La prévalence croissante du cancer stimule considérablement le marché mondial des conjugués anticorps-médicament (CAM), augmentant la demande de traitements ciblés et efficaces. Les CAM permettent un ciblage précis des cellules cancéreuses tout en réduisant les dommages aux tissus sains grâce à l'association d'anticorps monoclonaux et de puissants médicaments cytotoxiques. Face à la hausse des taux de cancer, les entreprises pharmaceutiques investissent massivement dans la technologie des CAM afin de répondre au besoin de traitements plus personnalisés, notamment pour les cancers difficiles. Cette croissance de la population de patients atteints de cancer accélère les essais cliniques, les autorisations réglementaires et les lancements commerciaux des CAM, faisant progresser le marché des CAM. La prévalence croissante du cancer stimule considérablement le marché mondial des CAM en augmentant la demande de thérapies ciblées et efficaces. Les CAM, grâce à leur capacité à délivrer des traitements puissants directement aux cellules cancéreuses tout en épargnant les tissus sains, sont devenus une solution prometteuse. Ce fardeau croissant du cancer stimule la recherche, le développement et les investissements dans la technologie des CAM, favorisant ainsi de nouvelles avancées et l'expansion du marché.
Accéder au rapport complet sur https://www.databridgemarketresearch.com/reports/global-antibody-drug-conjugates-market
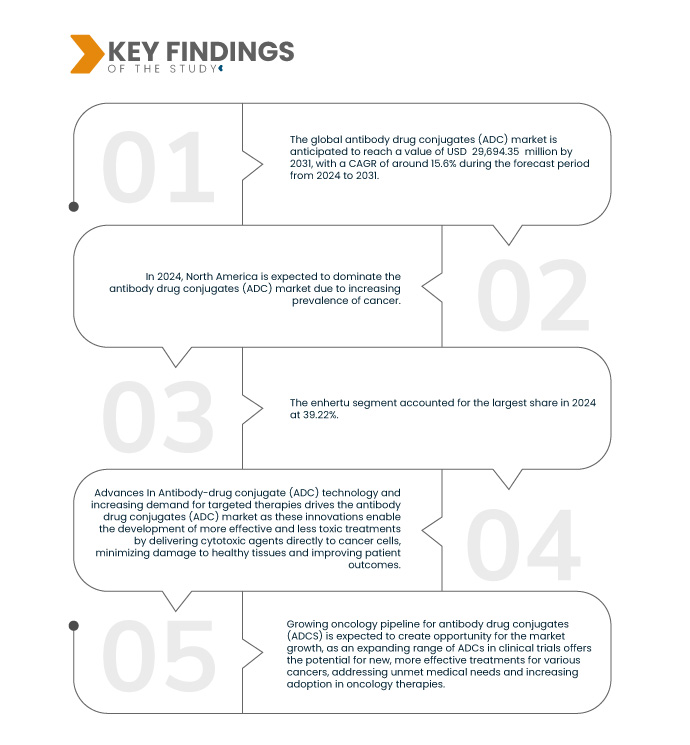
Data Bridge Market Research analyse que la taille du marché mondial des conjugués anticorps-médicaments (ADC) était évaluée à 9,33 milliards USD en 2023 et devrait atteindre 29,69 milliards USD d'ici 2031, avec un TCAC de 15,6 % au cours de la période de prévision de 2024 à 2031.
Principales conclusions de l'étude
Progrès dans la technologie des conjugués anticorps-médicament (ADC)
Les progrès de la technologie des conjugués anticorps-médicament (CAM) stimulent la croissance du marché mondial des CAM en améliorant l'efficacité et la sécurité des traitements contre le cancer. Parmi les innovations clés, on compte des liants améliorés pour une administration stable et précise des médicaments, ciblant les cellules cancéreuses tout en épargnant les tissus sains. Les CAM de nouvelle génération, dotés de charges utiles optimisées et d'une sélection des patients basée sur des biomarqueurs, garantissent que les traitements atteignent les personnes les plus avantagées, minimisant ainsi les effets secondaires. De plus, l'amélioration des procédés de fabrication réduit les coûts de production, améliore l'accès et encourage les investissements dans le développement des CAM, contribuant ainsi à la solidité du portefeuille de produits et à l'intérêt croissant du marché.
Des innovations telles que des systèmes de liaison améliorés et des charges utiles médicamenteuses efficaces améliorent la précision et la sécurité des traitements contre le cancer. Les stratégies guidées par les biomarqueurs optimisent le ciblage des patients pour une efficacité optimale. L'amélioration des techniques de fabrication a rendu les ADC plus accessibles et plus rentables, attirant ainsi les investissements et élargissant les options thérapeutiques. Globalement, ces avancées renforcent l'efficacité des ADC et stimulent la croissance du marché et l'intérêt de l'industrie pharmaceutique.
Portée du rapport et segmentation du marché
Rapport métrique
|
Détails
|
Période de prévision
|
2024 à 2031
|
Année de base
|
2023
|
Année historique
|
2022 (Personnalisable 2016-2021)
|
Unités quantitatives
|
Chiffre d'affaires en milliards USD
|
Segments couverts
|
Par produit (Enhertu, Kadcyla, Trodelvy, Polivy, Adcetris, Padcev, Besponsa, Elahere, Zylonta, Mylotarg, Tivdak et autres), composant antigène (récepteur HER2, Trop-2, CD79B, CD30, nectine 4, CD22, CD19, CD33, facteurs tissulaires et autres), composant anticorps (ADC de troisième génération, ADC de deuxième génération, ADC de quatrième génération et ADC de première génération), composant de liaison (lieurs clivables et non clivables), composants de charges utiles cytotoxiques ou d'ogives (agents endommageant l'ADN et agents perturbateurs des microtubules), technologie de liaison (lieurs peptidiques, liants thioéther, liants hydrazone et liants disulfure), technologie de conjugaison (conjugaison spécifique au site et conjugaison chimique), indication (cancer du sein, Cancer du sang (leucémie, lymphome), cancer du poumon, cancer gynécologique , cancer gastro-intestinal, cancer génito-urinaire et autres), utilisateur final (hôpitaux, centres spécialisés, cliniques, centres ambulatoires, soins à domicile et autres), canal de distribution (appels d'offres directs, ventes au détail et autres)
|
Pays couverts
|
États-Unis, Canada, Mexique, Allemagne, Royaume-Uni, France, Italie, Espagne, Russie, Belgique, Pays-Bas, Suisse, Autriche, Irlande, Pologne, Norvège, Hongrie, Lituanie, reste de l'Europe, Japon, Chine, Inde, Australie, Singapour, reste de l'Asie-Pacifique, Brésil, Argentine, reste de l'Amérique du Sud, Arabie saoudite, Émirats arabes unis, Israël, Afrique du Sud et reste du Moyen-Orient et de l'Afrique
|
Acteurs du marché couverts
|
DAIICHI SANKYO COMPANY, LIMITED (Japon), F. Hoffmann-La Roche Ltd (Suisse), Gilead Sciences, Inc. (États-Unis), Astellas Pharma Inc. (Japon), Takeda (Japon), Pfizer Inc. (États-Unis), Abbvie (États-Unis), ADC Therapeutics (Suisse), Amgen, Inc. (Californie), AstraZeneca (Angleterre), Bayer (Allemagne), Byondis (Pays-Bas), EISAI INC (Japon), GSK plc (Royaume-Uni), Johnson & Johnson Services, Inc. (États-Unis), Oxford BioTherapeutics (Angleterre), Remegen (Chine), Sanofi (France) et Sutra Biopharma, Inc. (États-Unis), entre autres
|
Points de données couverts dans le rapport
|
Outre les informations sur les scénarios de marché tels que la valeur marchande, le taux de croissance, la segmentation, la couverture géographique et les principaux acteurs, les rapports de marché organisés par Data Bridge Market Research incluent également une analyse approfondie des experts, une épidémiologie des patients, une analyse du pipeline, une analyse des prix et un cadre réglementaire.
|
Analyse des segments
Le marché mondial des conjugués anticorps-médicament (ADC) est segmenté en dix segments notables qui sont basés sur le produit, le composant antigène, le composant anticorps, le composant de liaison, les charges utiles cytotoxiques ou le composant d'ogives, la technologie de liaison, la technologie de conjugaison, l'indication, l'utilisateur final, le canal de distribution.
- Sur la base du produit, le marché est segmenté en enhertu, kadcyla, trodelvy, polivy, adcetris, padcev, besponsa, elahere, zylonta, mylotarg, tivdak et autres
En 2024, le segment enhertu devrait dominer le marché avec une part de marché de 39,22 %
En 2024, le segment enhertu devrait dominer le marché avec une part de marché de 39,22 % en raison de sa grande efficacité et de son profil de sécurité favorable dans le traitement des cancers HER2-positifs, ainsi que de son approbation élargie pour plusieurs types de cancer, ce qui augmente son adoption clinique et commerciale.
- Sur la base du composant antigénique, le marché est segmenté en récepteur HER2, Trop-2, CD79B CD30, nectine 4, CD22, CD19, CD33, facteurs tissulaires et autres
En 2024, le segment des récepteurs HER2 devrait dominer le marché avec une part de marché de 59,62 %
En 2024, le segment des récepteurs HER2 devrait dominer le marché avec une part de marché de 59,62 % en raison de la forte prévalence des cancers HER2-positifs, tels que les cancers du sein et de l'estomac, et de l'efficacité prouvée des ADC ciblant HER2 pour fournir des avantages cliniques significatifs pour ces affections.
- En fonction de la composition des anticorps, le marché est segmenté en ADC de troisième, deuxième, quatrième et première génération. En 2024, le segment des ADC de troisième génération devrait dominer le marché avec une part de marché de 56,17 %.
- En fonction de la composition des lieurs, le marché est segmenté en lieurs clivables et lieurs non clivables. En 2024, le segment des lieurs clivables devrait dominer le marché avec une part de marché de 79,31 %.
- En fonction des charges utiles cytotoxiques ou des composants des ogives, le marché est segmenté en agents endommageant l'ADN et agents perturbateurs des microtubules. En 2024, le segment des agents endommageant l'ADN devrait dominer le marché avec une part de marché de 62,67 %.
- Sur la base de la technologie des lieurs, le marché est segmenté en lieurs peptidiques, lieurs thioéther, lieurs hydrazone et lieurs disulfure. En 2024, le segment des lieurs peptidiques devrait dominer le marché avec une part de marché de 61,15 %
- En fonction de la technologie de conjugaison, le marché est segmenté en conjugaison spécifique de site et conjugaison chimique. En 2024, le segment de la conjugaison spécifique de site devrait dominer le marché avec une part de marché de 56,28 %.
- Selon les indications, le marché est segmenté en cancer du sein, cancers du sang (leucémie, lymphome), cancer du poumon, cancers gynécologiques, cancers gastro-intestinaux, cancers génito-urinaires, etc. En 2024, le segment du cancer du sein devrait dominer le marché avec une part de marché de 42,51 %.
- En fonction de l'utilisateur final, le marché est segmenté en hôpitaux, centres spécialisés, cliniques, centres ambulatoires, soins à domicile, etc. En 2024, le segment hospitalier devrait dominer le marché avec une part de marché de 59,81 %.
- En fonction du canal de distribution, le marché est segmenté en appels d'offres directs, vente au détail et autres. En 2024, le segment des appels d'offres directs devrait dominer le marché avec une part de marché de 66,23 %.
Acteurs majeurs
Data Bridge Market Research analyse DAIICHI SANKYO COMPANY, LIMITED (Japon), F. Hoffmann-La Roche Ltd (Suisse), Gilead Sciences, Inc. (États-Unis), Astellas Pharma Inc. (Japon) et Takeda (Japon) comme les principaux acteurs du marché.
Développement du marché
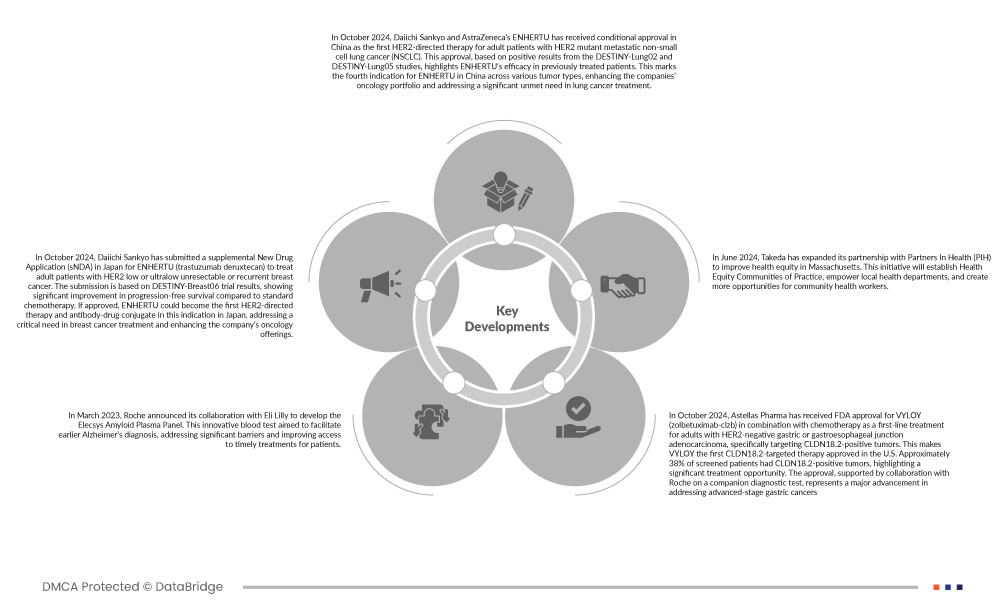
- En octobre 2024, ENHERTU, développé par Daiichi Sankyo et AstraZeneca, a reçu une autorisation de mise sur le marché conditionnelle en Chine. Il s'agit du premier traitement ciblant HER2 chez les patients adultes atteints d'un cancer du poumon non à petites cellules (CPNPC) métastatique muté HER2. Cette autorisation, fondée sur les résultats positifs des études DESTINY-Lung02 et DESTINY-Lung05, souligne l'efficacité d'ENHERTU chez les patients déjà traités. Il s'agit de la quatrième indication d'ENHERTU en Chine pour différents types de tumeurs, enrichissant ainsi le portefeuille oncologique des deux sociétés et répondant à un besoin important non satisfait dans le traitement du cancer du poumon.
- En octobre 2024, Daiichi Sankyo a déposé une demande d'autorisation de mise sur le marché (sNDA) au Japon pour ENHERTU (trastuzumab déruxtécan) pour le traitement des patients adultes atteints d'un cancer du sein non résécable ou récidivant à HER2 faible ou ultra-faible. Cette demande s'appuie sur les résultats de l'essai DESTINY-Breast06, qui montrent une amélioration significative de la survie sans progression par rapport à la chimiothérapie standard. S'il est approuvé, ENHERTU pourrait devenir le premier traitement et conjugué anticorps-médicament ciblant HER2 dans cette indication au Japon, répondant ainsi à un besoin crucial dans le traitement du cancer du sein et enrichissant l'offre oncologique de la société.
- En mars 2023, Roche a annoncé sa collaboration avec Eli Lilly pour le développement du panel de plasma amyloïde Elecsys. Ce test sanguin innovant vise à faciliter le diagnostic précoce de la maladie d'Alzheimer, à lever d'importants obstacles et à améliorer l'accès des patients à des traitements rapides.
- En octobre 2024, Astellas Pharma a reçu l'approbation de la FDA pour VYLOY (zolbetuximab-clzb) en association avec la chimiothérapie comme traitement de première intention chez les adultes atteints d'adénocarcinome gastrique ou de la jonction gastro-œsophagienne HER2 négatif, ciblant spécifiquement les tumeurs CLDN18.2 positives. VYLOY est ainsi le premier traitement ciblant CLDN18.2 approuvé aux États-Unis. Environ 38 % des patients dépistés présentaient des tumeurs CLDN18.2 positives, ce qui souligne une opportunité thérapeutique significative. Cette approbation, soutenue par une collaboration avec Roche sur un test de diagnostic compagnon, représente une avancée majeure dans la prise en charge des cancers gastriques à un stade avancé.
Analyse régionale
Géographiquement, les pays couverts par le rapport sur le marché mondial des conjugués anticorps-médicament (ADC) sont les États-Unis, le Canada, le Mexique, l'Allemagne, le Royaume-Uni, la France, l'Italie, l'Espagne, la Russie, la Belgique, les Pays-Bas, la Suisse, l'Autriche, l'Irlande, la Pologne, la Norvège, la Hongrie, la Lituanie, le reste de l'Europe, le Japon, la Chine, l'Inde, l'Australie, Singapour, le reste de l'Asie-Pacifique, le Brésil, l'Argentine, le reste de l'Amérique du Sud, l'Arabie saoudite, les Émirats arabes unis, Israël, l'Afrique du Sud et le reste du Moyen-Orient et de l'Afrique.
Selon l'analyse de Data Bridge Market Research :
L'Amérique du Nord est la région dominante du marché au cours de la période de prévision de 2024 à 2031
L'Amérique du Nord devrait dominer le marché en raison de l'infrastructure de soins de santé avancée de la région, de la forte prévalence du cancer, du solide soutien réglementaire et des investissements substantiels dans la recherche et le développement en oncologie, favorisant l'adoption de thérapies ADC innovantes.
L'Asie-Pacifique devrait être la région connaissant la croissance la plus rapide du marché au cours de la période de prévision de 2024 à 2031.
L'Asie-Pacifique devrait connaître une croissance au cours de la période de prévision en raison de l'augmentation des investissements dans les infrastructures de santé, de la prévalence croissante du cancer et de la demande croissante de thérapies ciblées dans les économies émergentes.
Pour plus d'informations sur le marché mondial des conjugués anticorps-médicaments (ADC), cliquez ici : https://www.databridgemarketresearch.com/reports/global-antibody-drug-conjugates-market