L'incidence croissante des accidents vasculaires cérébraux (AVC) est un facteur déterminant du marché mondial des AVC, influençant à la fois la demande de traitement et les infrastructures de santé. L'AVC, principale cause d'invalidité et de décès dans le monde, est de plus en plus fréquent en raison de divers facteurs de risque, notamment le vieillissement de la population, la sédentarité, l'hypertension artérielle, le diabète, le tabagisme et une mauvaise alimentation. Avec l'allongement de l'espérance de vie et le vieillissement de la population, la prévalence des pathologies contribuant aux AVC, telles que l'hypertension et la fibrillation auriculaire, a également augmenté, ce qui entraîne une augmentation du nombre de personnes victimes d'AVC nécessitant une prise en charge médicale immédiate et une rééducation à long terme.
Alors que le fardeau mondial des accidents vasculaires cérébraux (AVC) continue de s'alourdir, les gouvernements, les organismes de santé et les laboratoires pharmaceutiques se concentrent sur l'amélioration de la prévention, du diagnostic précoce et du traitement des AVC. Cette priorité a permis d'innover dans les traitements et d'élargir le marché des produits et services de santé liés à l'AVC. De plus, les campagnes de sensibilisation du public et les initiatives de santé contribuent également à la détection et au traitement précoces, permettant ainsi à davantage de patients de survivre à un AVC et d'améliorer leur qualité de vie. Globalement, l'incidence croissante des AVC est un moteur essentiel de la croissance du marché, créant une demande pour des traitements, des technologies et des solutions de réadaptation innovants.
Accéder au rapport complet sur https://www.databridgemarketresearch.com/reports/global-stroke-market
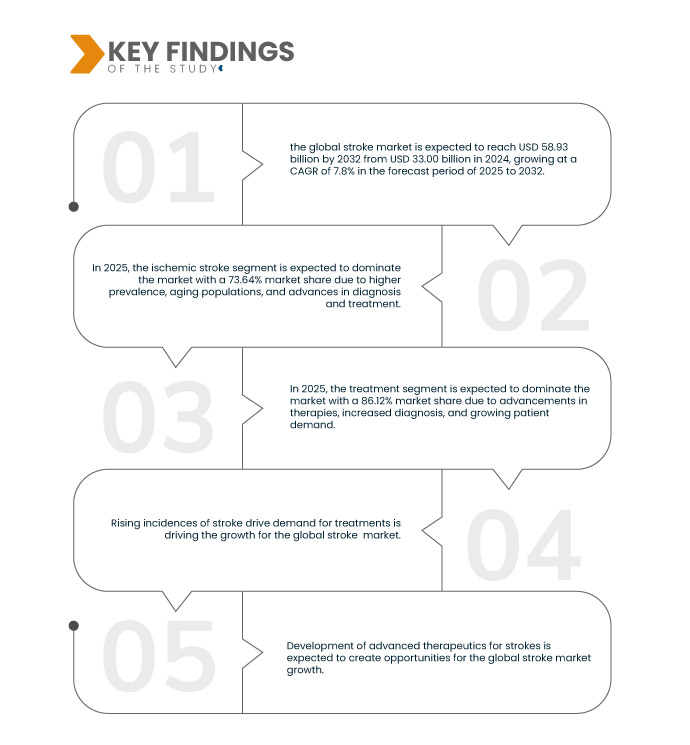
Data Bridge Market Research analyse que le marché mondial des accidents vasculaires cérébraux devrait atteindre 58,93 milliards USD d'ici 2032, contre 33,00 milliards USD en 2024, avec un TCAC de 7,8 % au cours de la période de prévision de 2025 à 2032.
Principales conclusions de l'étude
Augmentation du nombre de patients souffrant d'hypertension et de maladies coronariennes
L'hypertension artérielle, communément appelée hypertension artérielle, est une affection caractérisée par une force accrue du sang contre les parois des artères. Elle se définit généralement par une pression artérielle de 130/80 mm Hg ou plus et peut être qualifiée d'essentielle (primaire) ou secondaire, selon sa cause sous-jacente. Une hypertension prolongée peut entraîner divers problèmes de santé graves, dont l'un des plus importants est la maladie coronarienne. Cette maladie résulte de l'accumulation progressive de dépôts graisseux (athérosclérose) dans les artères coronaires, qui alimentent le muscle cardiaque en oxygène et en nutriments. Lorsque ces artères se rétrécissent ou se bouchent, le flux sanguin vers le cœur est réduit, ce qui entraîne des douleurs thoraciques (angine de poitrine) et, dans les cas graves, des crises cardiaques.
L'AVC est étroitement lié à la prévalence croissante de l'hypertension et des maladies coronariennes (MC), en raison de facteurs de risque communs et de mécanismes physiologiques sous-jacents. L'hypertension artérielle (HTA) augmente considérablement le risque d'AVC en endommageant les vaisseaux sanguins et en augmentant le risque de caillots sanguins. Une HT prolongée peut entraîner l'athérosclérose, c'est-à-dire l'accumulation de dépôts graisseux dans les artères, y compris celles qui irriguent le cerveau. Cela peut entraîner un rétrécissement des vaisseaux sanguins et une diminution du flux sanguin vers le cerveau, augmentant ainsi le risque d'AVC ischémique (causé par des obstructions) et hémorragique (causé par des saignements). L'HTA peut également endommager les vaisseaux sanguins cérébraux, les rendant plus sujets à la rupture ou à l'obstruction, augmentant ainsi le risque d'AVC.
Portée du rapport et segmentation du marché
Rapport métrique
|
Détails
|
Période de prévision
|
2025 à 2032
|
Année de base
|
2024
|
Année historique
|
2023 (Personnalisable 2013-2017)
|
Unités quantitatives
|
Chiffre d'affaires en milliards USD
|
Segments couverts
|
Type (accident vasculaire cérébral ischémique, accident ischémique transitoire (AIT) et accident vasculaire cérébral hémorragique ), diagnostic et traitement (traitement et diagnostic), sexe (femme et homme), utilisateur final (hôpitaux et cliniques, cliniques spécialisées, centres de chirurgie ambulatoire, soins à domicile, laboratoires et autres), canal de distribution (direct, vente au détail et en ligne)
|
Pays couverts
|
États-Unis, Canada, Mexique, Allemagne, France, Royaume-Uni, Italie, Espagne, Russie, Pays-Bas, Suisse, Turquie, Autriche, Irlande, Norvège, Pologne, reste de l'Europe, Japon, Chine, Inde, Corée du Sud, Australie, Singapour, Thaïlande, Malaisie, Indonésie, Vietnam, Philippines, Taïwan, reste de l'Asie-Pacifique, Brésil, Argentine, Chili, Pérou, reste de l'Amérique du Sud, Afrique du Sud, Arabie saoudite, Émirats arabes unis, Égypte, Koweït, Israël et reste du Moyen-Orient et de l'Afrique
|
Acteurs du marché couverts
|
Bristol-Myers Squibb Company (États-Unis), Boehringer Ingelheim International GmbH (Allemagne), F. Hoffmann-La Roche Ltd (Suisse), DAIICHI SANKYO COMPANY, LIMITED (Japon), Sanofi (France), Johnson & Johnson Services, Inc. (États-Unis), Bayer AG (Allemagne), Sandoz AG (Suisse), Pfizer Inc. (États-Unis), Medtronic (Irlande), Abbott (États-Unis), Viatris Inc. (États-Unis), AstraZeneca (Royaume-Uni), Penumbra, Inc. (États-Unis), GLENMARK PHARMACEUTICALS LTD (Inde), Fresenius SE & Co. KGaA (Allemagne), Teva Pharmaceuticals USA, Inc. (Israël), Lupin (Inde) et Amneal Pharmaceuticals LLC (États-Unis), entre autres
|
Points de données couverts dans le rapport
|
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research incluent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
|
Analyse des segments
Le marché mondial des accidents vasculaires cérébraux est segmenté en cinq segments notables en fonction du type, du diagnostic et du traitement, du sexe, de l'utilisateur final et du canal de distribution.
- Sur la base du type, le marché mondial des accidents vasculaires cérébraux est segmenté en accidents vasculaires cérébraux ischémiques, accidents vasculaires cérébraux hémorragiques et accidents ischémiques transitoires (AIT).
En 2025, le segment des accidents vasculaires cérébraux ischémiques devrait dominer le marché mondial des accidents vasculaires cérébraux
En 2025, le segment des accidents vasculaires cérébraux ischémiques devrait dominer le marché avec une part de marché de 73,64 % en raison d’une prévalence plus élevée, du vieillissement de la population et des progrès en matière de diagnostic et de traitement.
- Sur la base du diagnostic et du traitement, le marché est segmenté en traitement et diagnostic
En 2025, le segment du traitement devrait dominer le marché mondial des accidents vasculaires cérébraux
En 2025, le segment du traitement devrait dominer le marché avec une part de marché de 86,12 % en raison des progrès thérapeutiques, de l'augmentation des diagnostics et de la demande croissante des patients.
- Le marché mondial de l'AVC est segmenté en fonction du sexe, en fonction des sexes. En 2025, le segment féminin devrait dominer le marché avec une part de marché de 56,84 %.
- En fonction de l'utilisateur final, le marché mondial de l'AVC est segmenté en hôpitaux et cliniques, cliniques spécialisées, centres de chirurgie ambulatoire, soins à domicile et laboratoires, entre autres. En 2025, le segment des hôpitaux et cliniques devrait dominer le marché avec une part de marché de 51,57 %.
- En fonction des canaux de distribution, le marché mondial de l'AVC est segmenté en vente directe, vente au détail et en ligne. En 2025, le segment direct devrait dominer le marché avec une part de marché de 79,35 %.
Acteurs majeurs
Data Bridge Market Research analyse Bristol-Myers Squibb Company (États-Unis), Boehringer Ingelheim International GmbH (Allemagne), F. Hoffmann-La Roche Ltd (Suisse), DAIICHI SANKYO COMPANY, LIMITED (Japon) et Sanofi (France) comme les principaux acteurs du marché.
Évolution du marché
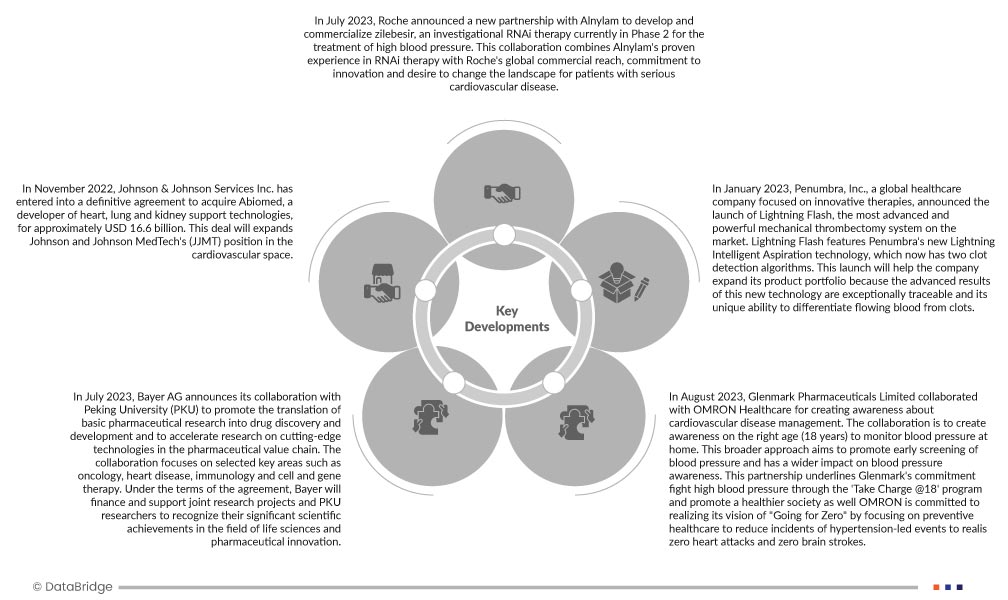
- En juillet 2023, Roche a annoncé un nouveau partenariat avec Alnylam pour le développement et la commercialisation du zilebesir, un traitement expérimental par ARN interférent actuellement en phase II pour le traitement de l'hypertension artérielle. Cette collaboration allie l'expérience éprouvée d'Alnylam en matière de traitement par ARN interférent à la portée commerciale mondiale de Roche, à son engagement en faveur de l'innovation et à sa volonté de transformer le quotidien des patients atteints de maladies cardiovasculaires graves.
- En septembre 2020, Daiichi Sankyo Company Limited a annoncé avoir déposé une demande complémentaire au Japon pour l'extension de l'autorisation de mise sur le marché de l'anticoagulant edoxaban (benzoate d'edoxaban hydraté) chez les patients âgés présentant une régurgitation non valvulaire et des saignements sévères. Risque. Cette demande s'appuie sur les résultats d'un essai clinique japonais de phase III (essai ELDERCARE-AF) mené auprès de 984 patients atteints de fibrillation atriale non valvulaire, âgés d'au moins 80 ans, présentant un risque hémorragique élevé et ne répondant pas aux autres traitements anticoagulants disponibles. Daiichi Sankyo prévoit de contribuer au traitement des patients âgés atteints de fibrillation atriale non valvulaire en proposant une nouvelle option thérapeutique.
- En mai 2021, Abbott a annoncé l'obtention du marquage CE pour son système d'implantation valvulaire aortique transcathéter (TAVI) de dernière génération, Navitor. Ce dispositif mini-invasif est désormais accessible aux personnes atteintes de sténose aortique sévère en Europe et présentant un risque chirurgical élevé ou extrême. Avec la valve Navitor, l'entreprise fait progresser les thérapies TAVI (également appelées TAVR ou remplacement valvulaire aortique transcathéter) grâce à des innovations, notamment une conception unique empêchant les fuites sanguines autour de la valve. Cette homologation renforce le portefeuille de solutions mini-invasives de l'entreprise pour le cœur structurel en offrant de nouvelles options et améliorations pour traiter une maladie cardiaque potentiellement mortelle.
- En avril 2021, Abbott a annoncé l'obtention du marquage CE pour son système de réparation de la valve tricuspide par cathéter TriClip de nouvelle génération, le premier dispositif mini-invasif de réparation de la valve tricuspide disponible en Europe pour le traitement de l'insuffisance tricuspide (IT). Ce traitement par clip, baptisé TriClip G4, est une solution non chirurgicale de réparation de la valve cardiaque, spécialement conçue pour le traitement de l'IT, ou fuite de la valve tricuspide, qui permet aux médecins d'adapter la réparation à l'anatomie spécifique de chaque patient. Cette innovation améliore les performances de l'entreprise et la capacité des cardiologues à réparer la valve tricuspide de manière sûre et efficace.
- En juillet 2023, Bayer AG annonce sa collaboration avec l'Université de Pékin (PKU) afin de promouvoir la traduction de la recherche pharmaceutique fondamentale en découverte et développement de médicaments et d'accélérer la recherche sur les technologies de pointe dans la chaîne de valeur pharmaceutique. Cette collaboration se concentre sur des domaines clés tels que l'oncologie, les maladies cardiaques, l'immunologie et la thérapie cellulaire et génique. Aux termes de cet accord, Bayer financera et soutiendra des projets de recherche conjoints et des chercheurs de la PKU afin de récompenser leurs avancées scientifiques majeures dans le domaine des sciences de la vie et de l'innovation pharmaceutique. Cet accord repose sur un partenariat académique stratégique entre Bayer et la PKU, qui a créé un centre de recherche commun à l'Université de Pékin afin de promouvoir la traduction du développement et de la recherche pharmaceutique en médicaments pour les patients.
Analyse régionale
Géographiquement, les pays couverts dans le rapport sur le marché mondial des accidents vasculaires cérébraux sont les États-Unis, le Canada, le Mexique, l'Allemagne, la France, le Royaume-Uni, l'Italie, l'Espagne, la Russie, les Pays-Bas, la Suisse, la Turquie, l'Autriche, l'Irlande, la Norvège, la Pologne, le reste de l'Europe, le Japon, la Chine, l'Inde, la Corée du Sud, l'Australie, Singapour, la Thaïlande, la Malaisie, l'Indonésie, le Vietnam, les Philippines, Taïwan, le reste de l'Asie-Pacifique, le Brésil, l'Argentine, le Chili, le Pérou, le reste de l'Amérique du Sud, l'Afrique du Sud, l'Arabie saoudite, les Émirats arabes unis, l'Égypte, le Koweït, Israël et le reste du Moyen-Orient et de l'Afrique.
Selon l'analyse de Data Bridge Market Research :
L'Amérique du Nord devrait dominer le marché mondial des accidents vasculaires cérébraux
L’Amérique du Nord domine le marché mondial des accidents vasculaires cérébraux (AVC), grâce à des systèmes de santé avancés, une prévalence élevée des AVC et une adoption significative des traitements.
L'Asie-Pacifique est la région qui connaît la croissance la plus rapide sur le marché mondial des accidents vasculaires cérébraux
L’Asie-Pacifique est la région qui connaît la croissance la plus rapide sur le marché mondial des accidents vasculaires cérébraux, en raison du vieillissement de la population, de l’augmentation des facteurs de risque et de l’amélioration des infrastructures de santé.
Pour plus d'informations sur le marché mondial des accidents vasculaires cérébraux, cliquez ici : https://www.databridgemarketresearch.com/reports/global-stroke-market