North America Glioblastoma Multiforme Treatment Market, By Type (Primary (De Novo), Secondary), Treatment (Surgery, Radiotherapy, Medications), Patient Type (Adult, Geriatric, Child), Drug Type (Generics, Branded), Route of Administration (Parenteral, Oral, Others), End User (Hospitals, Clinics, Home Healthcare, Others), Distribution Channel (Hospital Pharmacy, Retail Pharmacy, Online Pharmacy, Others) - Industry Trends and Forecast to 2029.
Market Analysis and Insights
Glioblastoma multiforme (GBM) is a grade IV WHO malignant tumor with astrocytic differentiation. As one of the most common clinically diagnosed central nervous system (CNS) oncological entries, there have been a wide variety of historical reports of the description and evolution of ideas regarding these tumors. The first recorded reports of gliomas were given in British scientific reports, by Berns in 1800 and in 1804 by Abernety, with the first comprehensive histomorphological description being given in 1865 by Rudolf Virchow. In 1926 Percival Bailey and Harvey Cushing gave the base for the modern classification of gliomas. Between 1934 and 1941 the most prolific researcher in glioma research was Hans-Joachim Scherer, who postulated some of the clinico-morphological aspects of GBM. With the introduction of molecular and genetic tests the true multifomity of GBM has been established, with different genotypes bearing the same histomorphological and IHC picture, as well as some of the aspects of gliomagenesis. For a GBM to develop, a specific trigger mutation needs to occur in a GBM stem cell - primary GBM, or a slow aggregation of individual mutations, without a distinct trigger mutation - secondary GBM. Knowledge of GBM has been closely related to general medical knowledge of the CNS since these malignancies were first described more than 200 years ago. Several great leaps have been made in that time, in the footsteps of both CNS and advancements in general medical knowledge. The demand for glioblastoma multiforme treatment is increasing, for which manufacturers are involved in the new product launches, increasing pipeline products and event participation in the market. These decisions are ultimately enhancing the growth of the market.

El informe de mercado sobre el tratamiento del glioblastoma multiforme proporciona detalles sobre la participación de mercado, los nuevos desarrollos, el impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de nuevas oportunidades de ingresos, cambios en las regulaciones del mercado, aprobaciones de productos, decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado, contáctenos para obtener un informe de analista; nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado. Las iniciativas estratégicas como la colaboración, el acuerdo y la firma de acuerdos de venta para inventar e innovar tratamientos farmacológicos son los principales impulsores que impulsaron la demanda del mercado en el período de pronóstico.
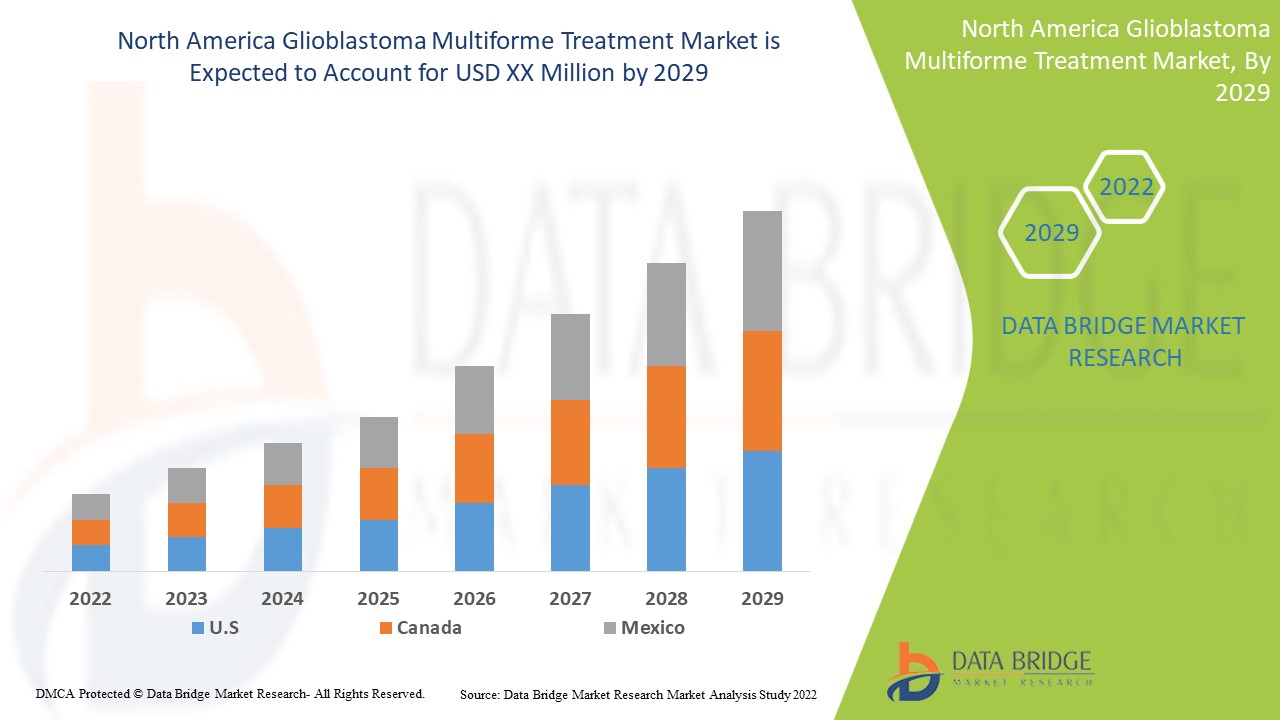
El mercado de tratamiento del glioblastoma multiforme es favorable y tiene como objetivo reducir la progresión de la enfermedad. Data Bridge Market Research analiza que el mercado de tratamiento del glioblastoma multiforme crecerá a una CAGR del 8,5 % durante el período de pronóstico de 2022 a 2029.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2022 a 2029 |
|
Año base |
2021 |
|
Años históricos |
2020 (Personalizable para 2019 - 2014) |
|
Unidades cuantitativas |
Ingresos en millones de USD, precios en USD |
|
Segmentos cubiertos |
Por tipo (primario (de novo), secundario), tratamiento (cirugía, radioterapia, medicamentos), tipo de paciente (adulto, geriátrico, niño), tipo de medicamento (genérico, de marca), vía de administración (parenteral, oral, otros), usuario final (hospitales, clínicas, atención médica domiciliaria, otros), canal de distribución (farmacia hospitalaria, farmacia minorista, farmacia en línea, otros) |
|
Países cubiertos |
Estados Unidos, Canadá, México |
|
Actores del mercado cubiertos |
F. Hoffmann-La Roche AG, Amgen Inc., Merck & Co., Inc., Pfizer Inc., Varian Medical Systems, Inc. (una subsidiaria de Siemens Healthcare), ZEISS International, Amneal Pharmaceuticals LLC, Elekta, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd., Eckert & Ziegler, Accord Healthcare, Angiochem, ANI Pharmaceuticals, Inc., Arbor Pharmaceuticals, LLC. (una subsidiaria de Azurity Pharmaceuticals, Inc.), AstraZeneca, Cantex Pharmaceuticals, Inc., CELON LABS, Diffusion Pharmaceuticals Inc., EnGeneIC, ERC.SA., Genenta science, Jazz Pharmaceuticals, Inc., Loxo Oncology (una subsidiaria de Eli Lilly), Novartis AG, VBL THERAPEUTICS, Viatris Inc. y Zydus Pharmaceuticals, Inc., entre otros. |
Definición de mercado
El glioblastoma multiforme (GBM) es el tumor cerebral maligno primario más común y agresivo y representa el 60% de los tumores cerebrales en adultos. Los GBM pueden surgir en el cerebro de novo o evolucionar a partir de un astrocitoma de grado inferior. En adultos, el GBM se presenta con mayor frecuencia en los hemisferios cerebrales, especialmente en los lóbulos frontal y temporal del cerebro. Se han estudiado muchos factores genéticos y ambientales en el glioblastoma multiforme, pero no se ha identificado ningún factor de riesgo que explique una gran proporción del GBM. Por lo tanto, como muchos otros cánceres, el GBM es esporádico, aunque algunos estudios indican una alta prevalencia (17%) de irradiación terapéutica previa entre los pacientes con GBM. La latencia entre la irradiación y el desarrollo de GBM varía de unos pocos años a varias décadas. No hay evidencia sustancial de asociación del GBM con factores de estilo de vida como el tabaquismo, el consumo de alcohol, el uso de drogas o la exposición a compuestos N-nitrosos. Los estudios han demostrado que el uso de teléfonos móviles no aumenta el riesgo de desarrollo de GBM; sin embargo, su asociación con el uso a largo plazo necesita más confirmación.
Dinámica del mercado del tratamiento del glioblastoma multiforme
Conductores
- Prevalencia creciente del glioblastoma multiforme
El glioblastoma multiforme (GBM) es el tumor cerebral primario maligno más común y representa entre el 77 % y el 81 % de todos los tumores malignos primarios del sistema nervioso central (SNC). La Organización Mundial de la Salud lo clasificó como un tumor astrocítico y oligodendroglial difuso de grado IV. La edad media de presentación del GBM primario es de 62 años y la supervivencia media es de aproximadamente 14,6 meses. El mal pronóstico asociado con el GBM está bien documentado, mientras que las tasas de supervivencia siguen siendo decepcionantemente bajas a pesar de los avances médicos y quirúrgicos. Según el estudio, los estudios internacionales revelan una tasa de incidencia anual aproximada de 0,59 a 5 por 100 000 personas; sin embargo, los estudios indican un aumento en la incidencia. Miranda-Filho et al. en 2017 describieron tasas crecientes de cánceres del SNC y del cerebro en países de América del Sur, Europa del Este y Europa del Sur, mientras que solo se informaron tasas decrecientes en Japón. Dobes et al. En 2011, también se observó una creciente incidencia de tumores GBM en dos de sus estudios multicéntricos australianos, con un aumento particular en los tumores GBM del lóbulo frontal y temporal. La mayor incidencia del glioblastoma multiforme aumenta la demanda de detección y diagnóstico tempranos mediante el uso de la última tecnología, lo que impulsa el mercado mundial de tratamiento del glioblastoma multiforme. Se espera que la creciente incidencia del glioblastoma en todo el mundo acelere la demanda de tratamiento del glioblastoma multiforme. Por lo tanto, se espera que las mayores tasas de incidencia del glioblastoma multiforme impulsen el crecimiento del mercado.
- Aumentar la investigación y el desarrollo (I+D)
El aumento de las actividades de investigación y desarrollo (I+D) en biotecnología molecular y terapia génica para el cáncer y enfermedades relacionadas ha facilitado el desarrollo de diversos fármacos biológicos. Estos fármacos ayudan a disminuir los efectos secundarios de los métodos de tratamiento existentes, creando así una aceptación más amplia entre los pacientes. Se espera que la heterogeneidad tumoral y la variación en el enfoque de tratamiento de paciente a paciente aumenten la demanda de un enfoque de tratamiento personalizado para controlar el glioblastoma multiforme. Se espera que la aprobación de nuevos tratamientos aumente la expectativa de vida de los pacientes que viven con glioblastoma multiforme. Además, se espera que una designación especial otorgada a los fármacos en investigación por la FDA agilice el proceso de aprobación y comercialización de terapias novedosas. Se espera que un aumento en las colaboraciones entre investigadores y actores del mercado impulse el desarrollo de opciones de tratamiento novedosas y efectivas para el glioblastoma multiforme. Se espera que la creciente aprobación de terapias novedosas y terapias combinadas impulse el mercado de tratamiento del glioblastoma multiforme.
Oportunidad
- Aumento de las aprobaciones de medicamentos
La creciente demanda de tratamientos para el glioblastoma multiforme debería generar más aprobaciones regulatorias para medicamentos asociados. El aumento de las aprobaciones regulatorias para medicamentos relacionados y productos recombinantes constituirá un aumento en el valor de mercado del tratamiento del glioblastoma multiforme en los próximos años. En el marco de una iniciativa de la Organización Panamericana de la Salud (OPS) para promover el reconocimiento de las Autoridades Reguladoras de Medicamentos, el proceso de evaluación de la ANMAT finalizó el 11 de diciembre de 2009. La industria del tratamiento del glioblastoma multiforme ha sido testigo de numerosas aprobaciones de medicamentos en los últimos años, impulsadas por la creciente tasa de mortalidad de la enfermedad. El aumento de las aprobaciones de medicamentos aumentará la demanda del mercado de tratamiento del glioblastoma multiforme.
Restricciones/Desafíos
El alto costo del tratamiento del glioblastoma multiforme
Las pruebas de diagnóstico del glioblastoma multiforme incluyen productos de tecnología muy avanzada. El desarrollo de esos productos implica una investigación y un desarrollo rigurosos por parte de la empresa desarrolladora. Por lo tanto, el costo del producto sigue siendo alto, lo que aumenta proporcionalmente el costo de las pruebas.
Las herramientas y técnicas diagnósticas utilizadas para el diagnóstico del glioblastoma multiforme incluyen:
Radioterapia, quimioterapia, entre otras. Las primeras etapas del glioblastoma multiforme suelen presentarse con síntomas mínimos o nulos; por lo tanto, el glioblastoma multiforme se diagnostica con frecuencia en etapas avanzadas, lo que resulta en un mal pronóstico. Por lo tanto, el alto costo del tratamiento del glioblastoma multiforme utilizando las modalidades y productos tecnológicos avanzados actuará como un importante factor restrictivo para el crecimiento del mercado mundial de tratamiento del glioblastoma multiforme.
Acontecimientos recientes
- En abril de 2022, Elekta y GE Healthcare anunciaron que habían firmado un acuerdo de colaboración comercial global en el campo de la oncología radioterápica, lo que les permitirá ofrecer a los hospitales una oferta integral de imágenes y tratamientos para pacientes con cáncer que requieran radioterapia. Esta asociación permitirá a las empresas promover conjuntamente soluciones para las necesidades de cada centro oncológico
- En julio de 2019, Amgen y Allergan plc anunciaron que MVASI (bevacizumab-awwb), un biosimilar de Avastin (bevacizumab), está disponible en los Estados Unidos (EE. UU.). Este lanzamiento mejorará las ventas del producto en la región.
Alcance del mercado del tratamiento del glioblastoma multiforme
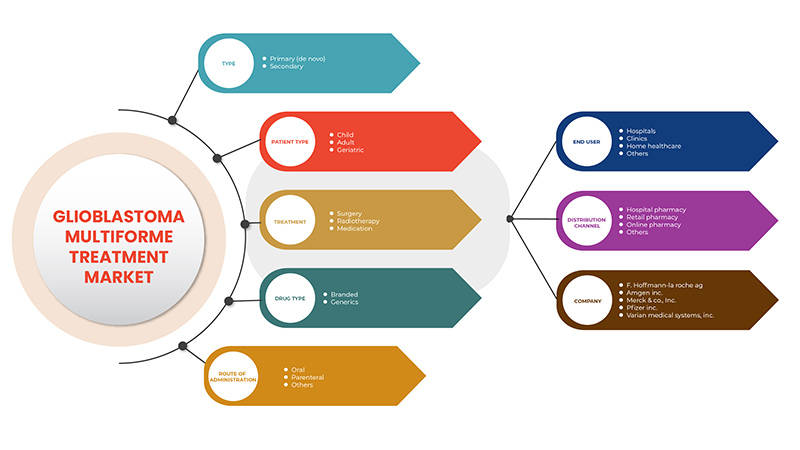
El mercado de tratamiento del glioblastoma multiforme se clasifica en siete segmentos notables que se basan en el tipo, el tratamiento, el tipo de paciente, el tipo de fármaco, la vía de administración, el usuario final y el canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
Tipo
- Primaria (De Novo)
- Secundario
Según el tipo, el mercado de tratamiento del glioblastoma multiforme se segmenta en primario (De Novo) y secundario.
Tratamiento
- Cirugía
- Radioterapia
- Medicamentos
Según el tratamiento, el mercado de tratamiento del glioblastoma multiforme está segmentado en cirugía, radioterapia y medicamentos.
Tipo de paciente
- Adulto
- Geriátrico
- Niño
Según el tipo de paciente, el mercado de tratamiento del glioblastoma multiforme se segmenta en adultos, geriátricos y niños.
Tipo de droga
- De marca
- Genéricos
Según el tipo de fármaco, el mercado de tratamiento del glioblastoma multiforme se segmenta en genéricos y de marca.
Vía de administración
- Oral
- Parenteral
- Otros
Según la vía de administración, el mercado de tratamiento del glioblastoma multiforme se segmenta en parenteral, oral y otros.
Usuario final
- Hospitales
- Clínicas
- Atención médica domiciliaria
- Otros
Sobre la base del usuario final, el mercado de tratamiento del glioblastoma multiforme está segmentado en hospitales, clínicas, atención médica domiciliaria y otros.
Canal de distribución
- Farmacia hospitalaria
- Farmacia minorista
- Farmacia en línea
- Otros
Sobre la base del canal de distribución, el mercado de tratamiento del glioblastoma multiforme está segmentado en farmacia hospitalaria, farmacia minorista y otras.
Análisis regional y perspectivas del mercado mundial de tratamiento del glioblastoma multiforme
Se analiza el mercado de tratamiento del glioblastoma multiforme y se proporcionan información y tendencias del tamaño del mercado por tipo, tratamiento, tipo de paciente, tipo de fármaco, vía de administración, usuario final y canal de distribución como se mencionó anteriormente.
Las regiones cubiertas en el informe del mercado de tratamiento del glioblastoma multiforme son EE. UU., Canadá y México.
En América del Norte, se espera que Estados Unidos domine el mercado debido a la creciente prevalencia de la enfermedad en la región.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en la regulación del mercado que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como el análisis de la cadena de valor ascendente y descendente, las tendencias técnicas y el análisis de las cinco fuerzas de Porter, los estudios de casos son algunos de los indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas globales y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los aranceles nacionales y las rutas comerciales se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado del tratamiento del glioblastoma multiforme
El panorama competitivo del mercado de tratamiento del glioblastoma multiforme proporciona detalles de los competidores. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia global, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y la variedad de productos y el dominio de la aplicación. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en el mercado de tratamiento del glioblastoma multiforme.
Algunos de los actores clave en el mercado son F. Hoffmann-La Roche AG, Amgen Inc., Merck & Co., Inc., Pfizer Inc., Varian Medical Systems, Inc. (una subsidiaria de Siemens Healthcare), ZEISS International, Amneal Pharmaceuticals LLC, Elekta, Sun Pharmaceutical Industries Ltd, Teva Pharmaceutical Industries Ltd., Eckert & Ziegler, Accord Healthcare, Angiochem, ANI Pharmaceuticals, Inc., Arbor Pharmaceuticals, LLC. (Una subsidiaria de Azurity Pharmaceuticals, Inc.), AstraZeneca, Cantex Pharmaceuticals, Inc., CELON LABS, Diffusion Pharmaceuticals Inc., EnGeneIC, ERC.SA., Genenta science, Jazz Pharmaceuticals, Inc., Loxo Oncology (Una subsidiaria de Eli Lilly), Novartis AG, VBL THERAPEUTICS, Viatris Inc. y Zydus Pharmaceuticals, Inc., entre otros.
Metodología de la investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con tamaños de muestra grandes. Los datos del mercado se analizan y estiman utilizando modelos estadísticos y coherentes del mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Aparte de esto, los modelos de datos incluyen la cuadrícula de posicionamiento de proveedores, el análisis de la línea de tiempo del mercado, la descripción general y la guía del mercado, la cuadrícula de posicionamiento de la empresa, el análisis de la participación de mercado de la empresa, los estándares de medición, el análisis global frente al regional y el análisis de la participación de los proveedores. Solicite una llamada de un analista en caso de tener más consultas.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TYPE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 EPIDEMIOLOGY
6 PIPELINE ANALYSIS
7 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: REGULATORY SCENARIO
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 GROWING PREVALENCE OF GLIOBLASTOMA MULTIFORME
8.1.2 INCREASING RESEARCH AND DEVELOPMENT (R&D)
8.1.3 PRESENCE OF A STRONG PIPELINE
8.1.4 GROWING GERIATRIC POPULATION
8.2 RESTRAINTS
8.2.1 HIGH COST OF GLIOBLASTOMA MULTIFORME TREATMENT
8.2.2 ADVERSE SIDE-EFFECTS OF GLIOBLASTOMA MULTIFORME TREATMENT
8.3 OPPORTUNITIES
8.3.1 INCREASING DRUG APPROVALS
8.3.2 PARTNERSHIP AND AGREEMENT BY MAJOR PLAYERS
8.3.3 INCREASING SUPPORT OF PRIVATE AND GOVERNMENT AGENCIES FOR TREATMENT
8.4 CHALLENGES
8.4.1 LACK OF NEW TREATMENT
8.4.2 ADVERSE EFFECTS AND RISKS ASSOCIATED WITH CANCER TREATMENT DRUGS
8.4.3 LACK OF EARLY DETECTION
9 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE
9.1 OVERVIEW
9.2 PRIMARY (DE NOVO)
9.3 SECONDARY
10 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT
10.1 OVERVIEW
10.2 SURGERY
10.3 RADIOTHERAPY
10.3.1 BRACHYTHERAPY
10.3.2 FRACTIONATED STEREOTACTIC RT (FSRT)
10.3.3 CONFORMAL OR INTENSITY-MODULATED RT
10.3.4 RADIOSURGERY
10.4 MEDICATIONS
10.4.1 TEMOZOLOMIDE
10.4.1.1 ORAL
10.4.1.1 INTRAVENOUS
10.4.2 NITROSOUREAS DRUGS
10.4.2.1 CARMUSTINE
10.4.2.1.1 PARENTERAL
10.4.2.1.2 IMPLANTABLE WAFERS
10.4.2.2 LOMUSTINE
10.4.2.3 NIMUSTINE
10.4.2.4 FOTEMUSTINE
10.4.3 TARGETED THERAPY
10.4.3.1 BEVACIZUMAB
10.4.3.2 OTHERS
10.4.4 ANTI-EPILEPTICS
10.4.4.1 LEVETIRACETAM
10.4.4.2 PHENYTOIN
10.4.4.3 CARBAMAZEPINE
10.4.5 CORTICOSTEROIDS
10.4.5.1 METHYLPREDNISOLONE
10.4.5.2 PREDNISONE
10.4.5.3 OTHERS
11 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE
11.1 OVERVIEW
11.2 ADULT
11.2.1 MALE
11.2.2 FEMALE
11.3 GERIATRIC
11.3.1 MALE
11.3.2 FEMALE
11.4 CHILD
12 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE
12.1 OVERVIEW
12.2 GENERICS
12.3 BRANDED
13 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION
13.1 OVERVIEW
13.2 PARENTERAL
13.3 ORAL
13.3.1 CAPSULES
13.3.2 TABLETS
13.3.3 POWDERS
13.4 OTHERS
14 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER
14.1 OVERVIEW
14.2 HOSPITAL
14.3 CLINICS
14.4 HOME HEALTHCARE
14.5 OTHERS
15 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 HOSPITAL PHARMACY
15.3 RETAIL PHARMACY
15.4 ONLINE PHARMACY
15.5 OTHERS
16 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 F.HOFFMAN-LA ROCHE
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENT
19.2 AMGEN INC.
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.2.5.1 PRODUCT APPROVAL
19.3 MERCK & CO., INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENTS
19.3.5.1 STRATETIC COLLABORATION
19.3.5.2 EVENTS
19.4 PFIZER INC.
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENT
19.4.5.1 MERGER
19.5 VARIAN MEDICAL SYSTEMS, INC. (A SUBSIDIARY OF SIEMENS HEALTHCARE)
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.5.5.1 PARTNERSHIP
19.5.5.2 ACQUISITION
19.6 ZEISS INTERNATIONAL
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.6.4.1 PRODUCT EXPANSION
19.7 AMNEAL PHARMACEUTICALS LLC
19.7.1 COMPANY SNAPSHOT
19.7.2 REVENUE ANALYSIS
19.7.3 PRODUCT PORTFOLIO
19.7.4 RECENT DEVELOPMENTS
19.7.4.1 EVENT
19.7.4.2 LAUNCH
19.7.4.3 ACQUISITION
19.8 ELEKTA
19.8.1 COMPANY SNAPSHOT
19.8.2 REVENUE ANALYSIS
19.8.3 PRODUCT PORTFOLIO
19.8.4 RECENT DEVELOPMENTS
19.8.4.1 PARTNERSHIP
19.9 SUN PHARMACEUTICAL INDUSTRIES LTD
19.9.1 COMPANY SNAPSHOT
19.9.2 REVENUE ANALYSIS
19.9.3 PRODUCT PORTFOLIO
19.9.4 RECENT DEVELOPMENT
19.9.4.1 AGREEMENT
19.1 TEVA PHARMACEUTICAL INDUSTRIES LTD
19.10.1 COMPANY SNAPSHOT
19.10.2 REVENUE ANALYSIS
19.10.3 PRODUCT PORTFOLIO
19.10.4 RECENT DEVELOPMENT
19.11 ECKERT & ZIEGLER
19.11.1 COMPANY SNAPSHOT
19.11.2 REVENUE ANALYSIS
19.11.3 PRODUCT PORTFOLIO
19.11.4 RECENT DEVELOPMENT
19.12 ACCORD HEALTHCARE
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 ANGIOCHEM
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENT
19.13.3.1 AGREMEENT
19.14 ANI PHARMACEUTICALS, INC.
19.14.1 COMPANY SNAPSHOT
19.14.2 REVENUE ANALYSIS
19.14.3 PRODUCT PORTFOLIO
19.14.4 RECENT DEVELOPMENTS
19.14.4.1 ACQUISITION
19.15 ARBOR PHARMACEUTICALS, LLC. A SUBSIDIARY OF AZURITY PHARMACEUTICALS, INC.
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENT
19.15.3.1 ACQUISITION
19.15.3.2 PRODUCT APPROVAL
19.16 ASTRAZENECA
19.16.1 COMPANY SNAPSHOT
19.16.2 REVENUE ANALYSIS
19.16.3 PRODUCT PORTFOLIO
19.16.4 RECENT DEVELOPMENT
19.16.4.1 AGREEMENT
19.17 CANTEX PHARMACEUTICALS, INC.
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENT
19.18 CELON LABS
19.18.1 COMPANY SNAPSHOT
19.18.2 PRODUCT PORTFOLIO
19.18.3 RECENT DEVELOPMENT
19.19 DIFFUSION PHARMACEUTICAL
19.19.1 COMPANY SNAPSHOT
19.19.2 SERVICES PORTFOLIO
19.19.3 RECENT DEVELOPMENT
19.2 ERC.SA
19.20.1 COMPANY SNAPSHOT
19.20.2 PRODUCT PORTFOLIO
19.20.3 RECENT DEVELOPMENT
19.20.3.1 PIPELINE UPDATE
19.21 ENGENEIC
19.21.1 COMPANY SNAPSHOT
19.21.2 PRODUCT PORTFOLIO
19.21.3 RECENT DEVELOPMENTS
19.21.3.1 AWARDS
19.22 GENENTA SCIENCE
19.22.1 COMPANY SNAPSHOT
19.22.2 PRODUCT PORTFOLIO
19.22.3 RECENT DEVELOPMENT
19.22.3.1 EVENT
19.23 JAZZ PHARMACEUTICALS, INC.
19.23.1 COMPANY SNAPSHOT
19.23.2 REVENUE ANALYSIS
19.23.3 PRODUCT PORTFOLIO
19.23.4 RECENT DEVELOPMENT
19.23.4.1 ACQUISITION
19.24 LOXO ONCOLOGY (A SUBSIDIARY OF ELI LILLY)
19.24.1 COMPANY SNAPSHOT
19.24.2 PRODUCT PORTFOLIO
19.24.3 RECENT DEVELOPMENT
19.25 NOVARTIS AG
19.25.1 COMPANY SNAPSHOT
19.25.2 REVENUE ANALYSIS
19.25.3 PRODUCT PORTFOLIO
19.25.4 RECENT DEVELOPMENT
19.26 VBL THERAPEUTICS
19.26.1 COMPANY SNAPSHOT
19.26.2 PRODUCT PORTFOLIO
19.26.3 RECENT DEVELOPMENT
19.26.3.1 EVENT
19.26.3.2 AWARD
19.27 VIATRIS INC
19.27.1 COMPANY SNAPSHOT
19.27.2 REVENUE ANALYSIS
19.27.3 PRODUCT PORTFOLIO
19.27.4 RECENT DEVELOPMENT
19.27.4.1 AGREEMENT
19.28 ZYDUS PHARMACEUTICALS, INC.
19.28.1 COMPANY SNAPSHOT
19.28.2 PRODUCT PORTFOLIO
19.28.3 RECENT DEVELOPMENTS
20 QUESTIONNAIRE
21 RELATED REPORTS
Lista de Tablas
TABLE 1 PIPELINE ANALYSIS FOR GLIOBLASTOMA MULTIFORME TREATMENT MARKET
TABLE 2 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA PRIMARY (DE NOVO) IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA SECONDARY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA SURGERY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA ADULTS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CHILD IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA GENERICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA BRANDED IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA PARENTERAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA HOSPITAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA CLINICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA HOME HEALTHCARE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA HOSPITAL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA RETAIL PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA ONLINE PHARMACY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA OTHERS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 56 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 57 NORTH AMERICA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 58 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 59 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 60 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 62 U.S. RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 63 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 64 U.S. TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 65 U.S. NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 66 U.S. CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 67 U.S. TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 68 U.S. ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 69 U.S. CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 70 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 71 U.S. ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 72 U.S. GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 73 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 74 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 75 U.S. ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 76 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 77 U.S. GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 78 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 80 CANADA RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 81 CANADA MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 82 CANADA TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 83 CANADA NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 84 CANADA CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 85 CANADA TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 86 CANADA ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 87 CANADA CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 88 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 89 CANADA ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 90 CANADA GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 91 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 92 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 93 CANADA ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 94 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 95 CANADA GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 96 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 97 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 98 MEXICO RADIOTHERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 99 MEXICO MEDICATIONS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 100 MEXICO TEMOZOLOMIDE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 101 MEXICO NITROSOUREAS DRUGS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 102 MEXICO CARMUSTINE IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 103 MEXICO TARGETED THERAPY IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 104 MEXICO ANTI-EPILEPTICS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 105 MEXICO CORTICOSTEROIDS IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY TREATMENT, 2020-2029 (USD MILLION)
TABLE 106 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 107 MEXICO ADULT IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 108 MEXICO GERIATRIC IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY PATIENT TYPE, 2020-2029 (USD MILLION)
TABLE 109 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DRUG TYPE, 2020-2029 (USD MILLION)
TABLE 110 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 111 MEXICO ORAL IN GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY ROUTE OF ADMINISTRATION, 2020-2029 (USD MILLION)
TABLE 112 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 113 MEXICO GLIOBLASTOMA MULTIFORME TREATMENT MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET : DATA TRIANGULATION
FIGURE 3 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MULTIVARIATE MODELLING
FIGURE 7 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: DBMR MARKET POSITION GRID
FIGURE 9 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: MARKET END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: VENDOR SHARE ANALYSIS
FIGURE 11 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SEGMENTATION
FIGURE 12 NORTH AMERICA IS EXPECTED TO DOMINATE AND ASIA-PACIFIC IS GROWING AT THE FASTEST PACE IN NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 13 INCREASE IN THE PREVALENCE OF GLIOBLASTOMA MULTIFORME AND INCREASE IN PIPELINE PRODUCTS ARE DRIVING THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 PRIMARY (DE NOVO) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET IN 2022 & 2029
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET
FIGURE 16 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2021
FIGURE 17 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, 2022-2029 (USD MILLION)
FIGURE 18 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 19 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE, LIFELINE CURVE
FIGURE 20 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2021
FIGURE 21 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TREATMENT, 2022-2029 (USD MILLION)
FIGURE 22 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, CAGR (2022-2029)
FIGURE 23 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT: BY TREATMENT, LIFELINE CURVE
FIGURE 24 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2021
FIGURE 25 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, 2022-2029 (USD MILLION)
FIGURE 26 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, CAGR (2022-2029)
FIGURE 27 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY PATIENT TYPE, LIFELINE CURVE
FIGURE 28 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2021
FIGURE 29 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, 2022-2029 (USD MILLION)
FIGURE 30 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, CAGR (2022-2029)
FIGURE 31 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DRUG TYPE, LIFELINE CURVE
FIGURE 32 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2021
FIGURE 33 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, 2022-2029 (USD MILLION)
FIGURE 34 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2022-2029)
FIGURE 35 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 36 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2021
FIGURE 37 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 38 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, CAGR (2022-2029)
FIGURE 39 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY END USER, LIFELINE CURVE
FIGURE 40 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 41 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 42 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 43 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 44 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: SNAPSHOT (2021)
FIGURE 45 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021)
FIGURE 46 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2022 & 2029)
FIGURE 47 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY COUNTRY (2021 & 2029)
FIGURE 48 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: BY TYPE (2022-2029)
FIGURE 49 NORTH AMERICA GLIOBLASTOMA MULTIFORME TREATMENT MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.