Marché du diagnostic du cancer du sein en Asie-Pacifique, par type de test (imagerie, biopsie, test génomique , test sanguin et autres), type (carcinome canalaire in situ, carcinome canalaire invasif, cancer du sein inflammatoire et cancer du sein métastatique), utilisateur final (hôpitaux, cliniques, instituts de recherche et universitaires, centres de diagnostic et autres), canal de distribution (appel d'offres direct, vente au détail et autres) Tendances et prévisions de l'industrie jusqu'en 2030.
Analyse et perspectives du marché du diagnostic du cancer du sein en Asie-Pacifique
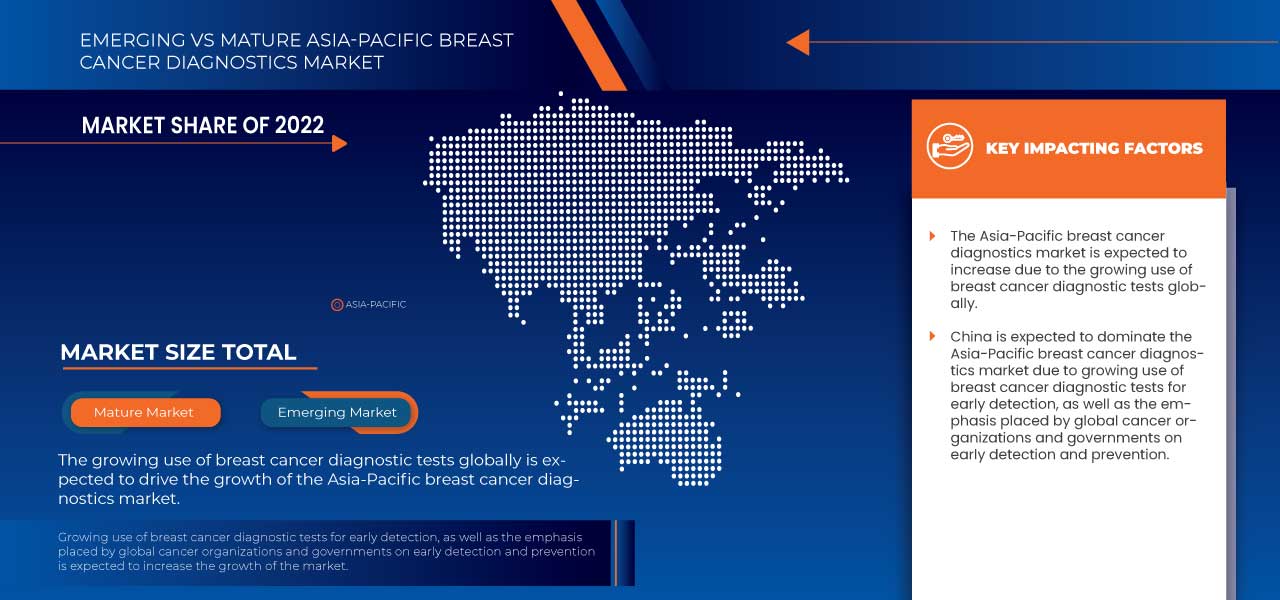
Le cancer du sein est la maladie la plus courante dans la population, survenant principalement chez les femmes atteintes de maladies prolifératives. Le cancer du sein se développe principalement dans les tissus mammaires. Les symptômes du cancer du sein comprennent des bosses dans le sein, une différence de taille et de forme des seins, un écoulement des mamelons et des plaques rouges irritées et squameuses sur la peau. L'incidence du cancer du sein chez les femmes augmente à chaque fois que la croissance du marché du cancer du sein augmente avec la croissance des médicaments. Méthodes avancées de diagnostic du cancer du sein avec des techniques développées. Améliorer les technologies développées dans le cancer du sein en augmentant l'efficacité, la précision, l'efficience, la fiabilité et la rapidité des résultats et en améliorant la détection des troubles. Des fonctionnalités avancées pour détecter le cancer en moins de temps. Le cancer est détecté à un stade précoce, ce qui aide les patients à récupérer plus rapidement, empêche les cellules cancéreuses de se propager à d'autres parties du corps et les traite avec de nouveaux médicaments sur le marché. L'augmentation du dépistage et de l'imagerie des tumeurs stimulera le marché du cancer du sein. Cela élargit le marché du cancer du sein avec un nombre croissant de patients chaque année, et le taux de guérison du cancer du sein augmente. Le soutien du gouvernement consiste à accroître la disponibilité de nouveaux projets de développement de médicaments et à accroître l’efficacité et l’efficience du traitement ainsi que l’intégration de nouveaux médicaments dans le domaine médical.
Cependant, le coût élevé des traitements de diagnostic du cancer et le manque de professionnels qualifiés sont les facteurs qui freinent la croissance du marché.
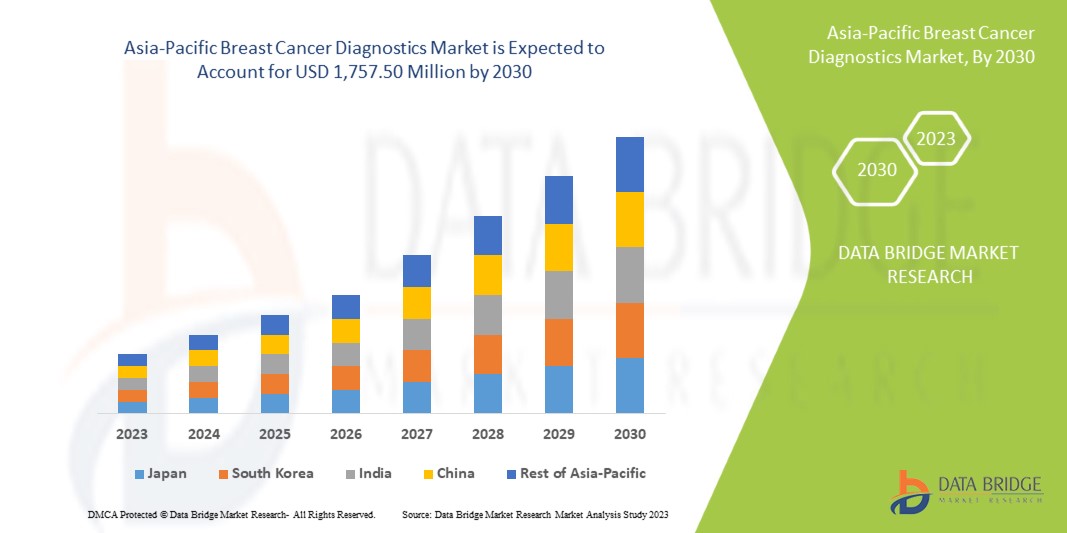
Data Bridge Market Research analyse que le marché du diagnostic du cancer du sein en Asie-Pacifique devrait atteindre la valeur de 1 757,50 millions USD d'ici 2030, à un TCAC de 10,3 % au cours de la période de prévision. Le type de test représente le segment de type de diagnostic le plus important du marché en raison de l'augmentation du nombre de patientes atteintes du cancer du sein et des avancées technologiques dans le traitement du cancer du sein en Asie-Pacifique. Ce rapport de marché couvre également en profondeur l'analyse des prix, l'analyse des brevets et les avancées technologiques.
|
Rapport métrique |
Détails |
|
Période de prévision |
2023 à 2030 |
|
Année de base |
2022 |
|
Années historiques |
2021 (personnalisable pour 2020-2016) |
|
Unités quantitatives |
Chiffre d'affaires en millions USD, volumes en unités, prix en USD |
|
Segments couverts |
Par type de test (imagerie, biopsie, test génomique, test sanguin et autres), type (carcinome canalaire in situ, carcinome canalaire invasif, cancer du sein inflammatoire et cancer du sein métastatique), utilisateur final (hôpitaux, cliniques, instituts de recherche et universitaires, centres de diagnostic et autres), canal de distribution (appel d'offres direct, vente au détail et autres). |
|
Pays couverts |
Chine, Japon, Inde, Australie, Corée du Sud, Singapour, Malaisie, Thaïlande, Indonésie, Philippines et reste de l'Asie-Pacifique. |
|
Acteurs du marché couverts |
Les principales entreprises présentes sur le marché sont F-Hoffmann La Roche Ltd., Siemens Healthcare GmbH, General Electric, Koninklijke Philips NV, FUJIFILM Corporation, Abbott, Hologic, Inc., OncoStem, Provista Diagnostics, Thermo Fisher Scientific Inc., Myriad Genetics, Inc., Illumina, Inc., Bio-Rad Laboratories, Inc., BD, NanoString., Cepheid, BIOMÉRIEUX, Exact Sciences Corporation, Biocept, Inc. et Abacus ALS, entre autres. |
Définition du marché du diagnostic du cancer du sein en Asie-Pacifique
Le cancer du sein est une maladie caractérisée par une croissance incontrôlée de cellules malignes dans le tissu mammaire, qui survient plus souvent chez les femmes que chez les hommes. Le cancer du sein est la division cellulaire incontrôlée des cellules mammaires, qui sont les cellules les plus courantes dans les glandes et les canaux mammaires. Certains symptômes du cancer du sein comprennent une grosseur ou une masse dans le sein, un écoulement sanguinolent du mamelon et un changement de forme du mamelon ou du sein. Le traitement du cancer du sein dépend du stade du cancer. Son traitement comprend la chimiothérapie, la radiothérapie, l'hormonothérapie et la chirurgie.
Le cancer du sein est un autre type de cancer qui est plus fréquent chez les femmes que chez les hommes. Un certain nombre de symptômes de la maladie comprennent des sécrétions saignantes de l'organe reproducteur, une grosseur ou un blocage dans le sein et des variations de texture ou de forme du sein ou du mamelon. Le traitement du cancer du sein dépend du stade du cancer. En outre, son traitement comprend la radiothérapie, la thérapie, l'hormonothérapie et la chirurgie. La détection précoce du carcinome est essentielle pour le traitement efficace de la maladie. La détection précoce de la maladie conduit également à de meilleurs résultats, notamment de nombreuses options de traitement, une meilleure survie et une meilleure qualité de vie. La pression et la demande croissantes de nouvelles formes ou méthodes de traitement sont dues à la propagation rapide des maladies.
Dynamique du marché du diagnostic du cancer du sein en Asie-Pacifique
Cette section traite de la compréhension des moteurs, des avantages, des opportunités, des contraintes et des défis du marché. Tout cela est discuté en détail ci-dessous :
Conducteurs
- Lancement de diagnostics et de thérapies technologiquement avancés pour favoriser la croissance
Le cancer du sein est la maladie la plus courante dans la population, survenant principalement chez les femmes atteintes d'une maladie proliférative. Le cancer du sein se développe dans les tissus mammaires. Les symptômes du cancer du sein comprennent des bosses dans le sein, des différences de taille et de forme du sein, des écoulements des mamelons et des plaques rouges squameuses qui démangent sur la peau. Cette maladie se propage dans le corps avec des symptômes croissants tels que des douleurs osseuses, des ganglions lymphatiques enflés, des difficultés respiratoires et bien d'autres. L'augmentation de la recherche et du développement avec de nouvelles technologies et de nouveaux traitements développés pour le cancer du sein contribuera considérablement à la croissance du marché.
De plus, les progrès technologiques en matière d’imagerie ouvrent de nouvelles possibilités d’amélioration du dépistage et de la détection précoce. L’une des avancées technologiques est la mammographie 3D, également appelée tomosynthèse mammaire. Cette procédure prend des images sous différents angles autour du sein et les construit en une image de type 3D.
Ces derniers temps, plusieurs avancées technologiques ont changé le destin du diagnostic du cancer. Comme mentionné ci-dessus, plusieurs autres technologies sont actuellement en phase d'essai et sont sur le point de changer le processus de diagnostic du cancer du sein. Ces avancées technologiques dans le domaine agissent comme un moteur majeur pour le marché du diagnostic du cancer du sein en Asie-Pacifique.
- Prévalence croissante du cancer du sein
L’un des principaux facteurs de croissance du marché de la région Asie-Pacifique est la prévalence croissante du cancer du sein dans le monde, ce qui devrait conduire à un grand nombre de patients ayant besoin d’options de traitement précises et efficaces. Le cancer du sein est l’un des cancers les plus courants au monde, et de meilleurs diagnostics devraient augmenter le nombre de patients diagnostiqués à l’avenir.
En outre, la demande croissante de soins préventifs et de traitements précoces a conduit à de nombreux dépistages du cancer, ce qui augmenterait également le nombre de patients ayant un besoin élevé de traitement au cours de la période de prévision.
Ainsi, la prévalence croissante du cancer du sein conduit à un plus grand nombre de diagnostics de cas et devrait donc stimuler la croissance du marché au cours de la période de prévision.
Retenue
- Effets indésirables des traitements contre le cancer du sein
Le dépistage consiste à rechercher un cancer avant que la personne ne présente des symptômes. Cela peut aider à détecter le cancer à un stade précoce. Lorsqu'un tissu anormal ou un cancer est détecté tôt, il peut être plus facile à traiter. Au moment où les symptômes apparaissent, le cancer peut avoir commencé à se propager. Plusieurs tests de dépistage sont utilisés pour détecter les cancers du sein, notamment l'imagerie par résonance magnétique du sein, la mammographie diagnostique, la biopsie, etc. Bien que ces tests soient considérés comme la norme pour le diagnostic du cancer du sein, ils comportent des risques associés, notamment des résultats faussement négatifs, des résultats faussement positifs et des effets secondaires tels qu'un stress psychologique excessif, une exposition excessive aux radiations et un risque grave de rupture de la tumeur et de propagation des cellules cancéreuses.
Opportunité
- Initiatives prises par le gouvernement
Le gouvernement s'efforce d'accroître les initiatives en matière de traitement du cancer du sein à l'aide de techniques avancées pour améliorer la santé des patients. Le nombre d'assurances, telles que les prestations sociales, a été augmenté afin que les citoyens ordinaires puissent choisir un traitement et profiter des médicaments et traitements disponibles pour améliorer leur qualité de vie. Dans les pays en développement comme la Chine et l'Inde, le gouvernement a lancé plusieurs programmes et initiatives pour sensibiliser à la maladie. Cela a considérablement accru la demande de diagnostic du cancer du sein et de thérapies de gestion de la maladie. L'augmentation des fusions et acquisitions entre les sociétés pharmaceutiques et les agences gouvernementales fait partie des principales tendances du marché du cancer du sein en Asie-Pacifique.
Le nombre de nouveaux cas de cancer du sein a augmenté de 0,6 % par an entre 2010 et 2019, et le taux de mortalité a augmenté en moyenne de 1,7 % par an sur la même période. À mesure que le nombre de patientes atteintes d'un cancer du sein augmente, le recours aux traitements et aux technologies avancées pour le diagnostic du cancer va augmenter, ce qui constitue une opportunité de croissance du marché.
Défi
- Manque d’infrastructures de diagnostic
Les maladies non transmissibles telles que le cancer du sein et d’autres types de cancer sont désormais reconnues par les Nations Unies et l’OMS comme une crise majeure de santé publique. Le cancer constitue la principale cause de ce problème et les systèmes de santé sont confrontés à un défi majeur pour améliorer les soins contre le cancer, maîtriser les coûts et accroître l’efficacité du système. Les différences d’approche et de résultats thérapeutiques entre les pays à revenu élevé et les pays à revenu faible et intermédiaire sont frappantes. Les raisons de cette disparité sont notamment le coût, l’accès aux soins, les lacunes en matière de main-d’œuvre et de formation, et le manque de sensibilisation du grand public et de la communauté médicale.
En outre, par rapport au diagnostic précoce, le dépistage du cancer est une stratégie de santé publique distincte et plus complexe qui nécessite des ressources, des infrastructures et une coordination supplémentaires. Les pays ruraux, en développement et à faible revenu des différentes régions ne disposent pas d'infrastructures suffisantes pour gérer les nouveaux kits et le stockage des échantillons. Ces pays ont besoin d'infrastructures de diagnostic pour assurer la détection précoce et le diagnostic rapide et précis de la maladie.
Impact post-COVID-19 sur le marché du diagnostic du cancer du sein en Asie-Pacifique
La pandémie de COVID-19 a entraîné des perturbations dans les services de santé électifs liés au dépistage du cancer du sein, aux soins pré-cancéreux et à la gestion des résultats de dépistage anormaux. Cela pourrait entraîner une augmentation de l’incidence du cancer du sein, aggravant ainsi les inégalités de santé existantes.
Les fabricants prennent diverses décisions stratégiques pour rebondir après la COVID-19. Les acteurs mènent de multiples activités de R&D, de lancement de produits et de partenariats stratégiques pour améliorer la technologie et les résultats des tests impliqués dans le marché.
Développements récents
- En novembre 2022, Koninklijke Philips NV a annoncé le lancement en Asie-Pacifique d'une solution d'échographie portable compacte de nouvelle génération lors de la réunion annuelle de la Radiological Society of North America (RSNA) afin d'apporter la qualité diagnostique associée aux systèmes d'échographie sur chariot haut de gamme à un plus grand nombre de patients. Elle est portable et polyvalente avec une bonne qualité d'image ou des performances. Elle est compatible avec les systèmes d'échographie Philips Affiniti et la sonde EPIQ. Cela a aidé l'entreprise à élargir son portefeuille de produits.
- En novembre 2022, Siemens Healthineers et Atrium Health, un important fournisseur de soins de santé à but non lucratif connu pour ses programmes de soins pédiatriques, oncologiques et cardiaques, ont annoncé un partenariat de valeur pluriannuel1. Cet accord stratégique vise à améliorer l'accès aux soins dans la zone de service d'Atrium Health dans le sud-est des États-Unis, à améliorer l'équité en matière de santé et à accroître la mobilité économique. Atrium Health acquiert pour plus de 140 millions de dollars d'équipements et d'appareils auprès de Siemens Healthineers, notamment des technologies d'imagerie avancées, de radio-oncologie et de robotique endovasculaire de précision. Cela a aidé l'entreprise à développer ses activités.
Portée du marché du diagnostic du cancer du sein en Asie-Pacifique
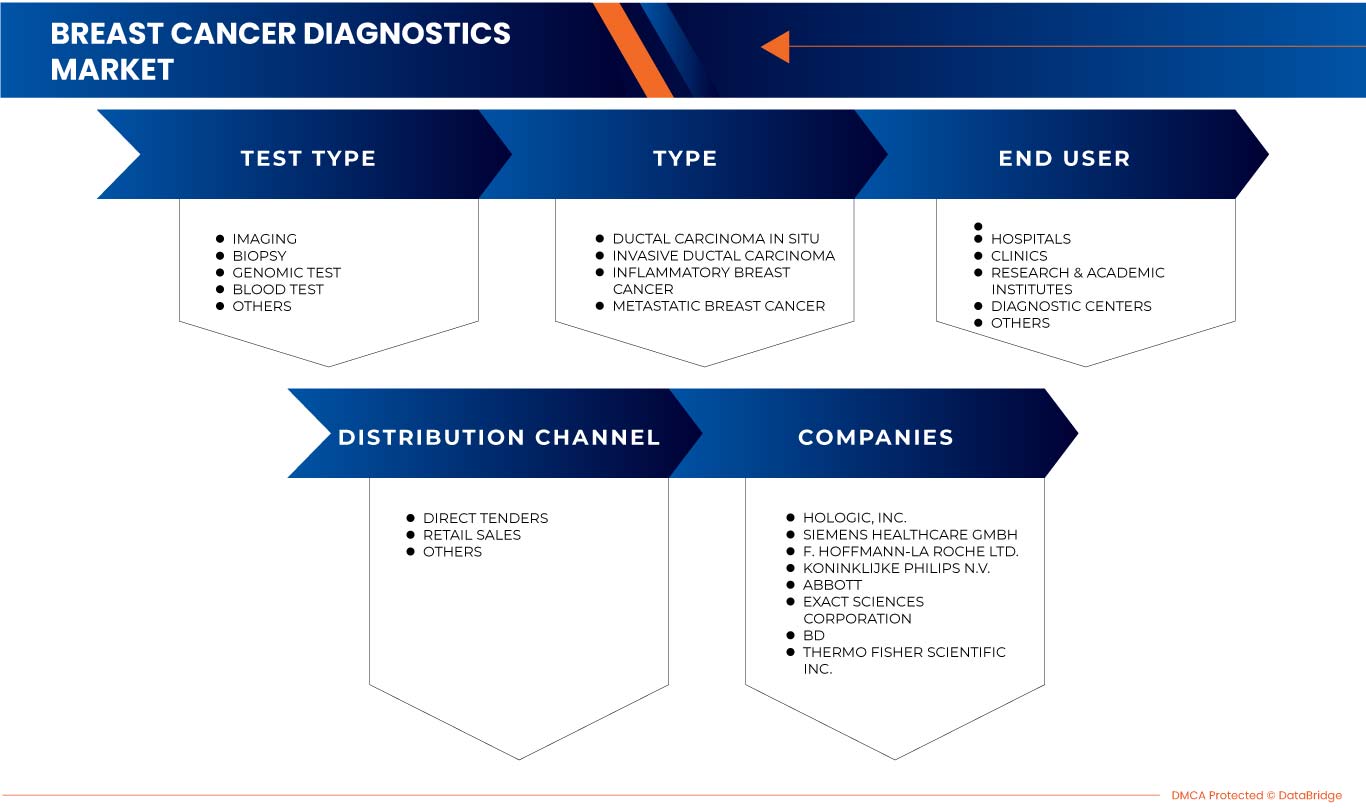
Le marché du diagnostic du cancer du sein en Asie-Pacifique est segmenté en type de test, type, utilisateur final et canal de distribution. La croissance entre les segments vous aide à analyser les niches de croissance et les stratégies pour aborder le marché et déterminer vos principaux domaines d'application et la différence entre vos marchés cibles.
MARCHÉ DU DIAGNOSTIC DU CANCER DU SEIN EN ASIE-PACIFIQUE, PAR TYPE DE TEST
- IMAGERIE
- BIOPSIE
- TEST GÉNOMIQUE
- ANALYSE DE SANG
- AUTRES
Sur la base du type de test, le marché du diagnostic du cancer du sein en Asie-Pacifique est segmenté en imagerie, biopsie, test génomique, tests sanguins et autres.
MARCHÉ DU DIAGNOSTIC DU CANCER DU SEIN EN ASIE-PACIFIQUE, PAR TYPE
- CARCINOME CANALAIRE IN SITU
- CARCINOME CANALAIRE INVASIF
- CANCER INFLAMMATOIRE DU SEIN
- CANCER DU SEIN MÉTASTATIQUE
Sur la base du type, le marché du diagnostic du cancer du sein en Asie-Pacifique est segmenté en carcinome canalaire in situ, carcinome canalaire invasif, cancer du sein inflammatoire et cancer du sein métastatique.
MARCHÉ DU DIAGNOSTIC DU CANCER DU SEIN EN ASIE-PACIFIQUE, PAR UTILISATEUR FINAL
- HÔPITAUX
- CLINIQUES
- INSTITUTS DE RECHERCHE ET D'ENSEIGNEMENT
- CENTRES DE DIAGNOSTIC
- AUTRES
Sur la base de l'utilisateur final, le marché du diagnostic du cancer du sein en Asie-Pacifique est segmenté en hôpitaux, cliniques, instituts de recherche et universitaires, centres de diagnostic et autres.
MARCHÉ DU DIAGNOSTIC DU CANCER DU SEIN EN ASIE-PACIFIQUE, PAR CANAL DE DISTRIBUTION
- APPEL D'OFFRES DIRECT
- VENTES AU DÉTAIL
- AUTRES
Sur la base du canal de distribution, le marché du diagnostic du cancer du sein en Asie-Pacifique est segmenté en appels d'offres directs, ventes au détail et autres.
Analyse/perspectives régionales du marché du diagnostic du cancer du sein en Asie-Pacifique
Le marché du diagnostic du cancer du sein en Asie-Pacifique est analysé et des informations sur la taille du marché sont fournies sur le type de test, le type, l’utilisateur final et le canal de distribution.
The countries covered in this market report China, Japan, India, Australia, South Korea, Singapore, Malaysia, Thailand, Indonesia, Philippines and rest of Asia-Pacific.
In 2023, China dominates Asia-Pacific region due to rise in breast cancer mainly among the women population as well as men.
The country section of the report also provides individual market impacting factors and changes in regulation in the market domestically that impact the current and future trends of the market. Data points such as new sales, replacement sales, country demographics, regulatory acts, and import-export tariffs are some of the major pointers used to forecast the market scenario for individual countries. Also, presence and availability of Asia-Pacific brands and their challenges faced due to large or scarce competition from local and domestic brands, and impact of sales channels are considered while providing forecast analysis of the country data.
Competitive Landscape and Asia-Pacific Breast Cancer Diagnostics Market Share Analysis
Asia-Pacific breast cancer diagnostics market competitive landscape provides details by competitor. Details included are company overview, company financials, revenue generated, market potential, investment in R&D, new market initiatives, production sites and facilities, company strengths and weaknesses, product launch, product trials pipelines, product approvals, patents, product width and breath, application dominance, technology lifeline curve. The above data points provided are only related to the company’s focus on the Asia-Pacific breast cancer diagnostics market.
Some of the major players operating in the Asia-Pacific breast cancer diagnostics market are F-Hoffmann La Roche Ltd., Siemens Healthcare GmbH, General Electric, Koninklijke Philips N.V., FUJIFILM Corporation, Abbott, Hologic, Inc., OncoStem, Provista Diagnostics, Thermo Fisher Scientific Inc., Myriad Genetics, Inc., Illumina, Inc., Bio-Rad Laboratories, Inc., BD, NanoString., Cepheid, BIOMÉRIEUX, Exact Sciences Corporation, Biocept, Inc., and Abacus ALS.
SKU-
Accédez en ligne au rapport sur le premier cloud mondial de veille économique
- Tableau de bord d'analyse de données interactif
- Tableau de bord d'analyse d'entreprise pour les opportunités à fort potentiel de croissance
- Accès d'analyste de recherche pour la personnalisation et les requêtes
- Analyse de la concurrence avec tableau de bord interactif
- Dernières actualités, mises à jour et analyse des tendances
- Exploitez la puissance de l'analyse comparative pour un suivi complet de la concurrence
Table des matières
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 TEST TYPE LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER'S 5 FORCES
5 EPIDEMIOLOGY
6 INDUSTRIAL INSIGHTS
7 REGULATORY FRAMEWORK
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 LAUNCH OF TECHNOLOGICALLY ADVANCED DIAGNOSIS & THERAPEUTICS TO AID GROWTH
8.1.2 INCREASING PREVALENCE OF BREAST CANCER
8.1.3 GROWING IMPORTANCE OF WOMEN'S HEALTH
8.1.4 RISING AWARENESS TOWARDS THE BREAST CANCER
8.2 RESTRAINTS
8.2.1 ADVERSE EFFECTS OF BREAST CANCER THERAPEUTICS
8.2.2 HIGH COST OF THE IMAGING SYSTEMS
8.3 OPPORTUNITIES
8.3.1 INITIATIVES TAKEN BY THE GOVERNMENT
8.3.2 STRATEGIC INITIATIVES TAKEN BY THE KEY MARKET PLAYERS
8.3.3 GROWTH IN RESEARCH AND DEVELOPMENT OF BREAST CANCER
8.4 CHALLENGES
8.4.1 LACK OF DIAGNOSTIC INFRASTRUCTURE
8.4.2 LACK OF SKILLED PROFESSIONALS FOR PROPER DIAGNOSIS OF BREAST CANCER
9 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE
9.1 OVERVIEW
9.2 IMAGING
9.2.1 IONIZING BREAST IMAGING TECHNOLOGIES
9.2.1.1 FULL-FIELD DIGITAL MAMMOGRAPHY (FFDM)
9.2.1.2 ANALOG MAMMOGRAPHY
9.2.1.3 3D BREAST TOMOSYNTHESIS
9.2.1.4 POSITRON EMISSION TOMOGRAPHY/ COMPUTED TOMOGRAPHY (PET/CT)
9.2.1.5 MOLECULAR BREAST IMAGING/ BREAST SPECIFIC GAMMA IMAGING (MBI/BSMI)
9.2.1.6 POSITRON EMISSION MAMMOGRAPHY
9.2.1.7 OTHERS
9.2.2 NON-IONIZING IMAGING TECHNOLOGIES
9.2.2.1 OPTICAL IMAGING
9.2.2.2 BREAST ULTRASOUND
9.2.2.3 BREAST MRI (MAGNETIC RESONANCE IMAGING)
9.2.2.4 AUTOMATED WHOLE BREAST ULTRASOUND (AWBU)
9.2.2.5 BREAST THERMOGRAPHY
9.2.3 BIOPSY
9.2.3.1 SURGICAL BIOPSY
9.2.3.2 FINE NEEDLE ASPIRATION BIOPSY
9.2.3.3 CORE NEEDLE BIOPSY
9.2.3.4 IMAGE-GUIDED BIOPSY
9.2.3.5 SENTINEL LYMPH NODE BIOPSY
9.2.4 GENOMIC TEST
9.2.4.1 MOLECULAR TESTING
9.2.4.1.1 PD-L1
9.2.4.1.2 MICROSATELLITE INSTABILITY-HIGH (MSI-H) OR DNA MISMATCH REPAIR DEFICIENCY (DMMR)
9.2.4.1.3 NTRK GENE FUSIONS
9.2.4.1.4 PI3KCA GENE MUTATION
9.2.4.2 MAMMAPRINT
9.2.4.3 ONCOTYPE DX
9.2.4.4 OTHERS
9.3 BLOOD TEST
9.4 OTHERS
10 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TYPE
10.1 OVERVIEW
10.2 INVASIVE DUCTAL CARCINOMA
10.3 DUCTAL CARCINOMA IN SITU
10.4 INFLAMMATORY BREAST CANCER
10.5 METASTATIC BREAST CANCER
11 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY END USER
11.1 OVERVIEW
11.2 HOSPITALS
11.3 DIAGNOSTICS CENTERS
11.4 CLINICS
11.5 RESEARCH & ACADEMIC INSTITUTES
11.6 OTHERS
12 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
12.1 OVERVIEW
12.2 DIRECT TENDER
12.3 RETAIL SALES
12.4 OTHERS
13 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY REGION
13.1 ASIA-PACIFIC
13.1.1 CHINA
13.1.2 INDIA
13.1.3 JAPAN
13.1.4 INDONESIA
13.1.5 PHILIPPINES
13.1.6 THAILAND
13.1.7 AUSTRALIA
13.1.8 MALAYSIA
13.1.9 SOUTH KOREA
13.1.10 SINGAPORE
13.1.11 REST OF ASIA-PACIFIC
14 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, COMPANY LANDSCAPE
14.1 COMPANY SHARE ANALYSIS: ASIA PACIFIC
15 SWOT ANALYSIS
16 COMPANY PROFILE
16.1 HOLOGIC, INC.
16.1.1 COMPANY SNAPSHOT
16.1.2 REVENUE ANALYSIS
16.1.3 COMPANY SHARE ANALYSIS
16.1.4 PRODUCT PORTFOLIO
16.1.5 RECENT DEVELOPMENTS
16.2 SIEMENS HEALTHCARE GMBH
16.2.1 COMPANY SNAPSHOT
16.2.2 REVENUE ANALYSIS
16.2.3 COMPANY SHARE ANALYSIS
16.2.4 PRODUCT PORTFOLIO
16.2.5 RECENT DEVELOPMENTS
16.3 F. HOFFMANN- LA ROCHE LTD
16.3.1 COMPANY SNAPSHOT
16.3.2 REVENUE ANALYSIS
16.3.3 COMPANY SHARE ANALYSIS
16.3.4 PRODUCT PORTFOLIO
16.3.5 RECENT DEVELOPMENTS
16.4 KONINKLIJKE PHILIPS N.V.
16.4.1 COMPANY SNAPSHOT
16.4.2 REVENUE ANALYSIS
16.4.3 COMPANY SHARE ANALYSIS
16.4.4 PRODUCT PORTFOLIO
16.4.5 RECENT DEVELOPMENTS
16.5 ABBOTT
16.5.1 COMPANY SNAPSHOT
16.5.2 REVENUE ANALYSIS
16.5.3 COMPANY SHARE ANALYSIS
16.5.4 PRODUCT PORTFOLIO
16.5.5 RECENT DEVELOPMENTS
16.6 ABACUS ALS
16.6.1 COMPANY SNAPSHOT
16.6.2 PRODUCT PORTFOLIO
16.6.3 RECENT DEVELOPMENTS
16.7 BD
16.7.1 COMPANY SNAPSHOT
16.7.2 REVENUE ANALYSIS
16.7.3 COMPANY SHARE ANALYSIS
16.7.4 PRODUCT PORTFOLIO
16.7.5 RECENT DEVELOPMENTS
16.8 BIOCEPT, INC.
16.8.1 COMPANY SNAPSHOT
16.8.2 REVENUE ANALYSIS
16.8.3 PRODUCT PORTFOLIO
16.8.4 RECENT DEVELOPMENTS
16.9 BIOMÉRIEUX
16.9.1 COMPANY SNAPSHOT
16.9.2 REVENUE ANALYSIS
16.9.3 PRODUCT PORTFOLIO
16.9.4 RECENT DEVELOPMENTS
16.1 BIO-RAD LABORATORIES, INC.
16.10.1 COMPANY SNAPSHOT
16.10.2 REVENUE ANALYSIS
16.10.3 PRODUCT PORTFOLIO
16.10.4 RECENT DEVELOPMENT
16.11 CEPHEID
16.11.1 COMPANY SNAPSHOT
16.11.2 REVENUE ANALYSIS
16.11.3 PRODUCT PORTFOLIO
16.11.4 RECENT DEVELOPMENT
16.12 EXACT SCIENCES CORPORATION
16.12.1 COMPANY SNAPSHOT
16.12.2 REVENUE ANALYSIS
16.12.3 COMPANY SHARE ANALYSIS
16.12.4 PRODUCT PORTFOLIO
16.12.5 RECENT DEVELOPMENTS
16.13 FUJIFILM CORPORATION
16.13.1 COMPANY SNAPSHOT
16.13.2 REVENUE ANALYSIS
16.13.3 PRODUCT PORTFOLIO
16.13.4 RECENT DEVELOPMENT
16.14 GENERAL ELECTRIC
16.14.1 COMPANY SNAPSHOT
16.14.2 REVENUE ANALYSIS
16.14.3 PRODUCT PORTFOLIO
16.14.4 RECENT DEVELOPMENTS
16.15 ILLUMINA, INC.
16.15.1 COMPANY SNAPSHOT
16.15.2 REVENUE ANALYSIS
16.15.3 PRODUCT PORTFOLIO
16.15.4 RECENT DEVELOPMENTS
16.16 MYRIAD GENETICS, INC.
16.16.1 COMPANY SNAPSHOT
16.16.2 REVENUE ANALYSIS
16.16.3 PRODUCT PORTFOLIO
16.16.4 RECENT DEVELOPMENT
16.17 NANOSTRING.
16.17.1 COMPANY SNAPSHOT
16.17.2 REVENUE ANALYSIS
16.17.3 PRODUCT PORTFOLIO
16.17.4 RECENT DEVELOPMENT
16.17.5 RECENT DEVELOPMENT
16.18 ONCOSTEM.
16.18.1 COMPANY SNAPSHOT
16.18.2 PRODUCT PORTFOLIO
16.18.3 RECENT DEVELOPMENT
16.19 PROVISTA DIAGNOSTICS.
16.19.1 COMPANY SNAPSHOT
16.19.2 PRODUCT PORTFOLIO
16.19.3 RECENT DEVELOPMENT
16.2 THERMO FISHER SCIENTIFIC INC.
16.20.1 COMPANY SNAPSHOT
16.20.2 REVENUE ANALYSIS
16.20.3 PRODUCT PORTFOLIO
16.20.4 RECENT DEVELOPMENT
17 QUESTIONNAIRE
18 RELATED REPORTS
Liste des tableaux
TABLE 1 INCIDENCE RATE OF BREAST CANCER (2020)
TABLE 2 MORTALITY RATE OF BREAST CANCER (2020)
TABLE 3 INCIDENCE OF DUCTAL CARCINOMA IN SITU (DCIS) BREAST CANCER
TABLE 4 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 5 ASIA PACIFIC IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 ASIA PACIFIC IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 7 ASIA PACIFIC IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 8 ASIA PACIFIC IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 9 ASIA PACIFIC NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 10 ASIA PACIFIC NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 11 ASIA PACIFIC BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 ASIA PACIFIC BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 13 ASIA PACIFIC BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 14 ASIA PACIFIC GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 ASIA PACIFIC GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 16 ASIA PACIFIC GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 17 ASIA PACIFIC MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 18 ASIA PACIFIC MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 19 ASIA PACIFIC BLOOD TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 20 ASIA PACIFIC OTHERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 22 ASIA PACIFIC INVASIVE DUCTAL CARCINOMA IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 ASIA PACIFIC DUCTAL CARCINOMA IN SITU IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 ASIA PACIFIC INFLAMMATORY BREAST CANCER IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 25 ASIA PACIFIC METASTATIC BREAST CANCER IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 27 ASIA PACIFIC HOSPITALS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 28 ASIA PACIFIC DIAGNOSTIC CENTERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 ASIA PACIFIC CLINICS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 ASIA PACIFIC RESEARCH AND ACADEMIC INSTITUTES IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 ASIA PACIFIC OTHERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 33 ASIA PACIFIC DIRECT TENDER IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 34 ASIA PACIFIC RETAIL SALES IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 ASIA PACIFIC OTHERS IN BREAST CANCER DIAGNOSTICS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 37 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 38 ASIA-PACIFIC IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 39 ASIA-PACIFIC IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 40 ASIA-PACIFIC IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 41 ASIA-PACIFIC NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 42 ASIA-PACIFIC NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 43 ASIA-PACIFIC BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 44 ASIA-PACIFIC BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 45 ASIA-PACIFIC GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 46 ASIA-PACIFIC GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 47 ASIA-PACIFIC MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 48 ASIA-PACIFIC MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 49 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 50 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 51 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 52 CHINA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 53 CHINA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 54 CHINA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 55 CHINA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 56 CHINA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 57 CHINA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 58 CHINA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 59 CHINA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 60 CHINA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 61 CHINA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 62 CHINA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 63 CHINA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 64 CHINA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 65 CHINA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 66 CHINA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 67 CHINA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 68 CHINA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 69 CHINA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 CHINA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 CHINA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 72 INDIA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 73 INDIA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 74 INDIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 75 INDIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 76 INDIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 77 INDIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 78 INDIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 79 INDIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 80 INDIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 81 INDIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 82 INDIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 83 INDIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 84 INDIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 85 INDIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 86 INDIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 87 INDIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 88 INDIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 89 INDIA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 90 INDIA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 91 INDIA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 92 JAPAN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 93 JAPAN IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 94 JAPAN IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 95 JAPAN IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 96 JAPAN IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 97 JAPAN NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 98 JAPAN NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 99 JAPAN NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 100 JAPAN BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 101 JAPAN BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 102 JAPAN BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 103 JAPAN GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 104 JAPAN GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 105 JAPAN GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 106 JAPAN MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 107 JAPAN MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 108 JAPAN MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 109 JAPAN BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 JAPAN BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 111 JAPAN BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 112 INDONESIA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 113 INDONESIA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 114 INDONESIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 115 INDONESIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 116 INDONESIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 117 INDONESIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 118 INDONESIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 119 INDONESIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 120 INDONESIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 121 INDONESIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 122 INDONESIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 123 INDONESIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 124 INDONESIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 125 INDONESIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 126 INDONESIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 127 INDONESIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 128 INDONESIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 129 INDONESIA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 130 INDONESIA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 131 INDONESIA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 132 PHILIPPINES BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 133 PHILIPPINES IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 134 PHILIPPINES IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 135 PHILIPPINES IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 136 PHILIPPINES IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 137 PHILIPPINES NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 138 PHILIPPINES NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 139 PHILIPPINES NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 140 PHILIPPINES BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 141 PHILIPPINES BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 142 PHILIPPINES BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 143 PHILIPPINES GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 144 PHILIPPINES GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 145 PHILIPPINES GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 146 PHILIPPINES MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 147 PHILIPPINES MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 148 PHILIPPINES MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 149 PHILIPPINES BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 150 PHILIPPINES BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 151 PHILIPPINES BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 152 THAILAND BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 153 THAILAND IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 154 THAILAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 155 THAILAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 156 THAILAND IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 157 THAILAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 158 THAILAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 159 THAILAND NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 160 THAILAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 161 THAILAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 162 THAILAND BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 163 THAILAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 164 THAILAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 165 THAILAND GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 166 THAILAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 167 THAILAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 168 THAILAND MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 169 THAILAND BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 THAILAND BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 171 THAILAND BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 172 AUSTRALIA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 173 AUSTRALIA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 174 AUSTRALIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 175 AUSTRALIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 176 AUSTRALIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 177 AUSTRALIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 178 AUSTRALIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 179 AUSTRALIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 180 AUSTRALIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 181 AUSTRALIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 182 AUSTRALIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 183 AUSTRALIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 184 AUSTRALIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 185 AUSTRALIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 186 AUSTRALIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 187 AUSTRALIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 188 AUSTRALIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 189 AUSTRALIA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 AUSTRALIA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 191 AUSTRALIA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 192 MALAYSIA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 193 MALAYSIA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 194 MALAYSIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 195 MALAYSIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 196 MALAYSIA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 197 MALAYSIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 198 MALAYSIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 199 MALAYSIA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 200 MALAYSIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 201 MALAYSIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 202 MALAYSIA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 203 MALAYSIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 204 MALAYSIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 205 MALAYSIA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 206 MALAYSIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 207 MALAYSIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 208 MALAYSIA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 209 MALAYSIA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 210 MALAYSIA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 211 MALAYSIA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 212 SOUTH KOREA BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 213 SOUTH KOREA IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 214 SOUTH KOREA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 215 SOUTH KOREA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 216 SOUTH KOREA IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 217 SOUTH KOREA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 218 SOUTH KOREA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 219 SOUTH KOREA NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 220 SOUTH KOREA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 221 SOUTH KOREA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 222 SOUTH KOREA BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 223 SOUTH KOREA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 224 SOUTH KOREA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 225 SOUTH KOREA GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 226 SOUTH KOREA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 227 SOUTH KOREA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 228 SOUTH KOREA MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 229 SOUTH KOREA BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 230 SOUTH KOREA BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 231 SOUTH KOREA BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 232 SINGAPORE BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 233 SINGAPORE IMAGING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 234 SINGAPORE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 235 SINGAPORE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 236 SINGAPORE IONIZING BREAST IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 237 SINGAPORE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 238 SINGAPORE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 239 SINGAPORE NON-IONIZING IMAGING TECHNOLOGIES IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 240 SINGAPORE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 241 SINGAPORE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 242 SINGAPORE BIOPSY IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 243 SINGAPORE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 244 SINGAPORE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 245 SINGAPORE GENOMIC TEST IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 246 SINGAPORE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
TABLE 247 SINGAPORE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 ASP
TABLE 248 SINGAPORE MOLECULAR TESTING IN BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (UNITS)
TABLE 249 SINGAPORE BREAST CANCER DIAGNOSTICS MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 250 SINGAPORE BREAST CANCER DIAGNOSTICS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 251 SINGAPORE BREAST CANCER DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 252 REST OF ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET, BY TEST TYPE, 2021-2030 (USD MILLION)
Liste des figures
FIGURE 1 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: ASIA PACIFIC VS REGIONAL MARKET ANALYSIS
FIGURE 5 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 GROWING AWARENESS OF BREAST CANCER AND INCREASING HEALTHCARE EXPENDITURE IS EXPECTED TO DRIVE THE GROWTH OF THE ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET FROM 2023 TO 2030
FIGURE 12 THE IMAGING SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC BREAST CANCER DIAGNOSTIC MARKET IN 2023 & 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET
FIGURE 14 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2022
FIGURE 15 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, 2023-2030 (USD MILLION)
FIGURE 16 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, CAGR (2023-2030)
FIGURE 17 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TEST TYPE, LIFELINE CURVE
FIGURE 18 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, 2022
FIGURE 19 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, 2023-2030 (USD MILLION)
FIGURE 20 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, CAGR (2023-2030)
FIGURE 21 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY TYPE, LIFELINE CURVE
FIGURE 22 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY END USER, 2022
FIGURE 23 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY END USER, 2023-2030 (USD MILLION)
FIGURE 24 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY END USER, CAGR (2023-2030)
FIGURE 25 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY END USER, LIFELINE CURVE
FIGURE 26 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2022
FIGURE 27 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 28 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 29 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET : BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 30 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET: SNAPSHOT (2022)
FIGURE 31 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022)
FIGURE 32 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 33 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 34 ASIA-PACIFIC BREAST CANCER DIAGNOSTICS MARKET: TEST TYPE (2023-2030)
FIGURE 35 ASIA PACIFIC BREAST CANCER DIAGNOSTICS MARKET: COMPANY SHARE 2022 (%)

Méthodologie de recherche
La collecte de données et l'analyse de l'année de base sont effectuées à l'aide de modules de collecte de données avec des échantillons de grande taille. L'étape consiste à obtenir des informations sur le marché ou des données connexes via diverses sources et stratégies. Elle comprend l'examen et la planification à l'avance de toutes les données acquises dans le passé. Elle englobe également l'examen des incohérences d'informations observées dans différentes sources d'informations. Les données de marché sont analysées et estimées à l'aide de modèles statistiques et cohérents de marché. De plus, l'analyse des parts de marché et l'analyse des tendances clés sont les principaux facteurs de succès du rapport de marché. Pour en savoir plus, veuillez demander un appel d'analyste ou déposer votre demande.
La méthodologie de recherche clé utilisée par l'équipe de recherche DBMR est la triangulation des données qui implique l'exploration de données, l'analyse de l'impact des variables de données sur le marché et la validation primaire (expert du secteur). Les modèles de données incluent la grille de positionnement des fournisseurs, l'analyse de la chronologie du marché, l'aperçu et le guide du marché, la grille de positionnement des entreprises, l'analyse des brevets, l'analyse des prix, l'analyse des parts de marché des entreprises, les normes de mesure, l'analyse globale par rapport à l'analyse régionale et des parts des fournisseurs. Pour en savoir plus sur la méthodologie de recherche, envoyez une demande pour parler à nos experts du secteur.
Personnalisation disponible
Data Bridge Market Research est un leader de la recherche formative avancée. Nous sommes fiers de fournir à nos clients existants et nouveaux des données et des analyses qui correspondent à leurs objectifs. Le rapport peut être personnalisé pour inclure une analyse des tendances des prix des marques cibles, une compréhension du marché pour d'autres pays (demandez la liste des pays), des données sur les résultats des essais cliniques, une revue de la littérature, une analyse du marché des produits remis à neuf et de la base de produits. L'analyse du marché des concurrents cibles peut être analysée à partir d'une analyse basée sur la technologie jusqu'à des stratégies de portefeuille de marché. Nous pouvons ajouter autant de concurrents que vous le souhaitez, dans le format et le style de données que vous recherchez. Notre équipe d'analystes peut également vous fournir des données sous forme de fichiers Excel bruts, de tableaux croisés dynamiques (Fact book) ou peut vous aider à créer des présentations à partir des ensembles de données disponibles dans le rapport.