Middle East and Africa e-Clinical Solutions Market, By Product (Electronic Data Capture and Clinical Trial Data Management Systems, Clinical Trial Management Systems, Clinical Analytics Platforms, Care Coordination Medical Records (CCMR), Randomization and Trial Supply Management, Clinical Data Integration Platforms, Electronic Clinical Outcome Assessment Solutions, Safety Solutions, Electronic Trial Master File Systems, Regulatory Information Management Solutions, and Others), Delivery Mode (Web- hosted (On-Demand) Solutions, Licensed Enterprise (On-Premises) Solutions and Cloud-Based (SAAS) Solutions), Clinical Trial Phase (Phase I, Phase II, Phase III, and Phase IV), Organization Size (Small & Medium and Large), User Device (Desktop, Tablet, Handheld PDA Device, Smart Phone, and Others), End User (Pharmaceutical and Biopharmaceutical Companies, Contract Research Organizations, Consulting Service Companies, Medical Device Manufacturers, Hospitals, and Academic Research Institutes), Industry Trends and Forecast to 2030.
Middle East and Africa e-Clinical Solutions Market Analysis and Size
The Middle East and Africa e-clinical solutions market is fragmented, as it consists of many global players such as Oracle, IQVIA Inc., Dassault Systemes, and Clario among others. The presence of these companies produces competitive prices for systems services and software across the region. Due to the presence of these players at regional and international levels, suppliers and manufacturers offer products with different specifications and characteristics in all budgets. The growing adoption of electronic data capture (EDC) systems and cloud-based is driving the market growth. Additionally, the growing focus on patient-centric clinical trials is expected to drive market growth.
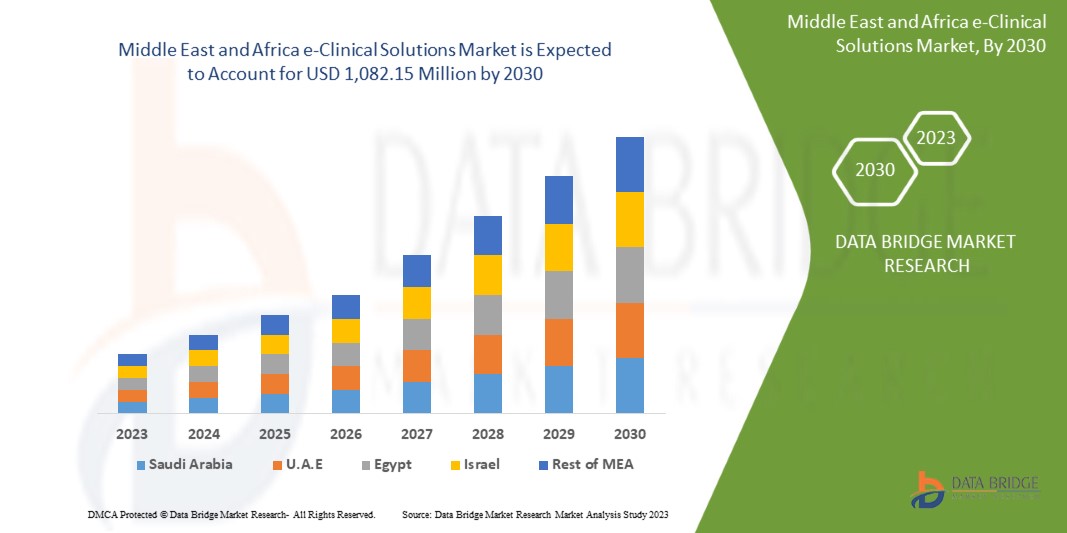
Data Bridge Market Research analyzes that the Middle East and Africa e-clinical solutions market is expected to reach a value of USD 1,082.15 million by 2030, at a CAGR of 11.9% during the forecast period. This market report also covers pricing analysis and technological advancements in depth.
|
Report Metric |
Details |
|
Forecast Period |
2023 to 2030 |
|
Base Year |
2022 |
|
Historic Years |
2021 (Customizable to 2015 – 2020) |
|
Quantitative Units |
Revenue in USD Million, Volumes in Units, and Pricing in USD |
|
Segments Covered |
Por producto (sistemas de captura electrónica de datos y gestión de datos de ensayos clínicos, sistemas de gestión de ensayos clínicos, plataformas de análisis clínico, registros médicos de coordinación de atención (CCMR), gestión de suministro de ensayos y aleatorización, plataformas de integración de datos clínicos, soluciones de evaluación electrónica de resultados clínicos, soluciones de seguridad, sistemas de archivos maestros de ensayos electrónicos, soluciones de gestión de información regulatoria y otros), modo de entrega (soluciones alojadas en la web (a pedido), soluciones empresariales con licencia (locales) y soluciones basadas en la nube (SAAS)), fase de ensayo clínico (fase I, fase II, fase III y fase IV), tamaño de la organización (pequeña, mediana y grande), dispositivo de usuario (computadora de escritorio, tableta, dispositivo PDA portátil, teléfono inteligente y otros), usuario final (compañías farmacéuticas y biofarmacéuticas, organizaciones de investigación por contrato, empresas de servicios de consultoría, fabricantes de dispositivos médicos, hospitales e institutos de investigación académica) |
|
Países cubiertos |
Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Egipto, Israel y el resto de Oriente Medio y África |
|
Actores del mercado cubiertos |
Los siguientes son algunos de los proveedores de servicios de salud que operan en el país: Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC, RealTime Software Solutions LLC, Quretec, Research Manager, Datatrack Int. e IQVIA Inc.
|
Definición de mercado
El objetivo de las soluciones clínicas electrónicas es revolucionar el campo de la investigación clínica. La organización de gestión clínica está tratando de mejorar la eficacia, la eficiencia y la accesibilidad de los datos de investigación clínica para tratar a los pacientes más rápidamente. La idea detrás de la creación de las soluciones clínicas electrónicas era identificar los problemas con los datos de investigación clínica, solucionarlos y ofrecer soluciones creativas para hacer que los datos de los ensayos clínicos sean útiles y fáciles de obtener, así como para facilitar la estandarización, la elaboración de informes y el funcionamiento del ámbito de la investigación clínica. Se espera que la creciente adopción y el enfoque creciente en las soluciones y el software clínicos electrónicos impulsen el crecimiento del mercado.
Las soluciones clínicas electrónicas, como la captura electrónica de datos (EDC), los resultados informados por los pacientes de forma electrónica (ePRO), los sistemas de gestión de ensayos clínicos (CTMS) y los sistemas de archivo maestro de ensayos electrónicos (eTMF), son programas informáticos que se utilizan en la investigación clínica para gestionar los datos clínicos. Sin embargo, se espera que las estrictas normas y regulaciones para la aprobación de estudios de ensayos clínicos y de productos limiten el crecimiento del mercado.
Dinámica del mercado de soluciones clínicas electrónicas en Oriente Medio y África
En esta sección se aborda la comprensión de los factores impulsores, las oportunidades, las limitaciones y los desafíos del mercado. Todo esto se analiza en detalle a continuación:
CONDUCTORES
Aumentar las actividades de I+D y el gasto correspondiente
La investigación y el desarrollo son cruciales en todas las industrias, incluidas las ciencias biológicas y la atención médica. La industria de las ciencias biológicas lleva a cabo una amplia investigación y desarrollo para generar ingresos y, a menudo, produce resultados que incluyen salvar o mejorar la vida de un paciente. El desarrollo de medicamentos requiere pasar por ensayos clínicos para verificar el análisis de datos clínicos y revisar su estudio y los nuevos datos.
Las soluciones clínicas electrónicas se utilizan para la revisión integral de datos, la automatización del proceso de transformación de datos y el análisis para acelerar los plazos. Las empresas farmacéuticas y biofarmacéuticas realizan numerosos ensayos clínicos para completar con éxito el desarrollo de medicamentos o productos biológicos. Las empresas farmacéuticas y biofarmacéuticas han aumentado su enfoque en la investigación y el desarrollo, lo que ha aumentado la demanda de soluciones clínicas electrónicas.
Mejora de la infraestructura sanitaria y creación de laboratorios avanzados
La infraestructura es un pilar fundamental que sustenta el objetivo fundamental de promover mejores estándares de atención y bienestar para todos los pacientes, junto con una buena experiencia en el sistema de atención de salud. Al mismo tiempo, el sistema de atención de salud y el personal deben respaldar la promoción de la salud, la prevención y el autocuidado efectivos de toda la población.
La infraestructura debe integrar el hospital, como centro de atención aguda y hospitalaria, en el sistema de atención sanitaria más amplio. Debe facilitar los siete dominios de la experiencia de calidad del paciente, la eficacia, la eficiencia, la puntualidad, la seguridad, la equidad y la sostenibilidad. La infraestructura incluye el entorno construido y los elementos de apoyo, como el equipo, el acceso, la tecnología de la información (TI), los sistemas y procesos, las iniciativas de sostenibilidad y el personal. Esta mejora de la infraestructura y el personal sanitario ofrece más oportunidades para que las soluciones clínicas electrónicas crezcan y den mejores resultados.
OPORTUNIDAD
Aumentan las inversiones de los distintos gobiernos en ensayos clínicos
La financiación de los ensayos clínicos procede de diversas fuentes, entre ellas el gobierno, inversores comerciales, organizaciones sin ánimo de lucro, instituciones académicas y otras organizaciones de investigación. Históricamente, los Institutos Nacionales de Salud (NIH) han realizado las mayores inversiones gubernamentales en investigación fundamental para el desarrollo de fármacos. La agencia de investigación avanzada de defensa (DARPA) también ha contribuido a la etapa de descubrimiento al aceptar algunas iniciativas biológicas de riesgo relativamente alto. Los gobiernos estatales también están tomando cada vez más la iniciativa en esta área, en parte debido a la frustración del público con el largo proceso de descubrimiento. Por lo tanto, se espera que las iniciativas e inversiones gubernamentales para descubrir nuevos fármacos con estudios de prueba creen una oportunidad para el crecimiento del mercado.
RESTRICCIÓN / DESAFÍO
Cuestiones de seguridad y privacidad de los datos
Los sistemas de información se preocupan mucho por la privacidad y la seguridad. El acceso a la información sanitaria individual es posible gracias a la digitalización de los servicios sanitarios desde cualquier dispositivo electrónico con conexión a Internet en cualquier parte del mundo. Los usuarios normalmente no son conscientes de cómo se manejan sus datos. Los datos sanitarios en la nube han llamado la atención de los piratas informáticos, que atacan los sistemas para lanzar ataques y robar datos confidenciales a cambio de beneficios económicos.
Las violaciones de datos han sido comunes desde 2019; en 2021, se han pirateado más de 50 millones de registros médicos electrónicos y se prevé que el número de violaciones de seguridad aumente cada año. La creación de acuerdos de intercambio de datos entre jurisdicciones y el almacenamiento y la manipulación de los datos se ven obstaculizados significativamente por las preocupaciones sobre la privacidad personal y la confidencialidad de la información y la reciente promulgación de leyes de privacidad y confidencialidad en las provincias y territorios (excepcionalmente, registros de pacientes). Por lo tanto, es una restricción para el mercado de soluciones clínicas electrónicas de Oriente Medio y África y se espera que obstaculice el crecimiento del mercado.
Impacto posterior a la COVID-19 en el mercado de soluciones clínicas electrónicas en Oriente Medio y África
La COVID-19 afectó positivamente al mercado de soluciones clínicas electrónicas de Oriente Medio y África. Las restricciones impuestas por el confinamiento dieron lugar a la aparición de diversas oportunidades, como el desarrollo de fármacos, la instalación de aplicaciones de registros médicos electrónicos y de historias clínicas electrónicas, entre otras.
Sin embargo, se espera que el aumento del apoyo gubernamental y las técnicas avanzadas e innovadoras brinden oportunidades lucrativas para el crecimiento del mercado. Además, se espera que el aumento de las asociaciones, adquisiciones y colaboraciones entre los actores del mercado impulse aún más el crecimiento del mercado. Además, el crecimiento ha sido alto desde que se abrió el mercado después de COVID-19, y se espera que haya un crecimiento considerable en el sector. Los actores del mercado están realizando múltiples actividades para mejorar las técnicas de estudio de prueba. Con esto, las empresas traerán avances e innovación al mercado.
Acontecimientos recientes
- En abril de 2023, Medidata, filial de Dassault Systèmes, anunció que Lambda Therapeutics está implementando los productos clínicos basados en la nube de Medidata, Rave EDC, Rave RTSM y Rave Imaging, según un comunicado de Medidata, filial de Dassault Systèmes. La automatización y optimización de las operaciones de gestión de datos y la entrega segura de datos de mayor calidad para obtener información más rápidamente mejorarán aún más la productividad de los ensayos clínicos. Esto ha ayudado a la empresa a promover sus ofertas en todo el mundo.
- En marzo de 2023, Clario lanzó una herramienta de visualización de imágenes basada en la nube que ayuda a los patrocinadores y las CRO a ver las imágenes de sus ensayos clínicos. Anteriormente, varias organizaciones tenían que participar en el procedimiento de transferencia de imágenes para ver las fotos de un ensayo clínico. Esto complicó el proceso, que ya era riesgoso, y aumentó la posibilidad de demoras y errores. Esto ha ayudado a la empresa a hacer crecer su oferta de servicios.
Alcance del mercado de soluciones clínicas electrónicas en Oriente Medio y África
El mercado de soluciones clínicas electrónicas de Oriente Medio y África se divide en seis segmentos importantes según el producto, el modo de entrega, la fase de ensayo clínico, el tamaño de la organización, el dispositivo del usuario y el usuario final. El crecimiento entre estos segmentos le ayudará a analizar los segmentos de crecimiento escaso en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos para ayudarlos a tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
Producto
- Sistemas de captura electrónica de datos y gestión de datos clínicos
- Sistemas de gestión de ensayos clínicos
- Plataformas de análisis clínicos
- Registro médico de coordinación de atención (CCMR)
- Aleatorización y gestión de suministros para ensayos
- Plataformas de integración de datos clínicos
- Soluciones de evaluación electrónica de resultados clínicos
- Soluciones de seguridad
- Sistemas de archivos maestros de juicios electrónicos
- Soluciones de gestión de información regulatoria
- Otros
Según el producto, el mercado está segmentado en captura electrónica de datos y gestión de datos clínicos, sistemas de gestión de ensayos clínicos, plataformas de análisis clínico, registros médicos de coordinación de atención (CCMR), gestión de suministro de ensayos y aleatorización, plataformas de integración de datos clínicos, soluciones de evaluación electrónica de resultados clínicos, soluciones de seguridad, sistemas de archivos maestros de ensayos electrónicos, soluciones de gestión de información regulatoria y otros.
Modo de entrega
- Soluciones alojadas en la Web (a pedido)
- Soluciones empresariales con licencia (locales)
- Soluciones basadas en la nube (SAAS)
Según el modo de entrega, el mercado está segmentado en soluciones alojadas en la web (a pedido), soluciones empresariales con licencia (en las instalaciones) y soluciones basadas en la nube (SAAS).
Fase de ensayo clínico
- Fase I
- Fase II
- Fase III
- Fase IV
Según la fase del ensayo clínico, el mercado se segmenta en Fase I, Fase II, Fase III y Fase IV.
Tamaño de la organización
- Pequeña y mediana
- Grande
Según el tamaño de la organización, el mercado se segmenta en pequeñas y medianas, y grandes.
Dispositivo de usuario
- De oficina
- Tableta
- Dispositivo PDA portátil
- Teléfono inteligente
- Otros
Según el dispositivo del usuario, el mercado está segmentado en computadoras de escritorio, tabletas, dispositivos PDA portátiles, teléfonos inteligentes y otros.
Usuario final
- Empresas farmacéuticas y biofarmacéuticas
- Organizaciones de investigación por contrato
- Empresas de servicios de consultoría
- Fabricantes de dispositivos médicos
- Hospitales
- Institutos de investigación académica
Según el usuario final, el mercado está segmentado en empresas farmacéuticas y biofarmacéuticas, organizaciones de investigación por contrato, empresas de servicios de consultoría, fabricantes de dispositivos médicos, hospitales e institutos de investigación académica.
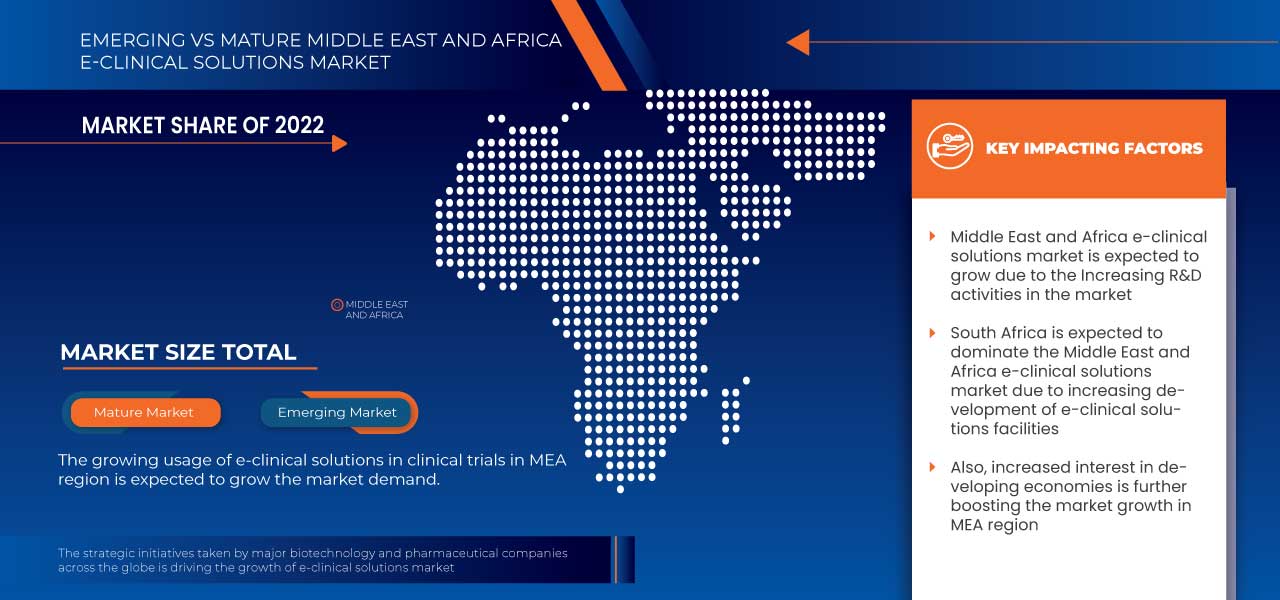
Análisis y perspectivas regionales del mercado de soluciones clínicas electrónicas en Oriente Medio y África
Se analiza el mercado de soluciones clínicas electrónicas de Oriente Medio y África, y se obtiene información sobre el tamaño del mercado en función del producto, el modo de entrega, la fase del ensayo clínico, el tamaño de la organización, el dispositivo del usuario y el usuario final.
Los países cubiertos en este informe de mercado son Sudáfrica, Arabia Saudita, Emiratos Árabes Unidos, Egipto, Israel y el resto de Medio Oriente y África.
Se espera que Sudáfrica domine la región de Medio Oriente y África debido a la creciente adopción de sistemas de captura electrónica de datos y soluciones basadas en la nube.
La sección de países del informe también proporciona factores individuales que impactan en el mercado y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas de Asia-Pacífico y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales y el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de las soluciones clínicas electrónicas en Oriente Medio y África
El panorama competitivo del mercado de soluciones clínicas electrónicas de Oriente Medio y África proporciona detalles sobre los competidores. Los detalles incluyen una descripción general de la empresa, información financiera, ingresos generados, potencial de mercado, nuevas iniciativas de mercado, presencia global, sitios e instalaciones de producción, capacidades de producción, fortalezas y debilidades de la empresa, lanzamiento de productos, amplitud y variedad de productos y dominio de aplicaciones. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de las empresas en relación con el mercado.
Algunos de los principales actores del mercado que operan en el mercado de soluciones clínicas electrónicas de Medio Oriente y África son Oracle, Signant Health, MaxisIT, Paraxel International Corporation, Dassault Systemes, Clario, Mednet, OpenClinica, LLC, 4G Clinical, Veeva Systems, Saama Technologies, LLC, Anju, Castor, Medrio, Inc., ArisGlobal, Merative, Advarra, eClinical Solutions, LLC, Y-Prime LLC y RealTime Software Solutions, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW
1.4 CURRENCY AND PRICING
1.5 LIMITATIONS
1.6 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 DBMR TRIPOD DATA VALIDATION MODEL
2.5 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.6 MULTIVARIATE MODELLING
2.7 MARKET END USER COVERAGE GRID
2.8 PRODUCT LIFELINE CURVE
2.9 DBMR MARKET POSITION GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER'S FIVE FORCES MODEL
4.2 ECOSYSTEMS ANALYSIS OF E CLINICAL SOLUTIONS
4.3 USE CASES
5 VALUE CHAIN ANALYSIS
6 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: REGULATIONS
7 MARKET OVERVIEW
7.1 DRIVERS
7.1.1 THE GROWING USAGE OF E-CLINICAL SOLUTIONS IN CLINICAL TRIALS
7.1.2 INCREASING R&D ACTIVITIES AND CORRESPONDING EXPENDITURE
7.1.3 STRATEGIC INITIATIVES TAKEN BY MAJOR BIOTECHNOLOGY AND PHARMACEUTICAL COMPANIES
7.1.4 IMPROVED HEALTHCARE INFRASTRUCTURE AND ESTABLISHMENT OF ADVANCED LABORATORIES
7.2 RESTRAINTS
7.2.1 DATA SAFETY AND PRIVACY ISSUES
7.2.2 LACK OF SKILLED PROFESSIONALS
7.2.3 LIMITED ADOPTION OF E-CLINICAL SOLUTIONS IN EMERGING COUNTRIES DUE TO BUDGETARY CONSTRAINTS AND POOR MANAGEMENT POLICIES
7.3 OPPORTUNITIES
7.3.1 RISING ADOPTION OF ELECTRONIC DATA CAPTURE (EDC) SYSTEMS AND CLOUD-BASED SOLUTIONS
7.3.2 INCREASING INVESTMENTS BY VARIOUS GOVERNMENTS IN CLINICAL TRIALS
7.3.3 GROWING FOCUS ON PATIENT-CENTRIC CLINICAL TRIALS
7.4 CHALLENGES
7.4.1 REGULATORY CHALLENGES ASSOCIATED WITH E-CLINICAL SOLUTIONS
7.4.2 HIGH IMPLEMENTATION COSTS
8 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT
8.1 OVERVIEW
8.2 ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS
8.3 RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT
8.4 CLINICAL TRIAL MANAGEMENT SYSTEMS
8.5 ELECTRONIC TRIAL MASTER FILE SYSTEMS
8.6 ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS
8.7 SAFETY SOLUTIONS
8.8 REGULATORY INFORMATION MANAGEMENT SOLUTIONS
8.9 CLINICAL DATA INTEGRATION PLATFORMS
8.1 CLINICAL ANALYTICS PLATFORMS
8.11 CARE COORDINATION MEDICAL RECORD (CCMR)
8.12 OTHERS
9 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE
9.1 OVERVIEW
9.2 WEB-HOSTED (ON-DEMAND) SOLUTIONS
9.3 CLOUD-BASED (SAAS) SOLUTIONS
9.4 LICENSED ENTERPRISE (ON-PREMISES) SOLUTIONS
10 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE
10.1 OVERVIEW
10.2 PHASE III
10.3 PHASE I
10.4 PHASE II
10.5 PHASE IV
11 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE
11.1 OVERVIEW
11.2 MEDIUM AND SMALL
11.3 LARGE
12 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE
12.1 OVERVIEW
12.2 DESKTOP
12.3 TABLET
12.4 SMART PHONE
12.5 HANDHELD PDA DEVICE
12.6 OTHERS
13 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER
13.1 OVERVIEW
13.2 CONTRACT RESEARCH ORGANIZATIONS
13.3 PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES
13.4 MEDICAL DEVICE MANUFACTURERS
13.5 HOSPITAL
13.6 CONSULTING SERVICE COMPANIES
13.7 ACADEMIC RESEARCH INSTITUTES
14 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY REGION
14.1 MIDDLE EAST AND AFRICA
14.1.1 SOUTH AFRICA
14.1.2 SAUDI ARABIA
14.1.3 U.A.E.
14.1.4 EGYPT
14.1.5 ISRAEL
14.1.6 REST OF MIDDLE EAST AND AFRICA
15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: MIDDLE EAST & AFRICA
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 ORACLE
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.2 DASSAULT SYSTEMES
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.3 CLARIO
17.3.1 COMPANY SNAPSHOT
17.3.2 COMPANY SHARE ANALYSIS
17.3.3 PRODUCT PORTFOLIO
17.3.4 RECENT DEVELOPMENT
17.4 VEEVA SYSTEMS
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENTS
17.5 ARISMIDDLE EAST & AFRICA
17.5.1 COMPANY SNAPSHOT
17.5.2 COMPANY SHARE ANALYSIS
17.5.3 PRODUCT PORTFOLIO
17.5.4 RECENT DEVELOPMENTS
17.6 4G CLINICAL
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENT
17.7 ADVARRA.
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENT
17.8 ANJU
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENTS
17.9 CASTOR.
17.9.1 COMPANY SNAPSHOT
17.9.2 PRODUCT PORTFOLIO
17.9.3 RECENT DEVELOPMENTS
17.1 DATATRAK INT.
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.11 ECLINICAL SOLUTIONS LLC.
17.11.1 COMPANY SNAPSHOT
17.11.2 PRODUCT PORTFOLIO
17.11.3 RECENT DEVELOPMENT
17.12 IQVIA INC
17.12.1 COMPANY SNAPSHOT
17.12.2 REVENUE ANALYSIS
17.12.3 PRODUCT PORTFOLIO
17.12.4 RECENT DEVELOPMENTS
17.13 MAXISIT
17.13.1 COMPANY SNAPSHOT
17.13.2 PRODUCT PORTFOLIO
17.13.3 RECENT DEVELOPMENT
17.14 MEDNET
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.15 MEDRIO, INC.
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENTS
17.16 MERATIVE
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENT
17.17 OPENCLINICA, LLC
17.17.1 COMPANY SNAPSHOT
17.17.2 PRODUCT PORTFOLIO
17.17.3 RECENT DEVELOPMENT
17.18 PAREXEL INTERNATIONAL CORPORATION
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENT
17.19 QURETEC
17.19.1 COMPANY SNAPSHOT
17.19.2 PRODUCT PORTFOLIO
17.19.3 RECENT DEVELOPMENT
17.2 REALTIME SOFTWARE SOLUTIONS, LLC
17.20.1 COMPANY SNAPSHOT
17.20.2 PRODUCT PORTFOLIO
17.20.3 RECENT DEVELOPMENT
17.21 RESEARCH MANAGER
17.21.1 COMPANY SNAPSHOT
17.21.2 PRODUCT PORTFOLIO
17.21.3 RECENT DEVELOPMENT
17.22 SAAMA TECHNOLOGIES, LLC
17.22.1 COMPANY SNAPSHOT
17.22.2 PRODUCT PORTFOLIO
17.22.3 RECENT DEVELOPMENT
17.23 SIGNANT HEALTH
17.23.1 COMPANY SNAPSHOT
17.23.2 PRODUCT PORTFOLIO
17.23.3 RECENT DEVELOPMENT
17.24 Y-PRIME, LLC.
17.24.1 COMPANY SNAPSHOT
17.24.2 PRODUCT PORTFOLIO
17.24.3 RECENT DEVELOPMENT
18 QUESTIONNAIRE
19 RELATED REPORTS
Lista de Tablas
TABLE 1 THE COSTS OF IMPLEMENTATION ARE AS FOLLOWS, ACCORDING TO SIMPLETRIALS:
TABLE 2 ACCORDING TO ECLINICAL WORKS – ECLINICAL OFFERS TWO ENTERPRISE PRICING PACKAGES:
TABLE 3 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 4 MIDDLE EAST & AFRICA ELECTRONIC DATA CAPTURE AND CLINICAL DATA MANAGEMENT SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 5 MIDDLE EAST & AFRICA RANDOMIZATION AND TRIAL SUPPLY MANAGEMENT IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 6 MIDDLE EAST & AFRICA CLINICAL TRIAL MANAGEMENT SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 7 MIDDLE EAST & AFRICA ELECTRONIC TRIAL MASTER FILE SYSTEMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 8 MIDDLE EAST & AFRICA ELECTRONIC CLINICAL OUTCOME ASSESSMENT SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 9 MIDDLE EAST & AFRICA SAFETY SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 10 MIDDLE EAST & AFRICA REGULATORY INFORMATION MANAGEMENT SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 11 MIDDLE EAST & AFRICA CLINICAL DATA INTEGRATION PLATFORMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 12 MIDDLE EAST & AFRICA CLINICAL ANALYTICS PLATFORMS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 13 MIDDLE EAST & AFRICA CARE COORDINATION MEDICAL RECORD (CCMR) IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 14 MIDDLE EAST & AFRICA OTHERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 16 MIDDLE EAST & AFRICA WEB-HOSTED (ON-DEMAND) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 17 MIDDLE EAST & AFRICA CLOUD-BASED (SAAS) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 18 MIDDLE EAST & AFRICA LICENSED ENTERPRISE (ON-PREMISES) SOLUTIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 19 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 20 MIDDLE EAST & AFRICA PHASE III IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 21 MIDDLE EAST & AFRICA PHASE I IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 22 MIDDLE EAST & AFRICA PHASE II IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 23 MIDDLE EAST & AFRICA PHASE IV IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 24 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 25 MIDDLE EAST & AFRICA MEDIUM AND SMALL IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 26 MIDDLE EAST & AFRICA LARGE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 27 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 28 MIDDLE EAST & AFRICA DESKTOP IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 29 MIDDLE EAST & AFRICA TABLET IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 30 MIDDLE EAST & AFRICA SMART PHONE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 31 MIDDLE EAST & AFRICA HANDHELD PDA DEVICE IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 32 MIDDLE EAST & AFRICA OTHERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 33 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 34 MIDDLE EAST & AFRICA CONTRACT RESEARCH ORGANIZATIONS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 35 MIDDLE EAST & AFRICA PHARMACEUTICAL AND BIOPHARMACEUTICAL COMPANIES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 36 MIDDLE EAST & AFRICA MEDICAL DEVICE MANUFACTURERS IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 37 MIDDLE EAST & AFRICA HOSPITAL IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 38 MIDDLE EAST & AFRICA CONSULTING SERVICE COMPANIES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 39 MIDDLE EAST & AFRICA ACADEMIC RESEARCH INSTITUTES IN E-CLINICAL SOLUTIONS MARKET, BY REGION, 2021-2030 (USD MILLION)
TABLE 40 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 41 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 42 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 43 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 44 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 45 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 46 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 48 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 49 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 50 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 51 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 52 SOUTH AFRICA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 53 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 54 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 55 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 56 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 57 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 58 SAUDI ARABIA E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 59 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 60 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 61 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 62 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 63 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 64 U.A.E. E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 EGYPT E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 66 EGYPT E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 67 EGYPT E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 68 EGYPT E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 69 EGYPT E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 70 EGYPT E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 71 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
TABLE 72 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY DELIVERY MODE, 2021-2030 (USD MILLION)
TABLE 73 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY CLINICAL TRIAL PHASE, 2021-2030 (USD MILLION)
TABLE 74 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY ORGANIZATION SIZE, 2021-2030 (USD MILLION)
TABLE 75 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY USER DEVICE, 2021-2030 (USD MILLION)
TABLE 76 ISRAEL E-CLINICAL SOLUTIONS MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 REST OF MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET, BY PRODUCT, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: SEGMENTATION
FIGURE 2 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DATA TRIANGULATION
FIGURE 3 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DROC ANALYSIS
FIGURE 4 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: MIDDLE EAST & AFRICA VS REGIONAL MARKET ANALYSIS
FIGURE 5 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: MARKET END USER COVERAGE GRID
FIGURE 8 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: DBMR MARKET POSITION GRID
FIGURE 9 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 MIDDLE EAST & AFRICA E- CLINICAL SOLUTIONS MARKET: SEGMENTATION
FIGURE 11 THE GROWING USAGE OF E-CLINICAL SOLUTIONS IN CLINICAL TRIALS AS WELL AS INCREASING R&D ACTIVITIES AND CORRESPONDING EXPENDITURE, IS EXPECTED TO DRIVE THE GROWTH OF THE MIDDLE EAST & AFRICA E- CLINICAL SOLUTIONS MARKET FROM 2023 TO 2030
FIGURE 12 PRODUCT SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET IN 2023 AND 2030
FIGURE 13 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF THE MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET
FIGURE 14 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, 2022
FIGURE 15 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, 2023-2030 (USD MILLION)
FIGURE 16 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, CAGR (2023-2030)
FIGURE 17 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 18 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, 2022
FIGURE 19 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, 2023-2030 (USD MILLION)
FIGURE 20 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, CAGR (2023-2030)
FIGURE 21 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY DELIVERY MODE, LIFELINE CURVE
FIGURE 22 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, 2022
FIGURE 23 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, 2023-2030 (USD MILLION)
FIGURE 24 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, CAGR (2023-2030)
FIGURE 25 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY CLINICAL TRIAL PHASE, LIFELINE CURVE
FIGURE 26 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, 2022
FIGURE 27 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, 2023-2030 (USD MILLION)
FIGURE 28 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, CAGR (2023-2030)
FIGURE 29 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY ORGANIZATION SIZE, LIFELINE CURVE
FIGURE 30 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, 2022
FIGURE 31 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, 2023-2030 (USD MILLION)
FIGURE 32 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, CAGR (2023-2030)
FIGURE 33 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY USER DEVICE, LIFELINE CURVE
FIGURE 34 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, 2022
FIGURE 35 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 36 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, CAGR (2023-2030)
FIGURE 37 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: BY END USER, LIFELINE CURVE
FIGURE 38 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: SNAPSHOT (2022)
FIGURE 39 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2022)
FIGURE 40 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2023 & 2030)
FIGURE 41 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: BY COUNTRY (2022 & 2030)
FIGURE 42 MIDDLE EAST AND AFRICA E-CLINICAL SOLUTIONS MARKET: PRODUCT (2023-2030)
FIGURE 43 MIDDLE EAST & AFRICA E-CLINICAL SOLUTIONS MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.