connaître la croissance la plus rapide entre 2025 et 2032, portée par l'augmentation des diagnostics chez les femmes adultes en Chine et en Inde. Les patientes atteintes de LAM sporadique bénéficient de plus en plus de techniques d'imagerie améliorées et d'un meilleur accès aux thérapies ciblées. La sensibilisation croissante aux maladies pulmonaires rares et la formation des pneumologues contribuent à un dépistage plus précoce et à une meilleure prise en charge de la maladie. La recherche clinique et les collaborations entre les hôpitaux locaux et les entreprises de biotechnologie internationales élargissent les options thérapeutiques. Ce segment connaît également une adoption croissante des programmes de suivi à domicile, permettant aux patientes de suivre leurs symptômes et leur observance thérapeutique. Le recours accru aux centres de diagnostic spécialisés dans les maladies rares soutient également la croissance rapide de ce segment.
- Par type
Le marché est segmenté, selon le type de traitement, en diagnostic et traitement. Le segment du traitement a dominé le marché en 2024, représentant la plus grande part de revenus (62,9 %), grâce à l'adoption croissante des inhibiteurs de mTOR et d'autres thérapies ciblées. Le traitement constitue l'intervention principale pour gérer la progression de la maladie et prévenir les complications graves. Les hôpitaux et les cliniques spécialisées sont les principaux utilisateurs finaux de ces traitements. La demande est également soutenue par les recommandations cliniques préconisant une intervention pharmacologique rapide après le diagnostic. Les entreprises pharmaceutiques japonaises, chinoises et sud-coréennes lancent activement de nouvelles options thérapeutiques. La sensibilisation accrue des patients et les programmes de formation des médecins favorisent l'adoption de ces traitements, assurant ainsi une domination durable du marché.
Le segment du diagnostic devrait connaître le taux de croissance annuel composé (TCAC) le plus rapide entre 2025 et 2032, grâce aux progrès technologiques tels que la tomodensitométrie à haute résolution, les tests génétiques et les méthodes de détection basées sur les biomarqueurs. Un diagnostic précoce est essentiel pour améliorer les résultats des traitements et réduire les complications graves. La sensibilisation croissante des pneumologues et des médecins généralistes dans les pays émergents d'Asie-Pacifique favorise l'orientation des patients vers les centres de diagnostic. L'investissement dans les infrastructures d'imagerie avancées et les capacités des laboratoires accélère encore l'adoption du marché. La multiplication des programmes de dépistage et des registres de maladies rares contribue également à la croissance rapide de ce segment. Les patients sont de plus en plus nombreux à rechercher des tests précoces grâce à une meilleure couverture d'assurance et aux programmes de soutien gouvernementaux.
- En raison de complications
En fonction des complications, le marché est segmenté en pneumothorax, chylothorax, tumeur rénale, épanchements pleuraux, œdème et accumulation de liquide, et autres. Le segment du pneumothorax a dominé le marché en 2024, du fait de sa forte incidence chez les patientes atteintes de LAM et de la nécessité fréquente d'une intervention clinique urgente. La prise en charge diagnostique et thérapeutique du pneumothorax repose notamment sur l'hospitalisation, l'imagerie et les interventions préventives. Les hôpitaux et les cliniques spécialisées sont les principaux utilisateurs. Les campagnes de sensibilisation et la formation des médecins ont permis d'améliorer le diagnostic et la prise en charge précoce du pneumothorax. Ce segment bénéficie également d'investissements importants en R&D de la part des entreprises pharmaceutiques, tant pour la prévention que pour le traitement. L'augmentation de la prévalence au Japon et en Chine conforte sa position dominante. Les programmes de suivi des patientes garantissent la poursuite de la croissance du marché.
Le segment du chylothorax devrait connaître la croissance la plus rapide entre 2025 et 2032, grâce à une détection et une sensibilisation accrues aux complications lymphatiques chez les patientes atteintes de LAM. La disponibilité de traitements peu invasifs, tels que la diétothérapie, le drainage et la prise en charge pharmacologique, favorise leur adoption rapide. Les hôpitaux et les cliniques spécialisées développent leurs capacités pour une prise en charge efficace du chylothorax. L'imagerie diagnostique et la recherche sur les biomarqueurs contribuent à l'augmentation des taux de détection. Les initiatives gouvernementales en matière de santé en Chine, en Inde et en Corée du Sud encouragent la prise en charge précoce des complications lymphatiques. L'éducation des patientes sur la surveillance des symptômes stimule également la croissance de ce segment.
- Par voie d'administration
Selon la voie d'administration, le marché est segmenté en voie orale et parentérale. Le segment oral a dominé le marché en 2024, porté par l'utilisation généralisée des inhibiteurs de mTOR par voie orale, tels que le sirolimus, pratiques et adaptés à une prise en charge au long cours. Les traitements oraux sont privilégiés dans les hôpitaux, les cliniques spécialisées et les services de soins à domicile en raison de leur facilité d'administration. Ce segment bénéficie des programmes d'observance thérapeutique et du suivi continu permettant d'optimiser la posologie. Les entreprises pharmaceutiques s'attachent à améliorer les formulations et la biodisponibilité. Les autorisations réglementaires au Japon et en Chine privilégient la disponibilité des traitements oraux. Les programmes de formation destinés aux médecins et aux patients contribuent à l'augmentation des taux d'adoption.
Le segment des traitements parentéraux devrait connaître le taux de croissance annuel composé le plus rapide entre 2025 et 2032, grâce à l'introduction de thérapies injectables pour les cas de LAM sévères ou à progression rapide. Les traitements parentéraux permettent un dosage précis et un effet thérapeutique rapide. Les hôpitaux et les cliniques spécialisées constituent les principaux lieux d'administration. Des essais cliniques menés en Chine, au Japon et en Inde élargissent les indications de la thérapie parentérale. De nouvelles formulations et de nouveaux protocoles de perfusion améliorent le confort et l'observance des patients. Le développement de programmes de traitement hospitaliers pour les cas complexes accélère encore l'adoption de ces traitements. Les programmes de formation destinés aux professionnels de santé améliorent la sécurité et l'efficacité des traitements, soutenant ainsi la croissance du segment.
- Par l'utilisateur final
En fonction de l'utilisateur final, le marché est segmenté en hôpitaux, cliniques spécialisées, centres de diagnostic, soins à domicile et autres. Le segment des hôpitaux a dominé le marché en 2024, générant la plus grande part de revenus, car les hôpitaux sont les principaux centres de diagnostic, de traitement et de prise en charge des complications de la LAM. Ils fournissent l'infrastructure nécessaire à l'imagerie, à l'administration des traitements et au suivi des patients. Les politiques gouvernementales au Japon, en Chine et en Corée du Sud soutiennent les programmes de traitement hospitaliers. Le volume élevé de patients et les capacités de prise en charge complètes maintiennent cette position dominante. La collaboration avec les entreprises pharmaceutiques garantit un approvisionnement constant en traitements. Les programmes de sensibilisation et de prise en charge des maladies rares ciblent souvent les hôpitaux comme points d'intervention clés.
Le segment des cliniques spécialisées devrait connaître la croissance la plus rapide entre 2025 et 2032, portée par l'intérêt croissant porté à la prise en charge des maladies rares et aux soins personnalisés. Ces cliniques proposent des plans de traitement ciblés, un suivi régulier et l'accès aux essais cliniques. Les protocoles de diagnostic et de thérapie avancés sont de plus en plus proposés dans les cliniques des centres urbains de Chine, du Japon et d'Inde. L'expertise croissante des médecins et la préférence des patients pour les soins spécialisés favorisent une adoption rapide. L'intégration du suivi à domicile améliore encore la prise en charge des patients. L'expansion des réseaux de cliniques dans les villes émergentes contribue à la croissance de ce segment.
- Par canal de distribution
En fonction du canal de distribution, le marché est segmenté en appels d'offres directs, pharmacies hospitalières, pharmacies de détail, pharmacies en ligne et autres. Le segment des appels d'offres directs a dominé le marché en 2024, porté par les achats en gros volumes effectués par les hôpitaux et les programmes de santé publique. Ce mode d'achat garantit un approvisionnement rapide et économique en traitements LAM aux établissements de santé. Il facilite également l'achat groupé de médicaments oraux et parentéraux pour plusieurs centres. Les gouvernements et les réseaux hospitaliers en Chine et au Japon privilégient les appels d'offres directs pour maintenir leurs niveaux de stock. Les contrats fournisseurs incluent souvent des programmes de suivi et de formation, favorisant ainsi l'adoption continue de ces traitements. Les entreprises pharmaceutiques privilégient les appels d'offres directs pour les traitements à forte valeur ajoutée.
Le segment des pharmacies en ligne devrait connaître la croissance la plus rapide entre 2025 et 2032, portée par l'essor du commerce électronique , les programmes de télémédecine et la commodité pour les patients en milieu urbain. Les pharmacies en ligne permettent la livraison à domicile de traitements oraux et le renouvellement des ordonnances. En Inde et en Corée du Sud, la préférence des patients pour l'accès à distance aux médicaments est croissante. L'intégration avec les applications de santé mobile améliore le suivi de l'observance thérapeutique. Les politiques gouvernementales soutenant les plateformes de santé numérique accélèrent l'adoption de ce segment. Les partenariats entre les entreprises pharmaceutiques et les plateformes en ligne optimisent la logistique et l'accessibilité.
Analyse régionale du marché de la lymphangioléiomyomatose (LAM) en Asie-Pacifique
- Le Japon a dominé le marché Asie-Pacifique du LAM avec la plus grande part de revenus (38 %) en 2024, grâce à une infrastructure de santé avancée, une forte adoption des diagnostics de maladies rares et la présence d'acteurs clés du secteur.
- Les patients et les professionnels de santé de la région accordent une grande importance à la disponibilité de solutions intégrées de diagnostic et de traitement, qui permettent une détection précoce, une surveillance continue et une meilleure prise en charge des complications telles que le pneumothorax et le chylothorax.
- Cette adoption généralisée est également soutenue par les initiatives gouvernementales en faveur des maladies rares, les investissements croissants dans les infrastructures de santé et la présence de cliniques spécialisées dans la LAM, faisant du Japon un pays leader dans le diagnostic et le traitement de cette maladie.
Analyse du marché japonais des LAM
Le marché japonais de la LAM est en plein essor grâce à des infrastructures de santé performantes, une forte sensibilisation aux maladies rares et une utilisation accrue des services de diagnostic et de traitement spécialisés. Les hôpitaux et les cliniques spécialisées au Japon proposent des programmes de prise en charge complets pour les patients atteints de LAM associée à la sclérose tubéreuse de Bourneville (TSC-LAM) et de LAM sporadique, intégrant le diagnostic, le traitement et le suivi. Le gouvernement et les programmes de lutte contre les maladies rares apportent un soutien supplémentaire, garantissant un accès rapide aux médicaments et aux interventions cliniques. Par ailleurs, la présence de grands groupes pharmaceutiques menant des recherches et des essais cliniques au Japon stimule l'innovation et l'adoption des thérapies. Le vieillissement de la population et l'urbanisation croissante devraient également contribuer à la croissance du marché, tant en milieu résidentiel qu'en milieu hospitalier.
Analyse du marché chinois des LAM
Le marché chinois de la LAM devrait connaître une croissance rapide grâce à une meilleure sensibilisation à la maladie, au développement des infrastructures de santé et à un accès accru aux diagnostics et aux thérapies spécialisées. Les hôpitaux et les centres spécialisés privilégient le dépistage et la prise en charge précoces des complications telles que le pneumothorax et le chylothorax. Les initiatives gouvernementales soutenant le traitement des maladies rares et le développement des programmes de défense des droits des patients stimulent l'adoption du marché. L'intégration des essais cliniques et des programmes de suivi des patients améliore les résultats et l'observance du traitement. Par ailleurs, la production locale et la disponibilité accrue des traitements oraux et parentéraux améliorent l'accessibilité financière pour les patients des zones urbaines et périurbaines.
Analyse du marché indien des LAM
Le marché indien de la LAM a représenté une part importante des revenus de la région Asie-Pacifique en 2024, grâce à une sensibilisation accrue aux soins de santé, à l'expansion des réseaux hospitaliers et à l'adoption croissante d'outils de diagnostic avancés. L'Inde observe une augmentation du nombre de cas détectés de LAM associée à la sclérose tubéreuse de Bourneville (TSC-LAM) et de LAM sporadique, les hôpitaux et les centres de diagnostic proposant des soins spécialisés. Le développement de programmes pour les maladies rares, le soutien gouvernemental et la disponibilité de traitements abordables proposés par les entreprises pharmaceutiques nationales et internationales sont des facteurs clés de la croissance de ce marché. Par ailleurs, la télémédecine et les programmes de suivi des soins à domicile facilitent un meilleur suivi des patients et une meilleure observance thérapeutique, contribuant ainsi à la croissance du marché.
Analyse du marché LAM en Corée du Sud
Le marché sud-coréen de la LAM devrait connaître une croissance soutenue grâce à une meilleure sensibilisation aux maladies pulmonaires rares et à un accès élargi aux services de santé spécialisés. Les hôpitaux et les cliniques spécialisées des zones urbaines offrent une prise en charge complète aux patients atteints de LAM associée à la sclérose tubéreuse de Bourneville (TSC-LAM) et de LAM sporadique, incluant le diagnostic, le traitement et la gestion des complications. Les initiatives gouvernementales en faveur des maladies rares et les programmes d'aide aux patients améliorent l'accès aux traitements. L'adoption de technologies d'imagerie avancées et de programmes de télésurveillance à domicile contribue à un diagnostic précoce et à de meilleurs résultats thérapeutiques. Les entreprises pharmaceutiques mènent activement des essais cliniques et lancent de nouveaux traitements dans le pays.
Part de marché de la lymphangioleiomyomatose (LAM) en Asie-Pacifique
Le secteur de la lymphangioleiomyomatose (LAM) en Asie-Pacifique est principalement dominé par des entreprises bien établies, notamment :
- Pfizer Inc. (États-Unis)
- Novartis AG (Suisse)
- Société pharmaceutique Takeda Limitée (Japon)
- GSK plc (Royaume-Uni)
- AstraZeneca (Royaume-Uni)
- Boehringer Ingelheim International GmbH (Allemagne)
- Cipla Ltd. (Inde)
- Sun Pharmaceutical Industries Ltd. (Inde)
- CSL Limited (Australie)
- Sanofi (France)
- Laboratoires Dr. Reddy's Ltd. (Inde)
- Hikma Pharmaceuticals PLC (Royaume-Uni)
- Zydus Pharma (Inde)
- Terumo Corporation (Japon)
- Intas Pharmaceuticals Ltd. (Inde)
- Apotex Inc. (Canada)
- Amneal Pharmaceuticals LLC (États-Unis)
- Société d'oxygène à domicile (États-Unis)
- CareDx, Inc. (États-Unis)
Quels sont les développements récents sur le marché de la lymphangioleiomyomatose (LAM) en Asie-Pacifique ?
- En février 2025, une enquête transversale menée par des chercheurs chinois auprès de 121 patientes atteintes de LAM a exploré leur compréhension et leur perception de la maladie en Asie sinophone, soulignant le besoin de soutien psychosocial et d'éducation dans la prise en charge de la LAM en Asie-Pacifique.
- En décembre 2024, une étude japonaise a montré que les patientes atteintes de LAM au Japon présentaient un risque significativement accru de cancer du poumon. Ceci souligne l'inquiétude croissante concernant les comorbidités chez les patientes atteintes de LAM en Asie-Pacifique et pourrait inciter à un dépistage et une prise en charge renforcés dans la région.
- En octobre 2023, l'Agence japonaise des produits pharmaceutiques et des dispositifs médicaux (PMDA) a publié un rapport d'évaluation indiquant que le médicament Sirolimus comprimés à 1 mg pour le traitement de la LAM était en cours d'examen en vue d'un nouveau dosage/indication, officialisant ainsi son utilisation élargie au Japon.
- En mai 2023, un article du Journal de la Société Européenne de Pneumologie a rapporté une hyperréactivité bronchique inattendue associée au traitement par sirolimus chez les patientes atteintes de LAM. Ces résultats influencent les stratégies thérapeutiques à l'échelle mondiale, notamment dans les régions Asie-Pacifique où le sirolimus est utilisé, et incitent à une surveillance accrue des effets indésirables respiratoires.
- En mai 2022, une étude rétrospective multicentrique coréenne portant sur 54 patientes coréennes atteintes de LAM a révélé que celles traitées initialement par inhibiteurs de mTOR présentaient un risque de progression de la maladie significativement plus faible que celles sous simple observation. Ces résultats plaident en faveur d'une intervention thérapeutique plus précoce chez les patientes atteintes de LAM dans la région Asie-Pacifique.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.