North America CRISPR Gene Detection and Diagnostic Market, By Class (Class 1- Multiple Effector Proteins and Class 2 -Single CrRNA-Binding Protein), Products & Services (Products and Services), Application (Biomedical Diagnostics, Genome Engineering, Drug Discovery, Agricultural Applications and Others), Workflow (Sample Preparation, Pre-Amplification, CrRNA, Cas Enzymes and Sensing), End User (Hospitals, Diagnostic Centers, Biotechnology Companies, Academic and Research Institutes and Others), Distribution Channel (Direct Tender, Retail Sales) Industry Trends and Forecast to 2029
Market Definition and Insights
CRISPR is clustered regularly interspaced short palindromic repeats and is a tool for genome editing, it allows researchers to alter DNA sequences and modify gene function easily. It has many potential applications, including correcting genetic defects and treating and preventing the spread of diseases. CRISPR-based diagnostics have been used for many biomedical applications, such as sensing nucleic-acid-based biomarkers of infectious and non-infectious diseases and detecting genetic diseases. The assay kits in CRISPR are composed of two components: a protein called Cas9 and a guide RNA, a string of nucleic acid molecules with a certain genetic code.
This CRISPR-Cas9 system has been modified for use in mammalian cells. We can either knock out specific genes by introducing a guide sequence (sgRNA) specific to our gene of interest by introducing frameshift mutations via Non-Homologous End Joining (NHEJ) or generate knock-in mutations.
CRISPR-Cas 9 systems have extended the scope of diagnostics and services in gene and cell therapies. Pharmaceutical companies invest heavily in R&D to develop new products, with a surge of gene and cell therapy agents entering early development. The market players investing would allow producing safe and effective treatments for patients in serious need.
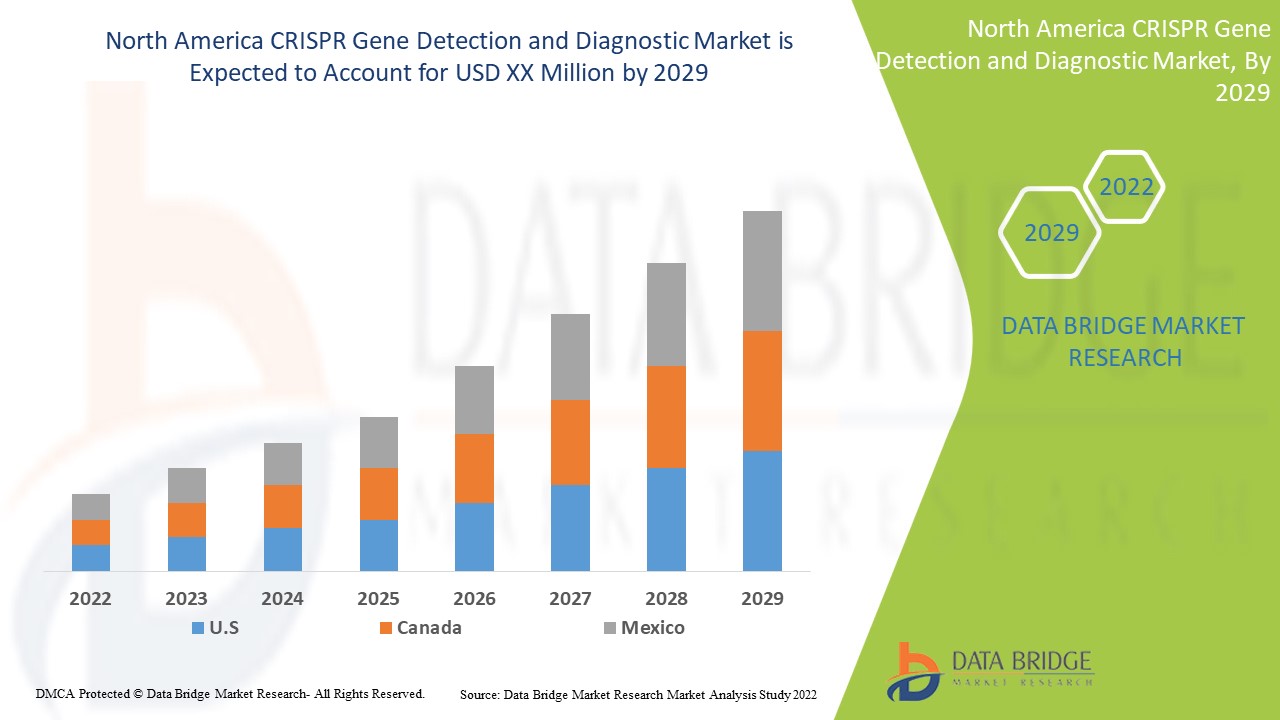
The North America CRISPR gene detection and diagnostic is supportive and aims to reduce the severity of the symptoms. Data Bridge Market Research analyses that the CRISPR gene detection and diagnostic market will grow at a CAGR of 19.6 % during the forecast period of 2022 to 2029.
|
Report Metric |
Details |
|
Forecast Period |
2022 to 2029 |
|
Base Year |
2021 |
|
Historic Years |
2020 (Customizable to 2019 - 2014) |
|
Quantitative Units |
Revenue in USD Million, Pricing in USD |
|
Segments Covered |
Por clase (Clase 1: proteínas efectoras múltiples y Clase 2: proteína de unión a ARNcr simple), Productos y servicios (Productos y servicios), Aplicación (Diagnóstico biomédico, Ingeniería genómica, Descubrimiento de fármacos, Aplicaciones agrícolas y otras), Flujo de trabajo (Preparación de muestras, Preamplificación, ARNcr, Enzimas Cas y detección), Usuario final (Hospitales, Centros de diagnóstico, Empresas de biotecnología, Institutos académicos y de investigación y otros), Canal de distribución (Licitación directa, Ventas minoristas) |
|
Países cubiertos |
Estados Unidos, Canadá y México |
|
Actores del mercado cubiertos |
GenScript, OriGene Technologies, Inc., Applied StemCell, GeneCopoeia, Inc., Agilent Technologies, Inc., Synthego, BioVision Inc., Hera Biolabs, Cellecta, Inc., New England Biolabs, 10x Genomics, addgene CasTag Biosciences, Merck KGaA, Integrated DNA Technologies, Inc. (una subsidiaria de Danaher), Thermo Fisher Scientific Inc., entre otros. |
Dinámica del mercado de detección y diagnóstico de genes mediante CRISPR en América del Norte
Conductores
- Aumento de la prevalencia e incidencia de enfermedades crónicas
Las enfermedades crónicas son afecciones de salud comunes, y uno de cada tres adultos padece alguna de ellas. Las enfermedades crónicas han afectado la salud y la calidad de vida de muchos ciudadanos.
CRISPR es la abreviatura de repeticiones palindrómicas cortas agrupadas y regularmente interespaciadas. En los últimos años, CRISPR se ha convertido en una herramienta de gran utilidad para la edición genética, que se utiliza para alterar las secuencias específicas de ADN en una célula. CRISPR tiene un uso importante en la investigación y el tratamiento de la enfermedad de Huntington, la distrofia muscular, el cáncer y el colesterol alto.
Por ejemplo,
- En 2021, los datos de NORD - National Organization for Rare Disorders, Inc. indicaron la incidencia diagnosticada de distrofia muscular de Duchenne (DMD). La distrofia muscular de Duchenne (DMD) es una enfermedad genética frecuente que afecta a 1 de cada 3500 nacimientos de varones en todo el mundo.
- Aumento de la inversión en investigación y desarrollo
Las tecnologías de edición genética, como el sistema CRISPR-Cas 9, han ampliado el alcance de los diagnósticos y servicios en terapias génicas y celulares. Las compañías farmacéuticas invierten mucho en I+D para desarrollar nuevos productos, y se ha producido un aumento de agentes de terapia génica y celular en las primeras etapas de desarrollo. La inversión de los actores del mercado permitiría alcanzar el objetivo de producir tratamientos seguros y eficaces para pacientes con necesidades graves.
Por ejemplo,
- En febrero de 2022, Synthego había recaudado 200 millones de dólares como inversión para investigación y desarrollo con el fin de impulsar el desarrollo de medicamentos basados en CRISPR desde la fase inicial de investigación hasta la fase clínica. Synthego utilizará el monto de inversión de la financiación de la Serie E para acelerar la creación de diagnósticos y servicios CRISPR.
Disponibilidad de financiación para el diagnóstico genético CRISPR
El Instituto Nacional de Salud (NIH) financia el diagnóstico y la investigación de genes mediante CRISPR. El sector privado también financia la detección e investigación de genes mediante CRISPR, pero esa inversión generalmente ocurre más tarde, durante la fase de prueba y desarrollo, y luego durante la investigación básica inicial. Como la edición genómica es un campo tan nuevo, un organismo gubernamental imparcial debe supervisarlos; la FDA es cautelosa y minuciosa, pero lucha sin descanso por obtener fondos, lo que hace una inversión a largo plazo que alinea el pago con los posibles beneficiarios futuros., mejorará aún más el crecimiento del mercado de detección y diagnóstico de genes mediante CRISPR.
Además, el avance en el diagnóstico genético CRISPR, las crecientes iniciativas de organizaciones públicas y privadas para difundir la conciencia y el aumento de la financiación gubernamental son los factores que expandirán el mercado de detección genética CRISPR. Otros factores, como el aumento de la demanda de terapias efectivas y la creciente conciencia sobre el diagnóstico oportuno, tendrán un impacto positivo en la tasa de crecimiento del mercado de detección y diagnóstico genético CRISPR. Además, los altos ingresos disponibles, el creciente número de enfermedades crónicas y el cambio en el estilo de vida darán como resultado la expansión del mercado de detección y diagnóstico genético CRISPR.
Oportunidades
- El aumento del gasto sanitario
Además, el aumento de las actividades de investigación y desarrollo y el aumento de las inversiones por parte del gobierno y de organizaciones privadas impulsarán nuevas oportunidades para la tasa de crecimiento del mercado.
- Iniciativa estratégica de los actores del mercado
La demanda de detección y diagnóstico de genes mediante CRISPR ha aumentado en los EE. UU. y, debido al tratamiento oportuno de enfermedades crónicas, estos factores favorables aumentan la necesidad de medicamentos y, para satisfacer la demanda del mercado, los actores menores y mayores del mercado están utilizando diversas estrategias.
Los principales actores también están tratando de diseñar estrategias específicas, como lanzamientos de productos, adquisiciones, aprobaciones, expansiones y asociaciones, para garantizar el buen funcionamiento del negocio, evitar riesgos y aumentar el crecimiento a largo plazo de las ventas del mercado.
Por ejemplo,
- En mayo de 2021, Horizon Discovery Ltd. amplió su cartera de modulación genética con el primer ARN guía sintético único y el represor dcas9 pendiente de patente para la interferencia CRISPR en Waltham. La expansión de la cartera había aumentado las ventas y los ingresos de la cartera de ARN guía sintético en la región de EE. UU. y el Reino Unido y había aumentado la colaboración con los actores del mercado.
Además, el lanzamiento de terapias efectivas y ensayos clínicos continuos brindarán oportunidades beneficiosas para el mercado de detección y diagnóstico de genes CRISPR en el período de pronóstico de 2022 a 2029. Además, la gran necesidad insatisfecha de tecnología actual y los avances en el campo de la atención médica aumentarán la tasa de crecimiento del mercado de detección y diagnóstico de genes CRISPR en el futuro.
Restricciones/Desafíos
Sin embargo, el alto costo de los diagnósticos CRISPR y los riesgos que se enfrentan al utilizarlos impedirán la tasa de crecimiento del mercado de detección y diagnóstico de genes CRISPR. Además, los riesgos que se corren al utilizar los dispositivos de resonancia magnética obstaculizarán el crecimiento del mercado de detección y diagnóstico de genes CRISPR. La falta de conocimientos especializados y regulaciones supondrán un desafío adicional para el mercado en el período de pronóstico mencionado anteriormente.
- Aumento del coste de los diagnósticos basados en CRISPR
El enorme potencial de las terapias basadas en CRISPR tiene un coste. Las terapias de edición genómica máxima requieren una mayor cantidad de tiempo para su desarrollo y producción, y de ahí el aumento de los costes. Además, los kits de ensayo y los medicamentos relacionados con la detección y el diagnóstico de genes mediante CRISPR son aplicables a un gran sector de la población. Estos costes recaen sobre los pacientes, por lo que se espera que los elevados costes actuales muestren una tendencia descendente en el futuro.
Por ejemplo,
- En julio de 2021, según Integrated DNA Technologies, Inc., el primer ensayo de diagnóstico basado en CRISPR disponible comercialmente para el SARS-CoV-2 que incluye LAMP de transcripción inversa (RT-LAMP) como preamplificación está actualmente disponible a USD 30,15 por reacción.
El informe de mercado de detección y diagnóstico de genes CRISPR proporciona detalles de nuevos desarrollos recientes, regulaciones comerciales, análisis de importación y exportación, análisis de producción, optimización de la cadena de valor, participación de mercado, impacto de los actores del mercado nacional y localizado, analiza oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, análisis estratégico del crecimiento del mercado, tamaño del mercado, crecimientos del mercado de categorías, nichos de aplicación y dominio, aprobaciones de productos, lanzamientos de productos, expansiones geográficas, innovaciones tecnológicas en el mercado. Para obtener más información sobre el mercado de detección y diagnóstico de genes CRISPR, comuníquese con Data Bridge Market Research para obtener un informe de analista, nuestro equipo lo ayudará a tomar una decisión de mercado informada para lograr el crecimiento del mercado.
Análisis de la epidemiología de los pacientes
Según un estudio de Globocan, en 2020, el cáncer de mama tuvo una alta incidencia de casos, alrededor del 11,7%, seguido del cáncer de pulmón con un 11,40%, el cáncer colorrectal con un 10,00% y el cáncer de cuello uterino y esófago con un menor número de casos incidentes.
El mercado de detección y diagnóstico de genes CRISPR también le proporciona un análisis detallado del mercado para el análisis de pacientes, el pronóstico y las curas. La prevalencia, la incidencia, la mortalidad y las tasas de adherencia son algunas de las variables de datos que están disponibles en el informe. Se analizan los análisis de impacto directo o indirecto de la epidemiología en el crecimiento del mercado para crear un modelo estadístico multivariado de cohorte más sólido para pronosticar el mercado en el período de crecimiento.
Impacto de la COVID-19 en el mercado de detección y diagnóstico de genes mediante CRISPR
El COVID-19 ha afectado negativamente al mercado. Los confinamientos y el aislamiento durante las pandemias complican el diagnóstico, la gestión y el tratamiento. La falta de acceso a los centros sanitarios para la administración rutinaria y de medicamentos afectará aún más al mercado. El aislamiento social aumenta el estrés, la desesperación y el apoyo social, todo lo cual puede provocar una reducción de la adherencia a la medicación anticonvulsiva durante la pandemia.
Desarrollo reciente
- En agosto de 2020, SHERLOCK BIOSCIENCES anunció una colaboración con Dartmouth-Hitchcock Health para llevar a cabo el ensayo clínico del kit de diagnóstico SHERLOCK para el SARS-CoV-2. El kit recibió la aprobación de emergencia de la Autorización de uso de emergencia (EUA) de la Administración de Alimentos y Medicamentos de los Estados Unidos (FDA).
Alcance del mercado de diagnóstico y detección de genes mediante CRISPR en América del Norte
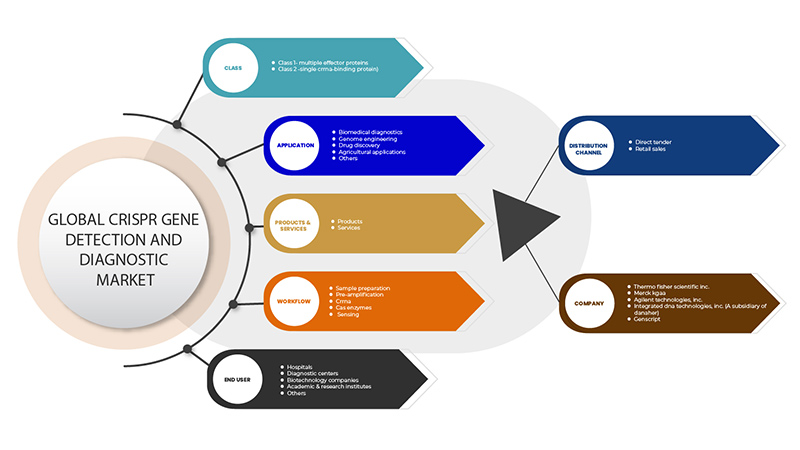
El mercado de detección y diagnóstico de genes mediante CRISPR está segmentado en base a seis segmentos: clase, productos y servicios, aplicación, flujo de trabajo, usuario final y canal de distribución. El crecimiento entre estos segmentos le ayudará a analizar los segmentos de crecimiento reducidos en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para ayudarlos a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Clase
- Clase 1: Proteínas efectoras múltiples
- Clase 2: proteína de unión a ARNcr simple
Sobre la base de la clase, el mercado de detección y diagnóstico de genes CRISPR se segmenta en clase 1: proteínas efectoras múltiples y clase 2: proteína de unión de ARNr único.
Productos y servicios
- Productos
- Servicios
Sobre la base de productos y servicios, el mercado de detección y diagnóstico de genes CRISPR se segmenta en productos y servicios.
Solicitud
- Diagnóstico biomédico
- Ingeniería Genómica
- Descubrimiento de fármacos
- Aplicaciones agrícolas
- Otros
Sobre la base de la aplicación, el mercado de detección y diagnóstico de genes CRISPR se segmenta en diagnóstico biomédico, ingeniería genómica, descubrimiento de fármacos, aplicaciones agrícolas y otros.
Flujo de trabajo
- Preparación de la muestra
- Preamplificación
- ARNcr
- Enzimas Cas
- Detección
Sobre la base del flujo de trabajo, el mercado de detección y diagnóstico de genes CRISPR se segmenta en preparación de muestras, preamplificación, CrRNA, enzimas Cas y detección.
Usuario final
- Hospitales
- Centros de diagnóstico
- Empresas de biotecnología
- Institutos académicos y de investigación
- Otros
Sobre la base del usuario final, el mercado de detección y diagnóstico de genes CRISPR está segmentado en hospitales, centros de diagnóstico, empresas de biotecnología, institutos académicos y de investigación y otros.
Canal de distribución
- Licitaciones directas
- Ventas al por menor
Sobre la base del canal de distribución, el mercado de detección y diagnóstico de genes CRISPR se segmenta en licitaciones directas y ventas minoristas.
Análisis y perspectivas regionales del mercado de detección y diagnóstico de genes mediante CRISPR
Se analiza el mercado de detección y diagnóstico de genes CRISPR de América del Norte y se proporcionan información y tendencias del tamaño del mercado por regiones, clase, productos y servicios, aplicación, flujo de trabajo, usuario final y canal de distribución como se menciona anteriormente.
Los países cubiertos en el informe del mercado de detección y diagnóstico de genes CRISPR son Estados Unidos, Canadá y México.
Estados Unidos domina el mercado de detección y diagnóstico de genes CRISPR debido al aumento del gasto en atención médica.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en las regulaciones en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, epidemiología de enfermedades y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de América del Norte y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de detección y diagnóstico de genes mediante CRISPR
El panorama competitivo del mercado de detección y diagnóstico de genes mediante CRISPR en América del Norte ofrece detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia en América del Norte, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y variedad de productos, y el dominio de las aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado de detección y diagnóstico de genes mediante CRISPR.
Algunos de los principales actores que operan en el mercado de detección y diagnóstico de genes CRISPR son GenScript, OriGene Technologies, Inc., Applied StemCell, GeneCopoeia, Inc., Agilent Technologies, Inc., Synthego, BioVision Inc., Hera Biolabs, Cellecta, Inc., New England Biolabs, 10x Genomics, addgene CasTag Biosciences, Merck KGaA, Integrated DNA Technologies, Inc. (una subsidiaria de Danaher), Thermo Fisher Scientific Inc. entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 CLASS SEGMENT LIFELINE CURVE
2.8 DBMR MARKET POSITION GRID
2.9 VENDOR SHARE ANALYSIS
2.1 MARKET END USER COVERAGE GRID
2.11 SECONDARY SOURCES
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL
4.2 PORTER'S FIVE FORCES MODEL
5 INTELLECTUAL PROPERTY LANDSCAPE (PATENT LANDSCAPE)
6 EPIDEMIOLOGY
7 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: REGULATORY SCENARIO
8 PIPELINE ANALYSIS FOR NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, OF CRISPR DIAGNOSTICS
9 MARKET OVERVIEW
9.1 DRIVERS
9.1.1 RISE IN PREVALENCE AND INCIDENCE OF CHRONIC DISEASES
9.1.2 RISE IN INVESTMENT IN RESEARCH AND DEVELOPMENT
9.1.3 AVAILABILITY OF FUNDING FOR CRISPR GENE DIAGNOSTICS
9.1.4 RISE IN GMP-CERTIFICATION APPROVALS FOR CRISPR GENE DIAGNOSTIC
9.1.5 RISE IN CLINICAL TRIALS FOR CRISPR BASED DIAGNOSTICS
9.2 RESTRAINTS
9.2.1 RISE IN COST OF CRISPR BASED DIAGNOSTICS
9.2.2 RISKS FACED WHILE USING CRISPR DIAGNOSIS
9.2.3 ETHICAL CONCERNS RELATED TO CRISPR GENE DETECTION AND DIAGNOSTIC RESEARCH
9.2.4 AVAILABILITY OF ALTERNATIVES
9.3 OPPORTUNITIES
9.3.1 STRATEGIC INITIATIVE BY MARKET PLAYERS
9.3.2 RISE IN HEALTHCARE EXPENDITURE
9.3.3 EMERGENCE OF TECHNOLOGICAL ADVANCEMENTS IN CRISPR BASED DIAGNOSTICS
9.4 CHALLENGES
9.4.1 LACK OF SKILLED PROFESSIONALS REQUIRED FOR CRISPR DIAGNOSTICS
9.4.2 STRINGENT REGULATIONS
10 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS
10.1 OVERVIEW
10.2 CLASS-2 SINGLE CRRNA-BINDING PROTEIN
10.2.1 BIOMEDICAL DIAGNOSTICS
10.2.2 AGRICULTURAL APPLICATIONS
10.2.3 GENOME ENGINEERING
10.2.4 DRUG DISCOVERY
10.2.5 OTHERS
10.3 CLASS-1 MULTIPLE EFFECTOR PROTEINS
11 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES
11.1 OVERVIEW
11.2 PRODUCTS
11.2.1 ASSAY KITS
11.2.1.1 SGRNA KIT
11.2.1.2 GENOMIC DETECTION KIT
11.2.1.3 OTHERS
11.2.2 PROTEINS
11.2.2.1 CAS9
11.2.2.2 CPF1
11.2.2.3 OTHERS
11.2.3 PLASMID AND VECTOR
11.2.4 LIBRARY
11.2.5 CONTROL KITS
11.2.6 DELIVERY SYSTEM PRODUCTS
11.2.7 DESIGN TOOLS
11.2.8 GENOMIC RNA
11.2.9 HDR BLOCKERS
11.2.9.1 AZIDOTHYMIDINE
11.2.9.2 TRIFLUOROTHYMIDINE
11.2.9.3 OTHERS
11.2.9.4 OTHERS
11.3 SERVICES
11.3.1 G-RNA DESIGN
11.3.2 CELL LINE ENGINEERING
11.3.3 MICROBIAL GENE EDITING
11.3.4 DNA SYNTHESIS
11.3.5 OTHERS
12 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION
12.1 OVERVIEW
12.2 BIOMEDICAL DIAGNOSTICS
12.2.1 CANCER
12.2.2 BLOOD DISORDERS
12.2.3 HEREDITARY DISORDERS
12.2.4 MUSCULAR DYSTROPHY
12.2.5 AIDS
12.2.6 NEURODEGENERATIVE CONDITION
12.2.7 OTHERS
12.3 AGRICULTURAL APPLICATIONS
12.4 GENOME ENGINEERING
12.4.1 CELL LINE ENGINEERING
12.4.2 HUMAN STEM CELLS
12.5 DRUG DISCOVERY
12.6 OTHERS
13 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW
13.1 OVERVIEW
13.2 CRRNA
13.3 CAS ENZYME
13.4 PRE-AMPLIFICATION
13.4.1 PCR
13.4.2 LAMP
13.4.3 RPA
13.5 SAMPLE PREPARATION
13.6 SENSING
13.6.1 FLUORESCENT PROBES
13.6.2 COLORIMETRIC
14 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER
14.1 OVERVIEW
14.2 BIOTECHNOLOGY COMPANIES
14.3 ACADEMIC AND RESEARCH INSTITUTES
14.4 DIAGNOSTIC CENTERS
14.5 HOSPITALS
14.6 OTHERS
15 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL
15.1 OVERVIEW
15.2 DIRECT TENDER
15.3 RETAIL SALES
16 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION
16.1 NORTH AMERICA
16.1.1 U.S.
16.1.2 CANADA
16.1.3 MEXICO
17 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY LANDSCAPE
17.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
18 SWOT ANALYSIS
19 COMPANY PROFILE
19.1 THERMO FISHER SCIENTIFIC INC.
19.1.1 COMPANY SNAPSHOT
19.1.2 REVENUE ANALYSIS
19.1.3 COMPANY SHARE ANALYSIS
19.1.4 PRODUCT PORTFOLIO
19.1.5 RECENT DEVELOPMENTS
19.2 MERCK KGA
19.2.1 COMPANY SNAPSHOT
19.2.2 REVENUE ANALYSIS
19.2.3 COMPANY SHARE ANALYSIS
19.2.4 PRODUCT PORTFOLIO
19.2.5 RECENT DEVELOPMENTS
19.3 AGILENT TECHNILOGIES, INC
19.3.1 COMPANY SNAPSHOT
19.3.2 REVENUE ANALYSIS
19.3.3 COMPANY SHARE ANALYSIS
19.3.4 PRODUCT PORTFOLIO
19.3.5 RECENT DEVELOPMENT
19.4 INTEGRATED DNA TECHNOLOGIES, INC. (A SUBSIDIARY OF DANAHER)
19.4.1 COMPANY SNAPSHOT
19.4.2 REVENUE ANALYSIS
19.4.3 COMPANY SHARE ANALYSIS
19.4.4 PRODUCT PORTFOLIO
19.4.5 RECENT DEVELOPMENTS
19.5 GENSCRIPT
19.5.1 COMPANY SNAPSHOT
19.5.2 REVENUE ANALYSIS
19.5.3 COMPANY SHARE ANALYSIS
19.5.4 PRODUCT PORTFOLIO
19.5.5 RECENT DEVELOPMENT
19.6 10 X GENOMICS
19.6.1 COMPANY SNAPSHOT
19.6.2 REVENUE ANALYSIS
19.6.3 PRODUCT PORTFOLIO
19.6.4 RECENT DEVELOPMENTS
19.7 APPLIED STEM CELL
19.7.1 COMPANY SNAPSHOT
19.7.2 PRODUCT PORTFOLIO
19.7.3 RECENT DEVELOPMENT
19.8 ADDGENE
19.8.1 COMPANY SNAPSHOT
19.8.2 PRODUCT PORTFOLIO
19.8.3 RECENT DEVELOPMENT
19.9 BIOVISION INC.
19.9.1 COMPANY SNAPSHOT
19.9.2 PRODUCT PORTFOLIO
19.9.3 RECENT DEVELOPMENT
19.1 CELLECTA, INC
19.10.1 COMPANY SNAPSHOT
19.10.2 PRODUCT PORTFOLIO
19.10.3 RECENT DEVELOPMENTS
19.11 CAS TAG BIOSCIENCES
19.11.1 COMPANY SNAPSHOT
19.11.2 PRODUCT PORTFOLIO
19.11.3 RECENT DEVELOPMENT
19.12 GENECOPOEIA, INC.
19.12.1 COMPANY SNAPSHOT
19.12.2 PRODUCT PORTFOLIO
19.12.3 RECENT DEVELOPMENT
19.13 HORIZON DISCOVERY LTD
19.13.1 COMPANY SNAPSHOT
19.13.2 PRODUCT PORTFOLIO
19.13.3 RECENT DEVELOPMENTS
19.14 HERA BIOLABS
19.14.1 COMPANY SNAPSHOT
19.14.2 PRODUCT PORTFOLIO
19.14.3 RECENT DEVELOPMENT
19.15 NEW ENGLAND BIOLABS
19.15.1 COMPANY SNAPSHOT
19.15.2 PRODUCT PORTFOLIO
19.15.3 RECENT DEVELOPMENTS
19.16 ORIGENE TECHNOLOGIES, INC.
19.16.1 COMPANY SNAPSHOT
19.16.2 PRODUCT PORTFOLIO
19.16.3 RECENT DEVELOPMENT
19.17 SYNTHEGO
19.17.1 COMPANY SNAPSHOT
19.17.2 PRODUCT PORTFOLIO
19.17.3 RECENT DEVELOPMENTS
19.18 TAKARA BIO INC.
19.18.1 COMPANY SNAPSHOT
19.18.2 REVENUE ANALYSIS
19.18.3 PRODUCT PORTFOLIO
19.18.4 RECENT DEVELOPMENT
19.19 TOOLGEN, INC.
19.19.1 COMPANY SNAPSHOT
19.19.2 PRODUCT PORTFOLIO
19.19.3 RECENT DEVELOPMENT
20 QUESTIONNAIRE
21 RELATED REPORTS
Lista de Tablas
TABLE 1 PIPELINE ANALYSIS FOR NORTH AMERICA CRISPR GENE THERAPEUTICS
TABLE 2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA CLASS-1 MULTIPLE EFFECTOR PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA AGRICULTURAL APPLICATIONS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA DRUG DISCOVERYIN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA CRRNA IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 NORTH AMERICA CAS ENZYME IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 NORTH AMERICA PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 NORTH AMERICA PRE-AMPLIFICATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 27 NORTH AMERICA SAMPLE PREPARATION IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 NORTH AMERICA SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 NORTH AMERICA SENSING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 30 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 31 NORTH AMERICA BIOTECHNOLOGY COMPANIES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 32 NORTH AMERICA ACADEMIC AND RESEARCH INSTITUTES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 NORTH AMERICA DIAGNOSTIC CENTERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 NORTH AMERICA HOSPITALS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 35 NORTH AMERICA OTHERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 36 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 37 NORTH AMERICA DIRECT TENDER IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 38 NORTH AMERICA RETAIL SALES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 39 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 40 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 41 NORTH AMERICA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 42 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 43 NORTH AMERICA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 44 NORTH AMERICA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 45 NORTH AMERICA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 46 NORTH AMERICA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 47 NORTH AMERICA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 48 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 49 NORTH AMERICA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 50 NORTH AMERICA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 51 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 52 NORTH AMERICA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 53 NORTH AMERICA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 54 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 55 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 56 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 57 U.S. CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 58 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 59 U.S. PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 60 U.S. ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 61 U.S. HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 62 U.S. PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 63 U.S. SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 64 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 65 U.S. GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 66 U.S. BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 67 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 68 U.S. PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 69 U.S. SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 70 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 71 U.S. CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 72 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 73 CANADA CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 74 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 75 CANADA PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 76 CANADA ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 77 CANADA HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 78 CANADA PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 79 CANADA SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 80 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 81 CANADA GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 82 CANADA BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 84 CANADA PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 85 CANADA SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 86 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 87 CANADA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 88 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY CLASS, 2020-2029 (USD MILLION)
TABLE 89 MEXICO CLASS-2 SINGLE CRRNA-BINDING PROTEIN IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 90 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCTS AND SERVICES, 2020-2029 (USD MILLION)
TABLE 91 MEXICO PRODUCTS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 92 MEXICO ASSAY KITS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 93 MEXICO HDR BLOCKERS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 94 MEXICO PROTEINS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY PRODUCT TYPE, 2020-2029 (USD MILLION)
TABLE 95 MEXICO SERVICES IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 96 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 97 MEXICO GENOME ENGINEERING IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 98 MEXICO BIOMEDICAL DIAGNOSTICS IN CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 99 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 100 MEXICO PRE-AMPLIFICATION IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 101 MEXICO SENSING IN GENE DETECTION AND DIAGNOSTIC MARKET, BY WORKFLOW, 2020-2029 (USD MILLION)
TABLE 102 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 103 MEXICO CRISPR GENE DETECTION AND DIAGNOSTIC MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: DBMR POSITION GRID
FIGURE 8 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: VENDOR SHARE ANALYSIS
FIGURE 9 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: END USER COVERAGE GRID
FIGURE 10 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SEGMENTATION
FIGURE 11 NORTH AMERICA IS ANTICIPATED TO DOMINATE THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET AND ASIA-PACIFIC IS ESTIMATED TO BE GROWING WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 INCREASED INCIDENCE OF CHRONIC DISEASES, RISE IN TECHNOLOGICAL ADVANCEMENTS IN CRISPR DIAGNOSTICS, AND GOVERNMENT FUNDING FOR THE DEVELOPMENT OF CRISPR DETECTION KITS ARE EXPECTED TO DRIVE THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 TO 2029
FIGURE 13 CLASS SEGMENT IS EXPECTED TO HAVE THE LARGEST SHARE OF THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET FROM 2022 & 2029
FIGURE 14 NORTH AMERICA CRISPR GENE PATENT SCENARIO, BY APPLICATION
FIGURE 15 CRISPR PATENT LANDSCAPE AND NUMBER OF APPLICATIONS OF NEW PATENT FAMILIES FILED WORLDWIDE, 2001 TO 2019
FIGURE 16 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET
FIGURE 17 INCIDENCE OF VARIOUS TYPES OF CANCER IN 2020
FIGURE 18 PREVALENCE OF HUNTINGTON’S DISEASE IN 2019
FIGURE 19 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2021
FIGURE 20 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, 2022-2029 (USD MILLION)
FIGURE 21 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, CAGR (2022-2029)
FIGURE 22 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS, LIFELINE CURVE
FIGURE 23 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2021
FIGURE 24 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, 2022-2029 (USD MILLION)
FIGURE 25 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, CAGR (2022-2029)
FIGURE 26 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY PRODUCTS AND SERVICES, LIFELINE CURVE
FIGURE 27 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2021
FIGURE 28 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, 2022-2029 (USD MILLION)
FIGURE 29 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 30 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 31 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2021
FIGURE 32 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, 2022-2029 (USD MILLION)
FIGURE 33 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, CAGR (2022-2029)
FIGURE 34 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY WORKFLOW, LIFELINE CURVE
FIGURE 35 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2021
FIGURE 36 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, 2022-2029 (USD MILLION)
FIGURE 37 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, CAGR (2022-2029)
FIGURE 38 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY END USER, LIFELINE CURVE
FIGURE 39 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 40 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, 2022-2029 (USD MILLION)
FIGURE 41 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 42 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 43 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: SNAPSHOT (2021)
FIGURE 44 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021)
FIGURE 45 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2022 & 2029)
FIGURE 46 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY COUNTRY (2021 & 2029)
FIGURE 47 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: BY CLASS (2022-2029)
FIGURE 48 NORTH AMERICA CRISPR GENE DETECTION AND DIAGNOSTIC MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.