North America Lentiviral Vector Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
744.76 Billion
USD
2,408.15 Billion
2025
2033
USD
744.76 Billion
USD
2,408.15 Billion
2025
2033
| 2026 –2033 | |
| USD 744.76 Billion | |
| USD 2,408.15 Billion | |
|
|
|
|
Segmentación del mercado de vectores lentivirales en Norteamérica, por componente (promotor lentiviral, etiquetas de fusión lentiviral, sistemas de empaquetado lentiviral y otros), tipo (producto y servicios), generación (4.ª generación, 3.ª generación, 2.ª generación y 1.ª generación), flujo de trabajo (procesamiento ascendente y descendente), método de administración (in vivo y ex vivo), indicación de enfermedad (cáncer, trastornos genéticos, enfermedades infecciosas , enfermedades veterinarias y otras), aplicación (terapia génica y vacunología), usuario final ( empresas de biotecnología , empresas farmacéuticas, organizaciones de investigación por contrato, organizaciones de desarrollo y fabricación por contrato (CDMO) e institutos académicos/de investigación): tendencias de la industria y pronóstico hasta 2033.
Tamaño del mercado de vectores lentivirales en América del Norte
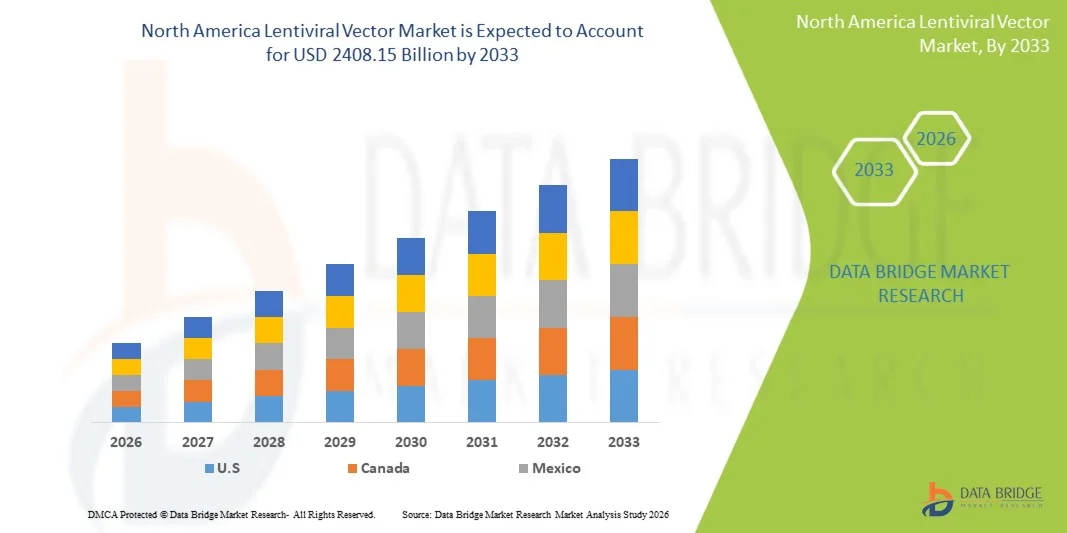
- El tamaño del mercado de vectores lentivirales de América del Norte se valoró en USD 744,76 millones en 2025 y se espera que alcance los USD 2408,15 millones para 2033 , con una CAGR del 15,80 % durante el período de pronóstico.
- El crecimiento del mercado está impulsado en gran medida por la creciente adopción de terapias genéticas y celulares, la creciente prevalencia de trastornos genéticos y los continuos avances tecnológicos en la ingeniería de vectores virales, lo que conduce a una mejor eficiencia y seguridad de la transducción en aplicaciones terapéuticas.
- Además, la creciente inversión en investigación y desarrollo, la expansión de los ensayos clínicos y la creciente demanda de medicina personalizada e inmunoterapias avanzadas están consolidando los vectores lentivirales como herramientas cruciales en la biotecnología y la atención médica modernas. Estos factores convergentes están acelerando la adopción de soluciones de vectores lentivirales, impulsando así significativamente el crecimiento de la industria.
Análisis del mercado de vectores lentivirales en América del Norte
- Los vectores lentivirales, ampliamente utilizados para la administración eficiente de genes en investigación, terapia génica y aplicaciones de terapia celular, son componentes cada vez más críticos de la biotecnología moderna debido a su capacidad para permitir la expresión genética estable y respaldar el desarrollo terapéutico avanzado en entornos preclínicos y clínicos.
- La creciente demanda de vectores lentivirales se debe principalmente a la rápida expansión de las líneas de desarrollo de terapias génicas y celulares, el aumento de las aprobaciones de terapias CAR-T y genéticamente modificadas, y las crecientes inversiones en medicina personalizada y de precisión en todo el mundo.
- Estados Unidos dominó el mercado de vectores lentivirales de América del Norte con la mayor participación en los ingresos de aproximadamente el 37,5 % en 2025, respaldado por una infraestructura biotecnológica avanzada, una sólida financiación de I+D, la adopción temprana de terapias genéticas y celulares, una gran cantidad de ensayos clínicos en curso y la presencia de empresas biofarmacéuticas líderes especializadas en la producción de vectores virales.
- Se espera que Canadá sea el país de más rápido crecimiento en el mercado de vectores lentivirales de América del Norte durante el período de pronóstico, impulsado por el aumento de las inversiones en investigación en biotecnología y terapia génica, la expansión de las actividades de ensayos clínicos, la creciente adopción de terapias avanzadas y políticas gubernamentales de apoyo que promueven el desarrollo de productos biológicos y las capacidades de fabricación nacional.
- El segmento de productos dominó el mercado con una participación en los ingresos del 64,3 % en 2025, impulsado por la alta demanda de vectores lentivirales, plásmidos y reactivos de transducción listos para usar.
Alcance del informe y segmentación del mercado de vectores lentivirales en América del Norte
|
Atributos |
Perspectivas clave del mercado de vectores lentivirales |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como el valor de mercado, la tasa de crecimiento, la segmentación, la cobertura geográfica y los principales actores, los informes de mercado seleccionados por Data Bridge Market Research también incluyen un análisis en profundidad de expertos, epidemiología de pacientes, análisis de la cartera de productos, análisis de precios y marco regulatorio. |
Tendencias del mercado de vectores lentivirales en América del Norte
Ampliación del uso de vectores lentivirales en terapias génicas y celulares avanzadas
- Una tendencia significativa y en aceleración en el mercado global de vectores lentivirales de América del Norte es la creciente utilización de vectores lentivirales en aplicaciones de terapia génica, terapia celular y medicina regenerativa, debido a su capacidad para administrar material genético tanto a células en división como a células que no se dividen con alta eficiencia.
- Por ejemplo, en junio de 2023, Thermo Fisher Scientific amplió sus capacidades de fabricación de vectores virales en EE. UU. y Europa para respaldar la creciente demanda mundial de vectores lentivirales utilizados en programas de terapia génica y celular en etapa clínica.
- El creciente número de ensayos clínicos dirigidos a trastornos genéticos raros, indicaciones oncológicas y enfermedades autoinmunes está reforzando la demanda de vectores lentivirales en instituciones de investigación y empresas biofarmacéuticas de todo el mundo.
- Además, los avances en ingeniería vectorial, incluidos perfiles de seguridad mejorados y una mayor eficiencia de transducción, están apoyando una adopción más amplia en el desarrollo clínico en etapa avanzada y la fabricación a escala comercial.
- Las crecientes colaboraciones entre centros de investigación académica, organizaciones de desarrollo y fabricación por contrato (CDMO) y compañías farmacéuticas están configurando aún más el panorama del mercado global.
Dinámica del mercado de vectores lentivirales en América del Norte
Conductor
Creciente demanda mundial de terapia génica, terapia celular y medicina personalizada
- El creciente enfoque global en la terapia genética y la medicina personalizada es un impulsor clave del mercado de vectores lentivirales de América del Norte, ya que estos vectores desempeñan un papel fundamental en la entrega de genes terapéuticos para la expresión a largo plazo.
- Por ejemplo, en septiembre de 2022, Oxford Biomedica firmó un acuerdo de suministro a largo plazo con una compañía farmacéutica global para fabricar vectores lentivirales para terapias con células CAR-T, lo que refleja una fuerte demanda comercial.
- La creciente prevalencia del cáncer, los trastornos genéticos raros y las enfermedades crónicas ha intensificado la necesidad de enfoques de tratamiento innovadores, acelerando así la adopción de vectores lentivirales en el desarrollo terapéutico.
- Los marcos regulatorios favorables para las terapias celulares y genéticas en regiones como América del Norte y Europa, combinados con el aumento de las inversiones en atención médica en Asia-Pacífico, están impulsando aún más la expansión del mercado.
- El aumento de la financiación pública y privada para la investigación biotecnológica, junto con las iniciativas gubernamentales que apoyan las terapias avanzadas, sigue fortaleciendo la demanda mundial de soluciones de vectores lentivirales.
Restricción/Desafío
Alta complejidad de fabricación, limitaciones de costos y estrictos requisitos regulatorios .
- Los altos costos de fabricación y los procesos de producción complejos representan desafíos importantes para el mercado global de vectores lentivirales de América del Norte, ya que la producción requiere instalaciones especializadas, personal calificado y un estricto cumplimiento de las normas GMP.
- Por ejemplo, en febrero de 2023, varias CDMO destacaron retrasos en los plazos de producción de vectores lentivirales debido a un mayor escrutinio regulatorio y limitaciones de capacidad, lo que afecta la escalabilidad de los programas de terapia génica.
- Los estrictos requisitos regulatorios relacionados con la bioseguridad, el control de calidad y la consistencia de los vectores virales aumentan los plazos de desarrollo y los costos operativos, en particular para las empresas de biotecnología pequeñas y medianas.
- La limitada capacidad de fabricación mundial y los cuellos de botella en la cadena de suministro restringen aún más la disponibilidad de vectores lentivirales para aplicaciones clínicas y comerciales a gran escala.
- Abordar estos desafíos mediante la optimización de procesos, la expansión de la infraestructura de fabricación, la capacitación de la fuerza laboral y la armonización regulatoria será fundamental para el crecimiento sostenido del mercado global.
Alcance del mercado de vectores lentivirales en América del Norte
El mercado está segmentado en función del componente, tipo, generación, flujo de trabajo, método de entrega, aplicación y usuario final.
• Por componente
Según sus componentes, el mercado norteamericano de vectores lentivirales se segmenta en promotores lentivirales, etiquetas de fusión lentivirales, sistemas de empaquetado lentivirales y otros. El segmento de sistemas de empaquetado lentivirales dominó la mayor cuota de mercado en ingresos, con un 41,6 % en 2025, impulsado por su papel esencial en la producción de vectores lentivirales con replicación deficiente, utilizados en terapias génicas y celulares. Los sistemas de empaquetado son fundamentales para el ensamblaje de partículas virales y garantizar una alta eficiencia de transducción, lo cual es vital para la fabricación de vectores de grado clínico. La fuerte demanda de los desarrolladores de terapias con células CAR-T impulsa significativamente este segmento. El creciente número de ensayos clínicos de terapia génica a nivel mundial incrementa el consumo de plásmidos y reactivos de empaquetado. Las empresas farmacéuticas y biotecnológicas prefieren sistemas de empaquetado estandarizados para garantizar la consistencia de los lotes y el cumplimiento normativo. Los avances en la tecnología de empaquetado, que mejoran el rendimiento y la seguridad, refuerzan aún más su dominio. La creciente externalización de la producción de vectores a CDMO también impulsa la demanda recurrente. La alta frecuencia de uso en los flujos de trabajo iniciales impulsa la generación de ingresos. La familiaridad regulatoria con los sistemas de empaquetado establecidos fomenta su adopción continua. La expansión de las instalaciones de fabricación GMP fortalece la penetración en el mercado. El aumento de las inversiones en infraestructura de terapia génica refuerza aún más su dominio. En general, la naturaleza crítica e insustituible de los sistemas de envasado consolida su liderazgo en el mercado.
Se prevé que el segmento de promotores lentivirales experimente el crecimiento más rápido, registrando una tasa de crecimiento anual compuesta (TCAC) del 18,9 % entre 2026 y 2033, impulsado por la creciente demanda de un control preciso de la expresión génica en terapias génicas avanzadas. Los promotores desempeñan un papel fundamental en la regulación de los niveles de expresión transgénica, lo cual es crucial para mejorar la eficacia y la seguridad terapéuticas. La creciente investigación en promotores inducibles y específicos de cada célula acelera su adopción. La expansión de las aplicaciones de biología sintética y medicina de precisión impulsa el crecimiento. Los institutos académicos y de investigación utilizan cada vez más promotores personalizados para lograr flexibilidad experimental. El creciente enfoque en la reducción de efectos no deseados impulsa la innovación en promotores. El aumento de la financiación para la ingeniería de vectores de última generación contribuye a la expansión del mercado. Las compañías farmacéuticas están invirtiendo en diseños de promotores patentados para mejorar la diferenciación de sus productos. La transición hacia terapias personalizadas aumenta la demanda de promotores especializados. Los avances en las tecnologías de cribado de promotores mejoran la velocidad de desarrollo. El aumento de la actividad de patentes refleja el impulso innovador. Estos factores, en conjunto, impulsan el rápido crecimiento de la TCAC del segmento.
• Por tipo
Según el tipo, el mercado norteamericano de vectores lentivirales se segmenta en productos y servicios. El segmento de productos dominó el mercado con una participación en los ingresos del 64,3 % en 2025, impulsado por la alta demanda de vectores lentivirales, plásmidos y reactivos de transducción listos para usar. Las empresas biotecnológicas y farmacéuticas dependen en gran medida de productos estandarizados para acelerar los plazos de desarrollo preclínico y clínico. Los productos ofrecen reproducibilidad y consistencia de calidad, esenciales para entornos regulados. Su amplio uso en la investigación académica y en las primeras etapas del descubrimiento de fármacos respalda su dominio en ingresos. La creciente disponibilidad de productos con certificación GMP fortalece aún más su adopción. El aumento de la inversión en terapias génicas y celulares impulsa la demanda recurrente de productos. Los fabricantes lanzan continuamente variantes mejoradas de productos, lo que impulsa la demanda de reemplazo. Los productos reducen la dependencia de las capacidades de producción interna de vectores. La amplia distribución a través de proveedores consolidados mejora la accesibilidad. El alto volumen de consumo en todos los flujos de trabajo mantiene el liderazgo en ingresos. El aumento de la actividad global de ensayos clínicos refuerza el dominio. En general, la fiabilidad y la escalabilidad de los productos mantienen el liderazgo del segmento.
Se anticipa que el segmento de servicios experimentará el crecimiento más rápido, con una CAGR del 20,4% entre 2026 y 2033, impulsada por las crecientes tendencias de externalización en la industria de la terapia génica. Las empresas farmacéuticas y biotecnológicas dependen cada vez más de las CDMO para el desarrollo de vectores, la ampliación y la fabricación conforme a las BPM. Los servicios reducen los gastos de capital y la complejidad operativa para los desarrolladores de terapias. El creciente número de ensayos clínicos aumenta significativamente la demanda de servicios por contrato. La experiencia en cumplimiento normativo hace que los proveedores de servicios sean muy atractivos. La expansión de las terapias personalizadas impulsa la demanda de servicios de vectores personalizados. La creciente complejidad del diseño de vectores requiere capacidades técnicas especializadas. Las nuevas empresas biotecnológicas emergentes prefieren los modelos de servicio por su rentabilidad. La escasez mundial de capacidad de fabricación interna acelera la externalización. Las alianzas estratégicas entre patrocinadores y CDMO respaldan el crecimiento. Las inversiones en infraestructura de servicios amplían la capacidad. Estos factores impulsan colectivamente un fuerte crecimiento de la CAGR para los servicios.
• Por Generación
En función de la generación, el mercado norteamericano de vectores lentivirales se segmenta en vectores de cuarta, tercera, segunda y primera generación. El segmento de tercera generación dominó el mercado con una participación en los ingresos del 38,7 % en 2025, gracias a su perfil de seguridad superior y su amplia aceptación clínica. Estos vectores ofrecen un menor riesgo de formación de virus con capacidad de replicación, lo que los hace adecuados para uso terapéutico. Su alta eficiencia de transducción facilita su uso en oncología y enfermedades raras. Las autoridades reguladoras están más familiarizadas con los vectores de tercera generación, lo que facilita los procesos de aprobación. La amplia información clínica respalda la confianza de médicos y desarrolladores. La sólida adopción de terapias CAR-T y con células madre refuerza el dominio. La compatibilidad con la fabricación a gran escala impulsa aún más la demanda. Los protocolos establecidos simplifican los procesos de desarrollo. La alta disponibilidad entre proveedores facilita la accesibilidad. El aumento de las aprobaciones de terapias génicas sustenta su uso. Su larga presencia en el mercado garantiza la confianza. Estos factores consolidan la posición dominante del segmento.
Se espera que el segmento de cuarta generación crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida, del 22,1 %, entre 2026 y 2033, impulsada por mejoras en la bioseguridad y el diseño de última generación. Estos vectores minimizan aún más los riesgos de la mutagénesis insercional, lo que mejora la seguridad del paciente. El creciente enfoque en terapias génicas avanzadas acelera su adopción. El aumento de la inversión en I+D impulsa la innovación rápida. Las instituciones académicas investigan activamente nuevos diseños de cuarta generación. Las compañías farmacéuticas adoptan estos vectores para programas clínicos de próxima fase. El énfasis regulatorio en la seguridad favorece a las generaciones avanzadas. Un mejor control de la expresión génica mejora los resultados terapéuticos. La demanda de indicaciones para enfermedades de alto riesgo aumenta su adopción. Los avances tecnológicos reducen la complejidad de la producción. Las colaboraciones estratégicas impulsan la comercialización. Estos factores, en conjunto, impulsan un rápido crecimiento.
• Por flujo de trabajo
Según el flujo de trabajo, el mercado norteamericano de vectores lentivirales se segmenta en procesamiento ascendente y descendente. El segmento de procesamiento ascendente dominó el mercado con una participación en los ingresos del 55,4 % en 2025, impulsado por su papel crucial en la producción de vectores. Actividades como el cultivo celular, la transfección y la amplificación de vectores generan una alta demanda de consumibles. El aumento de la escalabilidad de la fabricación de terapias génicas respalda este dominio. El alto uso de medios, reactivos y plásmidos impulsa los ingresos. Las inversiones continuas en optimización de la producción fortalecen la adopción. El fuerte enfoque en la mejora del rendimiento de los vectores respalda el gasto. La expansión de las instalaciones que cumplen con las BPM aumenta la actividad ascendente. La adopción de la automatización mejora la eficiencia y la repetibilidad. Las iteraciones frecuentes de los procesos impulsan las compras recurrentes. La alta dependencia técnica garantiza una demanda sostenida. El aumento del volumen de ensayos clínicos respalda el uso. Estos factores mantienen el dominio ascendente.
Se proyecta que el segmento de procesamiento posterior crecerá a la tasa de crecimiento anual compuesta (TCAC) más rápida, del 19,2 %, entre 2026 y 2033, impulsada por el creciente énfasis en la purificación y el control de calidad. Los requisitos regulatorios exigen vectores altamente purificados para uso clínico. La creciente adopción de tecnologías de cromatografía y filtración impulsa el crecimiento. La creciente complejidad de los vectores requiere soluciones avanzadas para el procesamiento posterior. La demanda de procesos de purificación escalables acelera su adopción. La expansión de los ensayos clínicos en etapas avanzadas aumenta la intensidad del procesamiento posterior. Las inversiones en nuevas tecnologías de purificación mejoran la eficiencia. Las CDMO amplían la capacidad para el procesamiento posterior para satisfacer la demanda. Los estrictos estándares de seguridad aumentan los requisitos de prueba. Las herramientas analíticas mejoradas impulsan el crecimiento. El alto valor por paso del proceso aumenta los ingresos. Estos factores, en conjunto, impulsan un rápido crecimiento de la TCAC.
• Por método de entrega
Según el método de administración, el mercado norteamericano de vectores lentivirales se segmenta en in vivo y ex vivo. El segmento ex vivo dominó la mayor cuota de mercado con un 58,3 % en 2025, impulsado por su amplio uso en terapias génicas CAR-T y otras terapias génicas celulares, en las que las células del paciente se modifican fuera del organismo. La administración ex vivo garantiza una mayor seguridad, un control preciso de la eficiencia de la transducción y una menor exposición sistémica, lo que la convierte en una opción muy popular en aplicaciones clínicas. Este segmento se beneficia de la creciente adopción de terapias celulares personalizadas, el creciente número de ensayos clínicos en oncología e inmunología, y la fuerte demanda de empresas biotecnológicas que desarrollan terapias CAR-T, TCR-T y con células madre. Los métodos ex vivo son más fáciles de supervisar para el cumplimiento normativo y el control de calidad, lo que anima a las empresas farmacéuticas a adoptar estos enfoques. La expansión de las capacidades de CDMO y la externalización del procesamiento ex vivo impulsan aún más el crecimiento de los ingresos. La alta adopción en entornos hospitalarios y de investigación refuerza el dominio del mercado. Los protocolos ex vivo estandarizados facilitan la reproducibilidad entre lotes. Además, el creciente enfoque en terapias celulares de última generación impulsa una mayor cuota de mercado para la administración ex vivo. La inversión en instalaciones de fabricación que cumplen con las normas GMP impulsa la expansión continua de este segmento. En general, la precisión, la seguridad y el conocimiento normativo hacen de la entrega ex vivo el método dominante.
Se espera que el segmento In Vivo experimente la tasa de crecimiento anual compuesta (TCAC) más rápida, del 19,2 %, entre 2026 y 2033, impulsada por el creciente interés en las aplicaciones de terapia génica directa, donde los vectores se administran directamente a los pacientes. La administración in vivo permite el tratamiento de enfermedades donde la manipulación celular ex vivo resulta impráctica, como ciertos trastornos genéticos y afecciones sistémicas. El aumento de la investigación en diana específica para tejidos, el desarrollo de vectores virales avanzados con tropismo mejorado y la creciente adopción en estudios preclínicos y clínicos contribuyen a este rápido crecimiento. Los marcos regulatorios favorables y la mayor financiación gubernamental para la investigación en terapia génica aceleran su adopción. Los avances en las tecnologías de administración, incluyendo cápsides virales más seguras y la optimización de promotores, impulsan la eficiencia. El creciente enfoque en trastornos genéticos raros impulsa la demanda de enfoques in vivo. Las compañías farmacéuticas están invirtiendo en plataformas de vectores patentadas para terapias in vivo dirigidas. La investigación colaborativa entre empresas biotecnológicas e instituciones académicas impulsa la innovación. La administración in vivo también se beneficia de procedimientos de administración simplificados en comparación con los métodos ex vivo. En general, la creciente cartera de candidatos a terapia génica posiciona la administración in vivo para un crecimiento significativo de la TCAC.
• Por indicación de enfermedad
Según la indicación de la enfermedad, el mercado norteamericano de vectores lentivirales se segmenta en cáncer, trastornos genéticos, enfermedades infecciosas, enfermedades veterinarias y otras. El segmento de cáncer dominó la mayor cuota de mercado en ingresos, con un 46,5 % en 2025, impulsado por la rápida adopción de terapias con CAR-T, TCR-T y células NK dirigidas a tumores hematológicos y sólidos. Las aplicaciones oncológicas representan la mayor parte de la demanda mundial de vectores lentivirales debido a la alta actividad de ensayos clínicos, las aprobaciones regulatorias y la creciente adopción comercial. El aumento de la prevalencia del cáncer, la expansión de la infraestructura de terapia génica y las crecientes inversiones de las empresas farmacéuticas y biotecnológicas impulsan aún más este segmento. Las terapias con CAR-T dirigidas a la leucemia, el linfoma y el mieloma múltiple son importantes impulsores de ingresos. La colaboración entre institutos de investigación y CDMO para la producción de vectores centrados en oncología mejora la capacidad de suministro. La modificación celular ex vivo avanzada y las estrategias de tratamiento personalizado refuerzan su dominio. El segmento se beneficia de un alto potencial de reembolso en los mercados desarrollados. La mayor concienciación de los pacientes y el apoyo gubernamental a las terapias contra el cáncer también contribuyen a la cuota de mercado. Las plataformas lentivirales estandarizadas para oncología mejoran la consistencia y la escalabilidad. La expansión del acceso a los mercados globales impulsa una adopción sostenida. En general, las aplicaciones oncológicas siguen siendo la indicación de enfermedad más dominante en el mercado.
Se prevé que el segmento de Trastornos Genéticos registre la tasa de crecimiento anual compuesta (TCAC) más rápida, del 18,7 %, entre 2026 y 2033, impulsada por la creciente prevalencia de enfermedades hereditarias como la anemia de células falciformes, la beta-talasemia y la fibrosis quística. Este crecimiento se sustenta en avances en medicina de precisión, edición genética y terapia génica ex vivo que corrige defectos genéticos. El aumento de los ensayos clínicos, las iniciativas de financiación gubernamental y la creciente adopción de plataformas de terapia personalizada impulsan la expansión del mercado. Las empresas biotecnológicas y farmacéuticas desarrollan activamente terapias génicas dirigidas a trastornos raros, lo que impulsa el crecimiento del segmento. Las innovaciones en el diseño de promotores y la ingeniería de vectores mejoran la eficacia y reducen los efectos no deseados. El segmento se beneficia de una mayor concienciación y un diagnóstico temprano de las enfermedades genéticas raras. Los institutos académicos y de investigación están explorando nuevos mecanismos de administración para estos trastornos. Las aprobaciones regulatorias para terapias para trastornos genéticos estimulan aún más su adopción. La expansión de las CDMO que ofrecen producción especializada de vectores fortalece las cadenas de suministro. La creciente demanda en los mercados emergentes contribuye al crecimiento del segmento. En general, los trastornos genéticos representan el segmento de indicaciones de enfermedades de más rápido crecimiento a nivel mundial.
• Por aplicación
En función de la aplicación, el mercado norteamericano de vectores lentivirales se segmenta en terapia génica y vaccinología. El segmento de terapia génica dominó la mayor cuota de mercado en ingresos, con un 62,4 % en 2025, impulsado por el amplio uso de vectores lentivirales en terapias CAR-T, TCR-T y con células madre. Las aplicaciones de terapia génica se benefician de una sólida cartera de productos clínicos, el aumento de las aprobaciones regulatorias y la creciente comercialización de terapias avanzadas. Su alta adopción en oncología, trastornos genéticos raros e inmunología respalda su dominio. La externalización de la producción de vectores a organizaciones de desarrollo de productos (CDMO), la inversión en instalaciones que cumplen con las normas GMP y la estandarización de los protocolos de fabricación contribuyen al crecimiento. Las empresas farmacéuticas y biotecnológicas priorizan los vectores lentivirales para aplicaciones ex vivo debido a su predecible eficiencia de transducción. Las instituciones académicas y de investigación también recurren cada vez más a la terapia génica para estudios preclínicos. La expansión de la medicina personalizada y las terapias de precisión consolida aún más el liderazgo del segmento. La familiaridad regulatoria con los flujos de trabajo de la terapia génica facilita su adopción. Los incentivos gubernamentales y los programas de financiación impulsan las iniciativas de investigación. En general, la terapia genética sigue siendo el segmento de aplicación dominante en el mercado de vectores lentivirales de América del Norte.
Se espera que el segmento de Vacunología experimente la CAGR más rápida del 20,1% entre 2026 y 2033, impulsada por la creciente demanda mundial de vacunas basadas en vectores virales, incluyendo para enfermedades infecciosas como el VIH, la COVID-19 y patógenos emergentes. El desarrollo acelerado de vacunas, la expansión de las iniciativas de salud pública y la financiación gubernamental para la preparación ante pandemias impulsan el crecimiento. Los avances en el diseño de vectores, la optimización de promotores y las mejoras en la inmunogenicidad aumentan la eficacia de las vacunas. Las colaboraciones estratégicas entre empresas de biotecnología, CDMO e institutos de investigación aceleran la comercialización. La creciente adopción en los mercados emergentes debido a las campañas de vacunación gubernamentales respalda la expansión. Los marcos regulatorios específicos para vacunas agilizan las aprobaciones. El aumento de la inversión en I+D en nuevas plataformas de vectores virales fomenta la innovación. El crecimiento de las inmunoterapias personalizadas también contribuye a la adopción del segmento. La escalabilidad de la producción de vectores mejora la eficiencia de la fabricación. En general, las aplicaciones de vacunología representan el segmento de más rápido crecimiento a nivel mundial.
• Por el usuario final
En función del usuario final, el mercado norteamericano de vectores lentivirales se segmenta en empresas de biotecnología, farmacéuticas, organizaciones de investigación por contrato (CRO), organizaciones de desarrollo y fabricación por contrato (CDMO) e institutos académicos y de investigación. El segmento de CDMO dominó la mayor cuota de mercado en ingresos, con un 48,2 % en 2025, gracias a su papel crucial en la producción subcontratada de vectores lentivirales de grado clínico y comercial. Las CDMO ofrecen experiencia especializada, instalaciones que cumplen con las normas GMP y una fabricación escalable para productos CAR-T, TCR-T y de terapia génica. El aumento de la subcontratación por parte de empresas biotecnológicas y farmacéuticas, las ventajas de costes y la necesidad de una producción que cumpla con las normativas refuerzan su dominio. Las sólidas alianzas con empresas farmacéuticas globales aumentan la demanda recurrente. La producción de vectores ex vivo a gran escala impulsa el crecimiento de los ingresos. La estandarización de los protocolos de producción garantiza la calidad y la reproducibilidad. La expansión de las instalaciones de CDMO en Norteamérica, Europa y Asia-Pacífico fortalece la penetración en el mercado. La inversión en plataformas vectoriales de última generación mejora la oferta de servicios. La familiaridad regulatoria y la experiencia con las cadenas de suministro clínicas impulsan la adopción. En general, las CDMO representan el segmento dominante de usuarios finales en el mercado norteamericano de vectores lentivirales.
Se espera que el segmento de las compañías farmacéuticas experimente la CAGR más rápida del 19,5% entre 2026 y 2033, impulsada por el aumento de las inversiones en líneas de desarrollo de terapia génica patentadas, nuevas plataformas de vectores lentivirales y la expansión global de las capacidades de producción de vectores virales. Las compañías farmacéuticas están integrando modelos de desarrollo interno y subcontratación para acelerar la comercialización de productos. El crecimiento se ve impulsado aún más por la demanda de productos avanzados CAR-T, TCR-T y de terapia génica. La creciente colaboración con las CDMO y las instituciones académicas mejora las capacidades tecnológicas. Las políticas regulatorias de apoyo y la creciente actividad de ensayos clínicos en oncología y enfermedades genéticas raras aceleran la adopción. Las adquisiciones y asociaciones estratégicas fortalecen las capacidades de fabricación. El enfoque en la innovación, la medicina de precisión y las terapias personalizadas respalda la expansión del segmento. La creciente demanda de soluciones de terapia génica en los mercados emergentes impulsa aún más el crecimiento. Los avances tecnológicos en ingeniería vectorial mejoran la seguridad y la eficacia. En general, las compañías farmacéuticas representan el segmento de usuarios finales de más rápido crecimiento.
Análisis regional del mercado de vectores lentivirales en América del Norte
- Se prevé que el mercado de vectores lentivirales de América del Norte crezca a una CAGR sólida del 23-24 % durante el período de pronóstico de 2026 a 2033, impulsado por la rápida expansión de la infraestructura biotecnológica, el aumento de las inversiones en investigación de terapias genéticas y celulares y el aumento de la actividad de ensayos clínicos en toda la región.
- Los gobiernos de América del Norte apoyan activamente el desarrollo de productos biológicos avanzados, medicina regenerativa y terapia génica mediante políticas de financiación favorables, incentivos fiscales y reformas regulatorias.
- Además, América del Norte está surgiendo como un centro clave de fabricación y subcontratación para la producción de vectores lentivirales, respaldado por ventajas de costos, capacidades de CDMO en expansión y una creciente demanda de las compañías biofarmacéuticas globales.
Perspectiva del mercado de vectores lentivirales en América del Norte y EE. UU.
El mercado de vectores lentivirales de América del Norte y Estados Unidos dominó el mercado de vectores lentivirales de América del Norte con la mayor participación en los ingresos de aproximadamente el 37,5 % en 2025, respaldado por una infraestructura biotecnológica avanzada, una sólida financiación de I+D, una adopción temprana de terapias genéticas y celulares, una gran cantidad de ensayos clínicos en curso y la presencia de empresas biofarmacéuticas líderes especializadas en la producción de vectores virales.
Perspectiva del mercado de vectores lentivirales de Canadá y América del Norte
Se espera que el mercado de vectores lentivirales de América del Norte de Canadá sea el país de más rápido crecimiento en el mercado de vectores lentivirales de América del Norte durante el período de pronóstico, impulsado por el aumento de las inversiones en investigación en biotecnología y terapia genética, la expansión de las actividades de ensayos clínicos, la creciente adopción de terapias avanzadas y políticas gubernamentales de apoyo que promueven el desarrollo de productos biológicos y las capacidades de fabricación nacional.
Cuota de mercado de vectores lentivirales en América del Norte
La industria de vectores lentivirales está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Grupo Lonza (Suiza)
- Takara Bio (Japón)
- Oxford Biomedica (Reino Unido)
- Sartorius AG (Alemania)
- FUJIFILM Diosynth Biotechnologies (Japón)
- Catalent (EE. UU.)
- Laboratorios Charles River (EE. UU.)
- AGC Biologics (Japón)
- Sirion Biotech (Alemania)
- Vigene Biosciences (EE. UU.)
- GeneCopoeia (EE. UU.)
- VectorBuilder (EE. UU.)
- Creative Biogene (EE. UU.)
- Bio-Techne (EE. UU.)
- Aldevron (EE. UU.)
- Terapias avanzadas WuXi (China)
- Bayer AG (Alemania)
- Genscript Biotech (China)
Últimos avances en el mercado de vectores lentivirales en América del Norte
- En abril de 2023, Yposkesi, una CDMO especializada en vectores virales, anunció el lanzamiento de LentiSure, una plataforma de producción de vectores lentivirales de próxima generación diseñada para impulsar la eficiencia y la solidez de la producción de células CAR-T y otras terapias inmunooncológicas basadas en células.
- En diciembre de 2023, VIVEbiotech reportó un crecimiento comercial significativo con un aumento del 70% en las ventas y capacidades ampliadas de fabricación de vectores lentivirales, respaldando aplicaciones tanto in vivo como ex vivo y suministrando vectores de grado GMP a socios globales.
- En junio de 2024, Charles River Laboratories anunció una colaboración estratégica con el Instituto Anschutz Gates de la Universidad de Colorado para fabricar vectores lentivirales, con el objetivo de acelerar el desarrollo de la terapia con células CAR-T para cánceres hematológicos y mejorar la capacidad de fabricación de terapias génicas avanzadas.
- En septiembre de 2024, Rentschler Biopharma lanzó servicios dedicados a la fabricación de vectores lentivirales en sus instalaciones de Stevenage (Reino Unido), presentando una nueva caja de herramientas de vectores lentivirales para complementar sus ofertas de AAV y respaldar el desarrollo de terapias celulares y génicas para enfermedades raras e inmunoterapia contra el cáncer.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.