Le marché américain des médicaments dermatologiques topiques se concentre sur le traitement des affections cutanées grâce à des crèmes, des gels et des patchs. Ces produits ciblent des affections telles que l'eczéma, le psoriasis , l'acné et les mycoses . Les médicaments topiques s'appliquent directement sur la peau, contribuant ainsi à la guérison et à la prévention des affections cutanées. Ils comprennent les antifongiques, les corticostéroïdes, les anesthésiques locaux, les antiviraux, les antibiotiques , les antiacnéiques, les antidémangeaisons et divers agents topiques. Ces médicaments sont largement utilisés pour les soins de la peau et jouent un rôle essentiel dans la santé dermatologique, offrant des solutions ciblées à divers problèmes cutanés.
Accéder au rapport complet sur https://www.databridgemarketresearch.com/reports/us-topical-derma-drugs-market
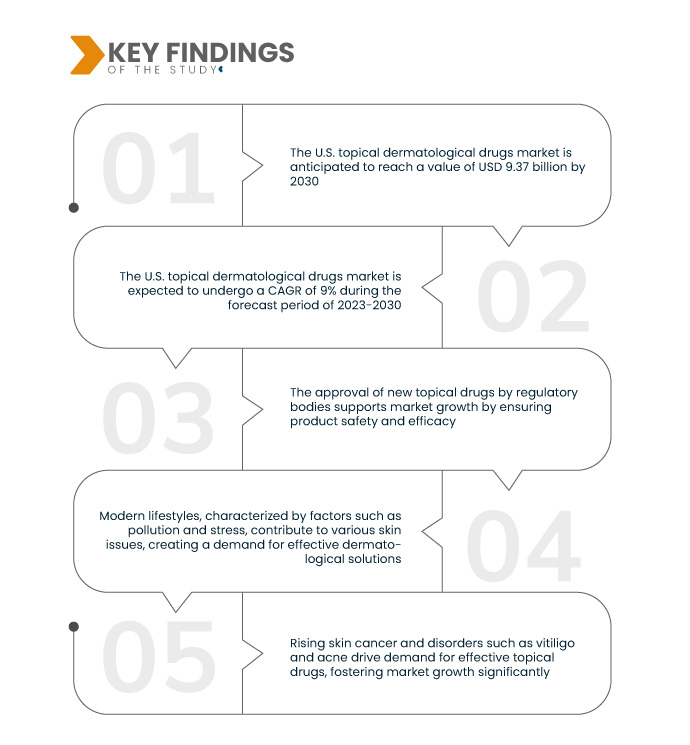
Data Bridge Market Research analyse que la valeur du marché américain des médicaments dermatologiques topiques était de 4,7 milliards USD en 2022 et devrait atteindre 9,37 milliards USD d'ici 2030, à un TCAC de 9 % de 2023 à 2030. Les produits dermatologiques en vente libre accessibles permettent de prendre soin de soi, favorisant la croissance du marché, en particulier pour traiter les affections cutanées légères et courantes sans avoir besoin d'une ordonnance, améliorant ainsi la commodité et le choix du consommateur.
Principales conclusions de l'étude
L’augmentation de la population âgée devrait stimuler le taux de croissance du marché
La population âgée, en constante augmentation, est plus vulnérable aux affections cutanées en raison du déclin fonctionnel lié à l'âge. Des problèmes tels qu'une sensibilité accrue aux UV, une sensibilité accrue aux infections, un retard de cicatrisation et une diminution de la graisse sous-cutanée accentuent la demande de traitements dermatologiques. La prévalence des affections cutanées augmente avec l'âge, soulignant le rôle crucial des interventions dermatologiques pour répondre aux besoins et aux défis spécifiques liés au vieillissement et favoriser la santé cutanée des personnes âgées.
Portée du rapport et segmentation du marché
Rapport métrique
|
Détails
|
Période de prévision
|
2023 à 2030
|
Année de base
|
2022
|
Années historiques
|
2021 (personnalisable de 2015 à 2020)
|
Unités quantitatives
|
Chiffre d'affaires en milliards USD, volumes en unités, prix en USD
|
Segments couverts
|
Gravité (légère à modérée, modérée à sévère), classe de médicaments (antifongiques, corticostéroïdes, anesthésiques locaux, antiviraux, antibiotiques, agents anti-acné, agents anti-démangeaisons, agents topiques divers, autres), formulation (patchs, gel, pommade, crème, mousses, autres), site (surface de la peau, épiderme et derme), application (acné, boutons de fièvre, urticaire, kératose actinique, anthrax, rosacée, eczéma, psoriasis, lupus, dermatite de contact, vitiligo, verrues, teigne, impétigo et autres), type de population (enfants, adultes), mode d'achat (sur ordonnance, en vente libre (OTC)), utilisateur final (hôpitaux, cliniques spécialisées, instituts universitaires et de recherche et autres), canal de distribution (pharmacie de détail et parapharmacie, pharmacie d'hôpital, pharmacie en ligne), Autres)
|
Acteurs du marché couverts
|
Sanofi (France), Novartis AG (Suisse), F. Hoffmann-La Roche Ltd (Suisse), Pfizer Inc. (États-Unis), Sanofi (France), Amgen Inc. (États-Unis), Lilly (États-Unis), AstraZeneca (Royaume-Uni), AbbVie Inc. (États-Unis), Johnson & Johnson Private Limited (États-Unis), LEO Pharma A/S (Danemark), Merck & Co., Inc. (États-Unis), Bausch Health Companies Inc. (Canada) et Bristol-Myers Squibb Company (États-Unis), Takeda Pharmaceutical Company Limited (Japon)
|
Points de données couverts dans le rapport
|
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research incluent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
|
Analyse des segments :
Le marché américain des médicaments topiques pour la peau est segmenté en fonction de la gravité, de la classe de médicament, de la formulation, du site, de l'application, du type de population, du mode d'achat, de l'utilisateur final et du canal de distribution.
- Sur la base de la gravité, le marché américain des médicaments topiques pour la peau est segmenté en léger à modéré et modéré à sévère.
- Sur la base de la classe de médicaments, le marché américain des médicaments topiques pour la peau est segmenté en antifongiques, corticostéroïdes, anesthésiques locaux, antiviraux, antibiotiques, agents anti-acné, agents anti-démangeaisons, divers agents topiques et autres.
- Sur la base de la formulation, le marché américain des médicaments topiques pour la peau est segmenté en patchs, gels, pommades, crèmes, mousses et autres.
- Sur la base du site, le marché américain des médicaments topiques pour la peau est segmenté en surface de la peau, épiderme et derme
- Sur la base de l'application, le marché américain des médicaments topiques pour la peau est segmenté en acné, boutons de fièvre, urticaire, kératose actinique, anthrax, rosacée, eczéma, psoriasis, lupus, dermatite de contact, vitiligo, verrues, teigne, impétigo et autres.
- Sur la base du type de population, le marché américain des médicaments topiques pour la peau est segmenté en enfants et adultes.
- Sur la base du mode d'achat, le marché américain des médicaments topiques pour la peau est segmenté en médicaments sur ordonnance et en vente libre (OTC).
- Sur la base de l'utilisateur final, le marché américain des médicaments topiques pour la peau est segmenté en hôpitaux, cliniques spécialisées, instituts universitaires et de recherche et autres.
- Sur la base du canal de distribution, le marché américain des médicaments topiques pour la peau est segmenté en pharmacies de détail et parapharmacies, pharmacies hospitalières, pharmacies en ligne et autres.
Acteurs majeurs
Data Bridge Market Research reconnaît les entreprises suivantes comme les principaux acteurs du marché américain des médicaments dermatologiques topiques : Sanofi (France), Novartis AG (Suisse), F. Hoffmann-La Roche Ltd (Suisse), Pfizer Inc. (États-Unis), Sanofi (France), Amgen Inc. (États-Unis), Lilly (États-Unis), AstraZeneca (Royaume-Uni), AbbVie Inc. (États-Unis), Johnson & Johnson Private Limited (États-Unis).
Évolution du marché
- En septembre 2022, la FDA américaine a approuvé Sotyktu (deucravacitinib), un médicament oral destiné aux adultes atteints de psoriasis en plaques modéré à sévère et bénéficiant d'un traitement systémique ou d'une photothérapie. Cette approbation réglementaire reconnaît Sotyktu comme une option thérapeutique viable, marquant une avancée significative dans la prise en charge des besoins thérapeutiques des personnes atteintes de cette forme spécifique de psoriasis.
- En juillet 2022, la FDA a approuvé la crème de roflumilast (ZORYVE) à 0,3 %, un inhibiteur topique de la phosphodiestérase-4 (PDE4). Cette approbation marque une innovation en dermatologie : la crème de roflumilast est désignée comme traitement ciblé du psoriasis en plaques chez les patients âgés de 12 ans et plus, offrant ainsi une nouvelle option thérapeutique pour la prise en charge de cette affection cutanée.
- En janvier 2022, AbbVie a reçu l'approbation de la FDA pour Rinvoq, élargissant ainsi son utilisation au traitement de la dermatite atopique réfractaire, modérée à sévère, chez les personnes de 12 ans et plus. Cette autorisation s'adresse aux patients n'ayant pas répondu ou ne tolérant pas les traitements systémiques oraux ou injectables antérieurs. L'approbation de Rinvoq offre une alternative intéressante pour la prise en charge des cas difficiles de dermatite atopique dans cette tranche d'âge spécifique, contribuant ainsi à l'amélioration des options thérapeutiques dans le paysage pharmaceutique dermatologique.
- En février 2020, Ortho Dermatologics a lancé dermatology.com, son premier service de médicaments non remboursés et son réseau de télémédecine. Cette plateforme innovante permet aux patients de consulter des professionnels de santé et d'acquérir des produits dermatologiques de marque en ligne. Cette initiative vise à renforcer la présence de l'entreprise sur le marché, en facilitant à la fois un accès élargi aux services médicaux et la vente de produits dermatologiques, améliorant ainsi le portefeuille de produits et la portée commerciale d'Ortho Dermatologics.
Pour plus d'informations sur le rapport sur le marché américain des médicaments dermatologiques topiques, cliquez ici : https://www.databridgemarketresearch.com/reports/us-topical-derma-drugs-market