Mercado de diagnóstico de accidentes cerebrovasculares en América del Norte, por gravedad (moderada, grave, leve), tipo (tomografía computarizada (TC), angiografía por tomografía computarizada (ATC), imágenes por resonancia magnética (IRM), angiografía por resonancia magnética (ARM), ecografía Doppler transcraneal , prueba de impulso cefálico por video (VHIT), otros), aplicación (accidente cerebrovascular isquémico, accidente cerebrovascular hemorrágico, ataques isquémicos transitorios (TIAS)), usuario final (hospitales, clínicas, centros quirúrgicos ambulatorios, atención médica domiciliaria), canal de distribución (licitación directa, distribuidores externos, otros), etapa (preoperatoria, perioperatoria, posoperatoria), país (EE. UU., Canadá, México), tendencias de la industria y pronóstico hasta 2028
 Análisis y perspectivas del mercado: mercado de diagnóstico de accidentes cerebrovasculares en América del Norte
Análisis y perspectivas del mercado: mercado de diagnóstico de accidentes cerebrovasculares en América del Norte
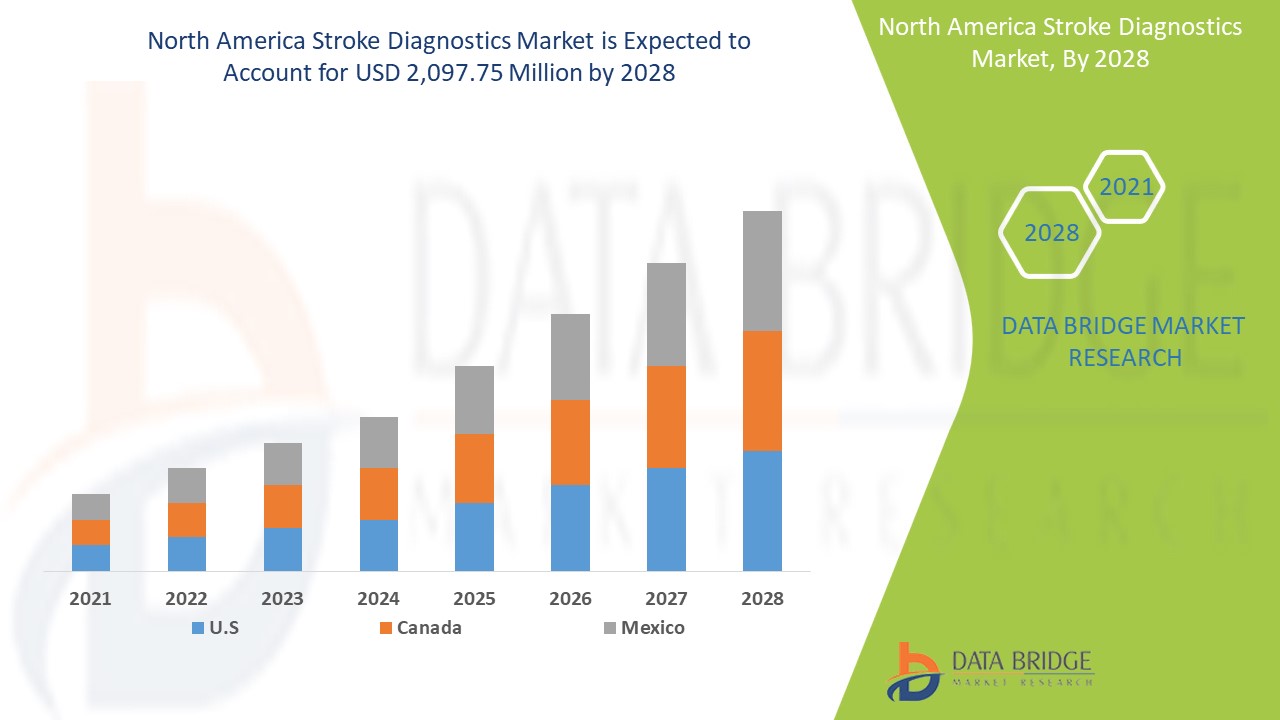
Se espera que el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte gane crecimiento de mercado en el período de pronóstico de 2021 a 2028. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 6,6% en el período de pronóstico de 2021 a 2028 y se espera que alcance los USD 2097,75 millones para 2028. El crecimiento de la I+D en la industria de la salud y un aumento en el gasto en atención médica son los principales impulsores que se prevé que impulsen la demanda del mercado en el período de pronóstico. Por el contrario, las retiradas de productos están restringiendo el mercado.
Un accidente cerebrovascular se produce cuando el suministro de sangre al cerebro disminuye o se bloquea por completo, lo que impide que el tejido cerebral reciba oxígeno y nutrientes. Existen varios tipos de dispositivos de diagnóstico que se utilizan para detectar un accidente cerebrovascular y sus síntomas iniciales, por ejemplo, la tomografía computarizada (TC), la angiografía por tomografía computarizada (ATC), la resonancia magnética (RM), la angiografía por resonancia magnética (ARM), la ecografía Doppler transcraneal y otros.
La creciente incidencia de accidentes cerebrovasculares y enfermedades cardiovasculares y neurológicas ha aumentado la demanda de diagnósticos de accidentes cerebrovasculares en los países en desarrollo. Además, el aumento del gasto en atención médica está impulsando el crecimiento del mercado. Por otro lado, el alto costo del diagnóstico puede obstaculizar el crecimiento del mercado. La presencia de actores del mercado y los lanzamientos de nuevos productos brindan una oportunidad. Sin embargo, la falta de profesionales capacitados puede suponer un desafío para el mercado.
El informe de mercado de diagnóstico de accidentes cerebrovasculares proporciona detalles sobre la participación de mercado, los nuevos desarrollos, el impacto de los actores del mercado nacional y localizado, análisis de oportunidades en términos de segmentos de ingresos emergentes, cambios en las regulaciones del mercado, aprobaciones de productos , decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado, contáctenos para obtener un resumen analítico. Nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado.
 Alcance y tamaño del mercado de diagnóstico de accidentes cerebrovasculares en América del Norte
Alcance y tamaño del mercado de diagnóstico de accidentes cerebrovasculares en América del Norte
El mercado de diagnóstico de accidentes cerebrovasculares de América del Norte está segmentado en función de la gravedad, el tipo, la aplicación, el usuario final, el canal de distribución y la etapa. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
- En función de la gravedad, el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte se segmenta en moderado, severo y leve. En 2021, se espera que el segmento moderado domine el mercado, con un aumento en las actividades de I+D.
- Según el tipo, el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte se segmenta en tomografía computarizada (TC), angiografía por tomografía computarizada (ATC), imágenes por resonancia magnética (IRM), angiografía por resonancia magnética (ARM), ecografía Doppler transcraneal, prueba de impulso cefálico por video (VHIT), otros. En 2021, se espera que el segmento de la tomografía computarizada (TC) domine el mercado con las crecientes iniciativas gubernamentales para proporcionar dispositivos más avanzados.
- En función de la aplicación, el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte se segmenta en accidentes cerebrovasculares isquémicos, accidentes cerebrovasculares hemorrágicos y ataques isquémicos transitorios (TIAS). En 2021, se espera que los accidentes cerebrovasculares isquémicos dominen el mercado con el creciente lanzamiento de productos por parte de los principales actores del mercado.
- En función de los usuarios finales, el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte está segmentado en hospitales, atención médica domiciliaria, centros quirúrgicos ambulatorios y clínicas. En 2021, se espera que el segmento hospitalario domine el mercado con la presencia de profesionales y personal capacitado.
- En función del canal de distribución, el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte se segmenta en licitación directa, distribuidor externo y otros. En 2021, se espera que la licitación directa domine el mercado debido al creciente número de pacientes con accidentes cerebrovasculares.
- En función de la etapa, el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte se segmenta en preoperatorio, perioperatorio y posoperatorio. En 2021, se espera que el preoperatorio domine el mercado debido al creciente avance en el desarrollo de productos.
Análisis a nivel de país del mercado de diagnóstico de accidentes cerebrovasculares
Se analiza el mercado de diagnóstico de accidentes cerebrovasculares y se proporciona información sobre el tamaño del mercado en función de la gravedad, el tipo, la aplicación, los usuarios finales, el canal de distribución y la etapa.
Los países cubiertos en el informe del mercado de diagnóstico de accidentes cerebrovasculares de América del Norte son EE. UU., Canadá y México.
Se espera que el segmento de diagnóstico de accidentes cerebrovasculares en la región de América del Norte crezca a la tasa de crecimiento más alta en el período de pronóstico de 2021 a 2028 debido al aumento del desarrollo de la investigación para mejorar la calidad del producto. Se espera que EE. UU. lidere el crecimiento del mercado de diagnóstico de accidentes cerebrovasculares en América del Norte, y se anticipa que el segmento de licitación directa domine debido a la creciente incidencia de la enfermedad en el país.
La sección de países del informe también proporciona factores de impacto individuales en el mercado y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de América del Norte y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
El aumento de los avances y la tecnología para el desarrollo de nuevos productos está impulsando el crecimiento del mercado de diagnóstico de accidentes cerebrovasculares
El mercado de diagnóstico de accidentes cerebrovasculares también le ofrece un análisis detallado del mercado para el crecimiento de cada país en la industria de diagnóstico de accidentes cerebrovasculares con las ventas de medicamentos para el diagnóstico de accidentes cerebrovasculares, el impacto del avance en la tecnología de diagnóstico de accidentes cerebrovasculares y los cambios en los escenarios regulatorios con su apoyo al mercado de diagnóstico de accidentes cerebrovasculares. Los datos están disponibles para el período histórico de 2011 a 2019.
Análisis del panorama competitivo y de la cuota de mercado de los diagnósticos de accidentes cerebrovasculares
El panorama competitivo del mercado de diagnóstico de accidentes cerebrovasculares proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y la extensión de los productos, el dominio de las aplicaciones y la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa relacionado con el mercado de diagnóstico de accidentes cerebrovasculares.
Las principales empresas que operan en el mercado de diagnóstico de accidentes cerebrovasculares de América del Norte son Siemens, Koninklijke Philips NV, Stryker, Hologic, Inc., ESAOTE SPA, Medtron AG, Shenzhen Anke High-Tech CO., Ltd, Aspect Imaging Ltd, Siemens, FONAR Corp, FUJIFILM Holdings Corporation, General Electric Company, ALPINION MEDICAL SYSTEMS Co., Ltd., Ltd, TERASON DIVISION TERATECH CORPORATION, Analogic Corporation, Carestream Health, IMRIS, Deerfield Imaging Inc., Canon Inc., SAMSUNG, SternMed GmbH, entre otras.
Numerosos lanzamientos de productos y acuerdos son iniciados por empresas de todo el mundo, lo que también acelera el mercado del diagnóstico de accidentes cerebrovasculares.
Por ejemplo,
- En febrero de 2021, Koninklijke Philips NV anunció que había completado con éxito la adquisición de BioTelemetry, Inc., un proveedor líder con sede en EE. UU. de diagnóstico y monitoreo cardíaco remoto. La adquisición ha agregado los nuevos productos de detección portátiles tecnológicos a su cartera de productos
- En septiembre de 2021, SAMSUNG anunció que había completado la adquisición de la empresa HARMAN. La adquisición ha aumentado la cartera de productos y el avance tecnológico de la empresa.
La colaboración, el lanzamiento de productos, la expansión comercial, los premios y reconocimientos, las empresas conjuntas y otras estrategias de los actores del mercado están mejorando el mercado de la empresa en el mercado de diagnóstico de accidentes cerebrovasculares, lo que también proporciona el beneficio para que la organización mejore su oferta de diagnóstico de accidentes cerebrovasculares.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA STROKE DIAGNOSTICS MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 NORTH AMERICA STROKE DIAGNOSTICS MARKET: REGULATIONS
6.1 REGULATION IN THE U.S.
6.2 REGULATION IN EUROPE
6.3 REGULATION IN CHINA
6.4 REGULATION IN JAPAN
6.5 REGULATION IN SOUTH AFRICA
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 TECHNOLOGICAL ADVANCEMENT
7.1.2 INCREASE IN THE INCIDENCE OF STROKE
7.1.3 INCREASE IN THE GERIATRIC POPULATION
7.1.4 INCREASE IN NUMBER OF PATIENTS WITH HYPERTENSION AND CORONARY HEART DISEASES
7.2 RESTRAINTS
7.2.1 HIGH COST OF DIAGNOSIS
7.2.2 INCREASE IN PRODUCT RECALL
7.3 OPPORTUNITIES
7.3.1 RISE IN HEALTHCARE SPENDING
7.3.2 INCREASE IN DIABETIC POPULATION AND OBESITY
7.3.3 INCREASE IN FDA APPROVAL AND PRODUCT LAUNCH
7.3.4 RISE IN AWARENESS REGARDING HEALTH AND STROKE
7.4 CHALLENGES
7.4.1 UNFAVORABLE REIMBURSEMENT SCENARIO
7.4.2 COMPLICATION RELATED TO DIAGNOSTIC DEVICES
8 IMPACT OF COVID-19 ON NORTH AMERICA STROKE DIAGNOSTICS MARKET
8.1 IMPACT ON PRICE
8.2 IMPACT ON DEMAND
8.3 IMPACT ON SUPPLY CHAIN
8.4 STRATEGIC INITIATIVES
8.5 CONCLUSION
9 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY SEVERITY
9.1 OVERVIEW
9.2 MODERATE
9.3 SEVERE
9.4 MILD
10 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY TYPE
10.1 OVERVIEW
10.2 COMPUTED TOMOGRAPHY (CT SCAN)
10.3 COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA)
10.4 MAGNETIC RESONANCE IMAGING (MRI)
10.5 MAGNETIC RESONANCE ANGIOGRAPHY (MRA)
10.6 TRANSCRANIAL DOPPLER ULTRASOUND
10.7 VIDEO HEAD IMPULSE TEST (VHIT)
10.8 OTHERS
10.8.1 CAROTID ULTRASOUND
10.8.2 CAROTID ANGIOGRAPHY
10.8.3 ELECTROCARDIOGRAPHY (EKG)
10.8.4 ECHOCARDIOGRAPHY
10.8.5 BLOOD TESTS
10.8.6 NUCLEAR NEUROIMAGING
10.8.7 OTHERS
11 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 ISCHEMIC STROKE
11.2.1 THROMBOTIC STROKES
11.2.2 EMBOLIC STROKES
11.3 HEMORRHAGIC STROKE
11.3.1 INTRACEREBRAL HEMORRHAGE
11.3.2 SUBARACHNOID HEMORRHAGE
11.4 TRANSIENT ISCHEMIC ATTACKS (TIAS)
12 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY END USER
12.1 OVERVIEW
12.2 HOSPITAL
12.3 CLINICS
12.4 AMBULATORY SURGICAL CENTERS
12.5 HOME HEALTHCARE
12.6 OTHERS
13 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 THIRD PARTY DISTRIBUTORS
13.4 OTHERS
14 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY STAGE
14.1 OVERVIEW
14.2 PRE OPERATIVE
14.3 PERI OPERATIVE
14.4 POST OPERATIVE
15 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY REGION
15.1 NORTH AMERICA
15.1.1 U.S.
15.1.2 CANADA
15.1.3 MEXICO
16 STROKE DIAGNOSTICS MARKET COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 SIEMENS
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 COMPANY SHARE ANALYSIS
18.1.4 PRODUCT PORTFOLIO
18.1.5 RECENT DEVELOPMENTS
18.2 KONINKLIJKE PHILIPS N.V.
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY SHARE ANALYSIS
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GENERAL ELECTRIC COMPANY
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 COMPANY SHARE ANALYSIS
18.3.4 PRODUCT PORTFOLIO
18.3.5 RECENT DEVELOPMENTS
18.4 CANON INC
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 COMPANY SHARE ANALYSIS
18.4.4 PRODUCT PORTFOLIO
18.4.5 RECENT DEVELOPMENTS
18.5 SHENZHEN MINDRAY BIO-MEDICAL ELECTRONICS CO., LTD
18.5.1 COMPANY SNAPSHOT
18.5.2 REVENUE ANALYSIS
18.5.3 COMPANY SHARE ANALYSIS
18.5.4 PRODUCT PORTFOLIO
18.5.5 RECENT DEVELOPMENTS
18.6 ALPINION MEDICAL SYSTEMS CO., LTD
18.6.1 COMPANY SNAPSHOT
18.6.2 PRODUCT PORTFOLIO
18.6.3 RECENT DEVELOPMENTS
18.7 ANALOGIC CORPORATION
18.7.1 COMPANY SNAPSHOT
18.7.2 PRODUCT PORTFOLIO
18.7.3 RECENT DEVELOPMENT
18.8 ASPECT IMAGING LTD
18.8.1 COMPANY SNAPSHOT
18.8.2 PRODUCT PORTFOLIO
18.8.3 RECENT DEVELOPMENT
18.9 BPL MEDICAL TECHNOLOGIES
18.9.1 COMPANY SNAPSHOT
18.9.2 PRODUCT PORTFOLIO
18.9.3 RECENT DEVELOPMENT
18.1 CARESTREAM HEALTH
18.10.1 COMPANY SNAPSHOT
18.10.2 REVENUE ANALYSIS
18.10.3 PRODUCT PORTFOLIO
18.10.4 RECENT DEVELOPMENTS
18.11 ESAOTE SPA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 FONAR CORP
18.12.1 COMPANY SNAPSHOT
18.12.2 REVENUE ANALYSIS
18.12.3 PRODUCT PORTFOLIO
18.12.4 RECENT DEVELOPMENT
18.13 FUJIFILM HOLDINGS CORPORATION
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 HOLOGIC, INC
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENTS
18.15 IMRIS, DEERFIELD IMAGING INC.
18.15.1 COMPANY SNAPSHOT
18.15.2 PRODUCT PORTFOLIO
18.15.3 RECENT DEVELOPMENT
18.16 MEDFIELD DIAGNOSTICS AB
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENT
18.17 MEDTRON AG
18.17.1 COMPANY SNAPSHOT
18.17.2 PRODUCT PORTFOLIO
18.17.3 RECENT DEVELOPMENT
18.18 NEUSOFT CORPORATION
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENT
18.19 SAMSUNG
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 PRODUCT PORTFOLIO
18.19.4 RECENT DEVELOPMENTS
18.2 SHENXHEN ANKE HIGH-TECH CO., LTD.
18.20.1 COMPANY SNAPSHOT
18.20.2 PRODUCT PORTFOLIO
18.20.3 RECENT DEVELOPMENT
18.21 SHIMADZU CORPORATION
18.21.1 COMPANY SNAPSHOT
18.21.2 REVENUE ANALYSIS
18.21.3 PRODUCT PORTFOLIO
18.21.4 RECENT DEVELOPMENTS
18.22 SIUI
18.22.1 COMPANY SNAPSHOT
18.22.2 PRODUCT PORTFOLIO
18.22.3 RECENT DEVELOPMENTS
18.23 STERNMED GMBH
18.23.1 COMPANY SNAPSHOT
18.23.2 PRODUCT PORTFOLIO
18.23.3 RECENT DEVELOPMENT
18.24 STRYKER
18.24.1 COMPANY SNAPSHOT
18.24.2 REVENUE ANALYSIS
18.24.3 PRODUCT PORTFOLIO
18.24.4 RECENT DEVELOPMENTS
18.25 TERASON DIVISION TERATECH CORPORATION
18.25.1 COMPANY SNAPSHOT
18.25.2 PRODUCT PORTFOLIO
18.25.3 RECENT DEVELOPMENT
19 QUESTIONNAIRE
20 RELATED REPORTS
Lista de Tablas
TABLE 1 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 2 NORTH AMERICA MODERATE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 3 NORTH AMERICA SEVERE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 4 NORTH AMERICA MILD IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 5 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 6 NORTH AMERICA COMPUTED TOMOGRAPHY (CT SCAN) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 7 NORTH AMERICA COMPUTED TOMOGRAPHY ANGIOGRAPHY (CTA) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 8 NORTH AMERICA MAGNETIC RESONANCE IMAGING (MRI) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 9 NORTH AMERICA MAGNETIC RESONANCE ANGIOGRAPHY (MRA) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 10 NORTH AMERICA TRANSCRANIAL DOPPLER ULTRASOUND IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 11 NORTH AMERICA VIDEO HEAD IMPULSE TEST (VHIT) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 12 NORTH AMERICA OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 13 NORTH AMERICA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 14 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 15 NORTH AMERICA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 16 NORTH AMERICA ISCHEMIC STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 17 NORTH AMERICA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 18 NORTH AMERICA HEMORRHAGIC STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 19 NORTH AMERICA TRANSIENT ISCHEMIC ATTACKS (TIAS) IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 20 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 21 NORTH AMERICA HOSPITAL IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 22 NORTH AMERICA CLINICS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 23 NORTH AMERICA AMBULATORY SURGICAL CENTERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 24 NORTH AMERICA HOME HEALTHCARE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 25 NORTH AMERICA OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 26 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 27 NORTH AMERICA DIRECT TENDER IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 28 NORTH AMERICA THIRD PARTY DISTRIBUTORS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 29 NORTH AMERICA OTHERS IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 30 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 31 NORTH AMERICA PRE OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 32 NORTH AMERICA PERI OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 33 NORTH AMERICA POST OPERATIVE IN STROKE DIAGNOSTICS MARKET, BY REGION, 2019-2028 (USD MILLION)
TABLE 34 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY COUNTRY, 2019-2028 (USD MILLION)
TABLE 35 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 36 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 37 NORTH AMERICA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 38 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 39 NORTH AMERICA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 40 NORTH AMERICA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 41 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 42 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 43 NORTH AMERICA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 44 U.S. STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 45 U.S. STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 46 U.S. OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 47 U.S. STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 48 U.S. ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 49 U.S. HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 50 U.S. STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 51 U.S. STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 52 U.S. STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 53 CANADA STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 54 CANADA STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 55 CANADA OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 56 CANADA STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 57 CANADA ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 58 CANADA HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 59 CANADA STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 60 CANADA STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 61 CANADA STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
TABLE 62 MEXICO STROKE DIAGNOSTICS MARKET, BY SEVERITY, 2019-2028 (USD MILLION)
TABLE 63 MEXICO STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 64 MEXICO OTHERS IN STROKE DIAGNOSTICS MARKET, BY TYPE, 2019-2028 (USD MILLION)
TABLE 65 MEXICO STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 66 MEXICO ISCHEMIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 67 MEXICO HEMORRHAGIC STROKE IN STROKE DIAGNOSTICS MARKET, BY APPLICATION, 2019-2028 (USD MILLION)
TABLE 68 MEXICO STROKE DIAGNOSTICS MARKET, BY END USER, 2019-2028 (USD MILLION)
TABLE 69 MEXICO STROKE DIAGNOSTICS MARKET, BY DISTRIBUTION CHANNEL, 2019-2028 (USD MILLION)
TABLE 70 MEXICO STROKE DIAGNOSTICS MARKET, BY STAGE, 2019-2028 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA STROKE DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA STROKE DIAGNOSTICS MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA STROKE DIAGNOSTICS MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA STROKE DIAGNOSTICS MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA STROKE DIAGNOSTICS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA STROKE DIAGNOSTICS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA STROKE DIAGNOSTICS MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA STROKE DIAGNOSTICS MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA STROKE DIAGNOSTICS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA STROKE DIAGNOSTICS MARKET: SEGMENTATION
FIGURE 11 THE INCREASE IN DEMAND FOR STROKE DIAGNOSTISIS EXPECTED TO DRIVE THE NORTH AMERICA STROKE DIAGNOSTICS MARKET IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 12 MODERATE IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA STROKE DIAGNOSTICS MARKET IN 2021 & 2028
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA STROKE DIAGNOSTICS MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2021 TO 2028
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA STROKE DIAGNOSTICS MARKET
FIGURE 15 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY SEVERITY, 2020
FIGURE 16 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY SEVERITY, 2020-2028 (USD MILLION)
FIGURE 17 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY SEVERITY, CAGR (2021-2028)
FIGURE 18 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY SEVERITY, LIFELINE CURVE
FIGURE 19 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY TYPE, 2020
FIGURE 20 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY TYPE, 2020-2028 (USD MILLION)
FIGURE 21 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY TYPE, CAGR (2021-2028)
FIGURE 22 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY TYPE, LIFELINE CURVE
FIGURE 23 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY APPLICATION, 2020
FIGURE 24 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY APPLICATION, 2020-2028 (USD MILLION)
FIGURE 25 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY APPLICATION, CAGR (2021-2028)
FIGURE 26 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 27 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY END USER, 2020
FIGURE 28 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY END USER, 2020-2028 (USD MILLION)
FIGURE 29 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY END USER, CAGR (2021-2028)
FIGURE 30 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY END USER, LIFELINE CURVE
FIGURE 31 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2020
FIGURE 32 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, 2020-2028 (USD MILLION)
FIGURE 33 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, CAGR (2021-2028)
FIGURE 34 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 35 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY STAGE, 2020
FIGURE 36 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY STAGE, 2020-2028 (USD MILLION)
FIGURE 37 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY STAGE, CAGR (2021-2028)
FIGURE 38 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY STAGE, LIFELINE CURVE
FIGURE 39 NORTH AMERICA STROKE DIAGNOSTICS MARKET: SNAPSHOT (2020)
FIGURE 40 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY COUNTRY (2020)
FIGURE 41 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY COUNTRY (2021 & 2028)
FIGURE 42 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY COUNTRY (2020 & 2028)
FIGURE 43 NORTH AMERICA STROKE DIAGNOSTICS MARKET: BY SEVERITY (2021-2028)
FIGURE 44 STROKE DIAGNOSTICS MARKET COMPANY SHARE 2020 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.













