Mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte, por tecnología (métodos de detección de CTC, métodos de enriquecimiento de CTC, selección positiva ex vivo, tecnologías basadas en moléculas (ARN), ensayo de invasión celular in vitro funcional, modelos de xenotrasplante, microchips, microcanal de espiral simple, selección negativa y tecnologías inmunocitoquímicas), aplicación (investigación de células madre cancerosas, anomalías cromosómicas múltiples y otras), usuario final (institutos de investigación y académicos, laboratorios de referencia y hospitales y laboratorios médicos), país (EE. UU., Canadá, México) - Tendencias y pronóstico de la industria -2029
Análisis y perspectivas del mercado: mercado de biopsia líquida de células tumorales circulantes (CTC) en América del Norte
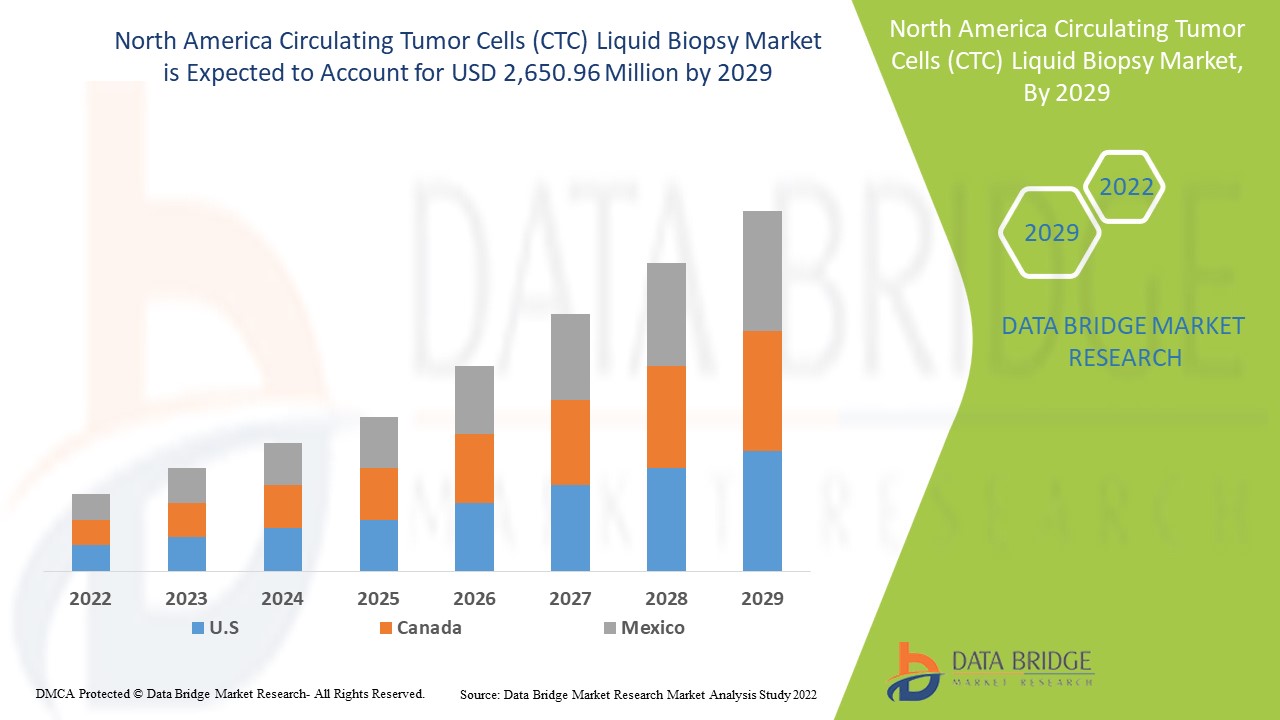
Se espera que el mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte gane crecimiento de mercado en el período de pronóstico de 2022 a 2029. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 25,9% en el período de pronóstico de 2022 a 2029 y se espera que alcance los USD 2650,96 millones para 2029 desde USD 442,64 millones en 2021. Es probable que la alta prevalencia de enfermedades crónicas y el aumento de las actividades de I+D para su aplicación efectiva sean los principales impulsores que impulsen la demanda del mercado en el período de pronóstico.
La biopsia líquida es un análisis de sangre no invasivo que detecta las células tumorales circulantes y los fragmentos de ADN tumoral que se liberan en la sangre desde los tumores primarios y las metástasis. Es una alternativa sencilla y precisa a la biopsia quirúrgica, que permite al cirujano detectar el cáncer en una etapa muy temprana.
Las células tumorales circulantes son un subconjunto poco común de células que funcionan como semilla de metástasis. Se encuentran en la sangre de pacientes que han desarrollado tumores sólidos. El análisis de células tumorales circulantes permite la detección y cuantificación de células tumorales en la sangre de pacientes con cáncer. Los diversos tipos de fenotipos biológicos de las células tumorales circulantes (CTC) incluyen células madre o mixtas, mesenquimales o epiteliales. Estos fenotipos están presentes en la sangre en una cantidad muy pequeña. Debido a esto, su detección necesita una fase de aislamiento-enriquecimiento. Después de eso, una segunda fase de detección.
La creciente demanda de biopsia líquida de células tumorales circulantes (CTC) debido a su eficacia y la alta prevalencia del cáncer son los principales impulsores de la demanda del mercado de biopsia líquida de células tumorales circulantes (CTC) en el período de pronóstico. Sin embargo, el escenario regulatorio y de reembolso poco claro y la escasez de personal calificado están restringiendo el crecimiento del mercado de biopsia líquida de células tumorales circulantes (CTC) en el período de pronóstico.
El informe de mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte proporciona detalles de la participación de mercado, los nuevos desarrollos y el impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de bolsas de ingresos emergentes, cambios en las regulaciones del mercado, aprobaciones de productos, decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado, contáctenos para obtener un resumen de analista. Nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado.
 Alcance y tamaño del mercado de biopsia líquida de células tumorales circulantes (CTC) en América del Norte
Alcance y tamaño del mercado de biopsia líquida de células tumorales circulantes (CTC) en América del Norte
El mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte se clasifica en tres segmentos notables que se basan en la tecnología, la aplicación y el usuario final. El crecimiento entre segmentos lo ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
- Sobre la base de la tecnología, el mercado de biopsia líquida de células tumorales circulantes (CTC) está segmentado en métodos de detección de CTC, métodos de enriquecimiento de CTC, selección positiva ex vivo, tecnologías basadas en moléculas (ARN), ensayo de invasión celular funcional in vitro, métodos de xenotrasplante, microchips, microcanal de espiral simple, selección negativa y tecnologías inmunocitoquímicas. En 2021, se espera que el segmento de métodos de detección de CTC domine el mercado debido al uso creciente de esta tecnología en los centros académicos y de investigación para las pruebas de biopsia líquida.
- En función de la aplicación, el mercado de biopsia líquida de células tumorales circulantes (CTC) se segmenta en investigación de células madre cancerosas, anomalías cromosómicas múltiples y otras. En 2021, se espera que el segmento de investigación de células madre cancerosas domine el mercado debido a la creciente demanda de diagnóstico y tratamiento tempranos del cáncer.
- En función del usuario final, el mercado de biopsia líquida de células tumorales circulantes (CTC) se segmenta en institutos de investigación y académicos, laboratorios de referencia y hospitales y laboratorios médicos. En 2021, se espera que el segmento de institutos académicos y de investigación domine el mercado debido a que los institutos de investigación y académicos desempeñan un papel esencial para acelerar la investigación y el desarrollo en las diversas áreas terapéuticas relacionadas con las biopsias líquidas.
Análisis a nivel de país del mercado de biopsia líquida de células tumorales circulantes (CTC) en América del Norte
Se analiza el mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte y se proporciona información sobre el tamaño del mercado por país, tecnología, aplicación y usuario final.
Los países cubiertos en el informe del mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte son EE. UU., Canadá y México.
Estados Unidos está dominando la biopsia líquida de células tumorales circulantes (CTC) en la región de América del Norte debido al aumento en el número de pacientes con cáncer y la presencia de actores importantes en el mercado.
La sección de países del informe también proporciona factores de impacto individuales en el mercado y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de América del Norte y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
La presencia de tecnología avanzada y las iniciativas estratégicas adoptadas por los actores están creando nuevas oportunidades en el mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte
El mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte también le proporciona un análisis detallado del mercado para el crecimiento de cada país en una industria en particular con ventas de productos, el impacto del avance en el mercado y los cambios en los escenarios regulatorios con su apoyo al mercado de instrumentos dentales. Los datos están disponibles para el período histórico de 2011 a 2020.
Análisis del panorama competitivo y de la cuota de mercado de biopsia líquida de células tumorales circulantes (CTC) en América del Norte
El panorama competitivo del mercado de biopsia líquida de células tumorales circulantes (CTC) de América del Norte proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y la extensión de los productos, el dominio de las aplicaciones y la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa relacionado con el mercado de biopsia líquida de células tumorales circulantes (CTC).
Las principales empresas que ofrecen biopsia líquida de células tumorales circulantes (CTC) son Eurofins Genomics (una subsidiaria de Eurofins Scientific), MDx Health, Guardant Health, IMMUCOR, Thermo Fisher Scientific, Inc., Menarini Silicon Biosystems, QIAGEN, Exact Sciences Corporation, Myriad Genetics, Inc., LungLife AI, Inc., Bio-Rad Laboratories, Inc., Illumina, Inc., Natera Inc., ExoDx (una subsidiaria de Bio-Techne Corporation), Biocept, Inc., F. Hoffman-La Roche Ltd., FOUNDATION MEDICINE, INC., Lucence Health, Inc., Inivata Ltd, Biolidics Limited, Vortex Biosciences, entre otras.
Por ejemplo,
- En noviembre de 2020, Lucence Health, Inc. anunció el lanzamiento del primer estudio de detección temprana que evalúa el uso de su tecnología de biopsia líquida. Esto ayudará a la empresa a avanzar en el lanzamiento de nuevos productos.
La colaboración, las empresas conjuntas y otras estrategias del actor del mercado están mejorando el mercado de la empresa en el mercado de biopsia líquida de células tumorales circulantes (CTC), lo que también proporciona el beneficio para que la organización mejore su oferta para el mercado de biopsia líquida de células tumorales circulantes (CTC).
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 TECHNOLOGY LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTERS FIVE FORCES
5 REGULATIONS: NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
5.1 ROLE OF FDA
5.2 ROLE OF CDC AND HCFA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 HIGH PREVALENCE OF CANCER
6.1.2 ADVANTAGES OF LIQUID BIOPSY OVER SURGICAL BIOPSY
6.1.3 GOVERNMENT INITIATIVES TO SPREAD AWARENESS ABOUT EARLY DIAGNOSIS OF CANCER
6.1.4 RISE IN FDA APPROVAL
6.1.5 HEALTHCARE REIMBURSEMENT FOR LIQUID BIOPSY
6.2 RESTRAINTS
6.2.1 DOWNSIDES OF LIQUID BIOPSY
6.2.2 RAPID DEVELOPMENT OF ULTRASENSITIVE IMAGING TECHNOLOGIES SUCH AS MAGNETIC RESONANCE IMAGING
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.3.2 RISE IN HEALTHCARE EXPENDITURE AND DISPOSABLE INCOME
6.3.3 INCREASE IN RESEARCH AND DEVELOPMENT ACTIVITIES
6.3.4 HUGE MARKET POTENTIAL IN DEVELOPING COUNTRIES
6.4 CHALLENGES
6.4.1 SHORTAGE OF SKILLED PERSONNEL
6.4.2 LACK OF ACCESSIBILITY
7 COVID-19 IMPACT ON NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
7.1 IMPACT ON PRICE
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY
7.4 KEY INITIATIVES BY MARKET PLAYER DURING COVID 19
7.5 CONCLUSION:
8 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY
8.1 OVERVIEW
8.2 CTC DETECTION METHODS
8.3 CTC ENRICHMENT METHODS
8.4 EX VIVO POSITIVE SELECTION
8.5 MOLECULAR (RNA)-BASED TECHNOLOGIES
8.6 FUNCTIONAL IN VITRO CELL INVASION ASSAY
8.7 XENOTRANSPLANTATION MODELS
8.8 MICROCHIPS
8.9 SINGLE SPIRAL MICROCHANNEL
8.1 NEGATIVE SELECTION
8.11 IMMUNOCYTOCHEMICAL TECHNOLOGIES
9 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION
9.1 OVERVIEW
9.2 CANCER STEM CELL RESEARCH
9.3 MULTIPLE CHROMOSOME ABNORMALITIES
9.4 OTHERS
10 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER
10.1 OVERVIEW
10.2 RESEARCH & ACADEMIC INSTITUTES
10.3 REFERENCE LABORATORIES
10.4 HOSPITALS AND PHYSICIAN LABORATORIES
11 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION
11.1 NORTH AMERICA
11.1.1 U.S.
11.1.2 CANADA
11.1.3 MEXICO
12 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: COMPANY LANDSCAPE
12.1 COMPANY SHARE ANALYSIS: NORTH AMERICA
13 SWOT ANALYSIS
14 COMPANY PROFILE
14.1 GUARDANT HEALTH
14.1.1 COMPANY SNAPSHOT
14.1.2 REVENUE ANALYSIS
14.1.3 COMPANY SHARE ANALYSIS
14.1.4 PRODUCT PORTFOLIO
14.1.5 RECENT DEVELOPMENTS
14.2 EUROFINS GENOMICS (A SUBSIDIARY OF EUROFINS SCIENTIFIC)
14.2.1 COMPANY SNAPSHOT
14.2.2 REVENUE ANALYSIS
14.2.3 COMPANY SHARE ANALYSIS
14.2.4 PRODUCT PORTFOLIO
14.2.5 RECENT DEVELOPMENTS
14.3 FOUNDATION MEDICINE, INC.
14.3.1 COMPANY SNAPSHOT
14.3.2 COMPANY SHARE ANALYSIS
14.3.3 PRODUCT PORTFOLIO
14.3.4 RECENT DEVELOPMENTS
14.4 ILLUMINA, INC.
14.4.1 COMPANY SNAPSHOT
14.4.2 REVENUE ANALYSIS
14.4.3 COMPANY SHARE ANALYSIS
14.4.4 PRODUCT PORTFOLIO
14.4.5 RECENT DEVELOPMENT
14.5 NATERA, INC.
14.5.1 COMPANY SNAPSHOT
14.5.2 REVENUE ANALYSIS
14.5.3 COMPANY SHARE ANALYSIS
14.5.4 PRODUCT PORTFOLIO
14.5.5 RECENT DEVELOPMENT
14.6 BIO-RAD LABORATORIES, INC.
14.6.1 COMPANY SNAPSHOT
14.6.2 REVENUE ANALYSIS
14.6.3 PRODUCT PORTFOLIO
14.6.4 RECENT DEVELOPMENT
14.7 QIAGEN
14.7.1 COMPANY SNAPSHOT
14.7.2 REVENUE ANALYSIS
14.7.3 PRODUCT PORTFOLIO
14.7.4 RECENT DEVELOPMENTS
14.8 THERMO FISHER SCIENTIFIC INC.
14.8.1 COMPANY SNAPSHOT
14.8.2 REVENUE ANALYSIS
14.8.3 PRODUCT PORTFOLIO
14.8.4 RECENT DEVELOPMENTS
14.9 BIOCEPT, INC.
14.9.1 COMPANY SNAPSHOT
14.9.2 REVENUE ANALYSIS
14.9.3 PRODUCT PORTFOLIO
14.9.4 RECENT DEVELOPMENT
14.1 BIOLIDICS LIMITED
14.10.1 COMPANY SNAPSHOT
14.10.2 REVENUE ANALYSIS
14.10.3 PRODUCT PORTFOLIO
14.10.4 RECENT DEVELOPMENTS
14.11 EXACT SCIENCES CORPORATION
14.11.1 COMPANY SNAPSHOT
14.11.2 REVENUE ANALYSIS
14.11.3 PRODUCT PORTFOLIO
14.11.4 RECENT DEVELOPMENT
14.12 EXODX (A SUBSIDIARY OF BIO-TECHNE CORPORATION)
14.12.1 COMPANY SNAPSHOT
14.12.2 REVENUE ANALYSIS
14.12.3 PRODUCT PORTFOLIO
14.12.4 RECENT DEVELOPMENTS
14.13 F. HOFFMANN-LA ROCHE LTD
14.13.1 COMPANY SNAPSHOT
14.13.2 RECENT FINANCIALS
14.13.3 PRODUCT PORTFOLIO
14.13.4 RECENT DEVELOPMENTS
14.14 IMMUCOR
14.14.1 COMPANY SNAPSHOT
14.14.2 PRODUCT PORTFOLIO
14.14.3 RECENT DEVELOPMENTS
14.15 INIVATA LTD
14.15.1 COMPANY SNAPSHOT
14.15.2 PRODUCT PORTFOLIO
14.15.3 RECENT DEVELOPMENTS
14.16 LUCENCE HEALTH, INC.
14.16.1 COMPANY SNAPSHOT
14.16.2 PRODUCT PORTFOLIO
14.16.3 RECENT DEVELOPMENTS
14.17 LUNGLIFE AI, INC.
14.17.1 COMPANY SNAPSHOT
14.17.2 PRODUCT PORTFOLIO
14.17.3 RECENT DEVELOPMENTS
14.18 MDXHEALTH
14.18.1 COMPANY SNAPSHOT
14.18.2 REVENUE ANALYSIS
14.18.3 PRODUCT PORTFOLIO
14.18.4 RECENT DEVELOPMENT
14.19 MENARINI SILICON BIOSYSTEMS
14.19.1 COMPANY SNAPSHOT
14.19.2 PRODUCT PORTFOLIO
14.19.3 RECENT DEVELOPMENTS
14.2 MYRIAD GENETICS, INC.
14.20.1 COMPANY SNAPSHOT
14.20.2 REVENUE ANALYSIS
14.20.3 PRODUCT PORTFOLIO
14.20.4 RECENT DEVELOPMENTS
14.21 VORTEX BIOSCIENCES
14.21.1 COMPANY SNAPSHOT
14.21.2 PRODUCT PORTFOLIO
14.21.3 RECENT DEVELOPMENTS
15 QUESTIONNAIRE
16 RELATED REPORTS
Lista de Tablas
TABLE 1 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 2 NORTH AMERICA CTC DETECTION METHODS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 3 NORTH AMERICA CTC ENRICHMENT METHODS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 NORTH AMERICA EX VIVO POSITIVE SELECTION IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 5 NORTH AMERICA MOLECULAR (RNA)-BASED TECHNOLOGIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 NORTH AMERICA FUNCTIONAL IN VITRO CELL INVASION ASSAY IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 7 NORTH AMERICA XENOTRANSPLANTATION MODELS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 8 NORTH AMERICA MICROCHIPS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 NORTH AMERICA SINGLE SPIRAL MICROCHANNEL IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 NORTH AMERICA NEGATIVE SELECTION IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 11 NORTH AMERICA IMMUNOCYTOCHEMICAL TECHNOLOGIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 13 NORTH AMERICA CANCER STEM CELL RESEARCH IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 NORTH AMERICA MULTIPLE CHROMOSOME ABNORMALITIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 NORTH AMERICA OTHERS IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 16 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 17 NORTH AMERICA RESEARCH & ACADEMIC INSTITUTES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 NORTH AMERICA REFERENCE LABORATORIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 NORTH AMERICA HOSPITALS AND PHYSICIAN LABORATORIES IN CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 21 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 22 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 23 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 24 U.S. CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 25 U.S. CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 26 U.S. CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 27 CANADA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 28 CANADA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 29 CANADA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 30 MEXICO CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY TECHNOLOGY, 2020-2029 (USD MILLION)
TABLE 31 MEXICO CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 32 MEXICO CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET, BY END USER, 2020-2029 (USD MILLION)
Lista de figuras
FIGURE 1 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: SEGMENTATION
FIGURE 2 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: DATA TRIANGULATION
FIGURE 3 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: DROC ANALYSIS
FIGURE 4 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET : NORTH AMERICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: DBMR MARKET POSITION GRID
FIGURE 8 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: SEGMENTATION
FIGURE 11 THE HIGH PREVALENCE OF CANCER IS EXPECTED TO DRIVE THE NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 CTC DETECTION METHODS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET
FIGURE 15 PREVALENCE OF BREAST CANCER IN EUROPE, INDIA, AND IN THE U.S.
FIGURE 16 PREVALENCE OF LUNG CANCER IN VARIOUS COUNTRIES, WITH HUNGARY BEING THE HIGHEST PREVALENCE RATE IN WOMEN AND MEN
FIGURE 17 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, 2021
FIGURE 18 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, 2020-2029 (USD MILLION)
FIGURE 19 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, CAGR (2022-2029)
FIGURE 20 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY, LIFELINE CURVE
FIGURE 21 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, 2021
FIGURE 22 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, 2020-2029 (USD MILLION)
FIGURE 23 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 24 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 25 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, 2021
FIGURE 26 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 27 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, CAGR (2022-2029)
FIGURE 28 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY END USER, LIFELINE CURVE
FIGURE 29 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: SNAPSHOT (2021)
FIGURE 30 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY COUNTRY (2021)
FIGURE 31 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY COUNTRY (2022 & 2029)
FIGURE 32 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY COUNTRY (2021 & 2029)
FIGURE 33 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: BY TECHNOLOGY (2022-2029)
FIGURE 34 NORTH AMERICA CIRCULATING TUMOR CELLS (CTC) LIQUID BIOPSY MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.