Global Software As A Medical Device Samd Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
1.58 Billion
USD
6.87 Billion
2024
2032
USD
1.58 Billion
USD
6.87 Billion
2024
2032
| 2025 –2032 | |
| USD 1.58 Billion | |
| USD 6.87 Billion | |
|
|
|
|
Segmentación del mercado global de software como dispositivo médico (SaMD), por tipo (en la nube y local), aplicación (detección y diagnóstico, monitoreo y alerta, y manejo de enfermedades crónicas): tendencias de la industria y pronóstico hasta 2032
Tamaño del mercado de software como dispositivo médico (SaMD)
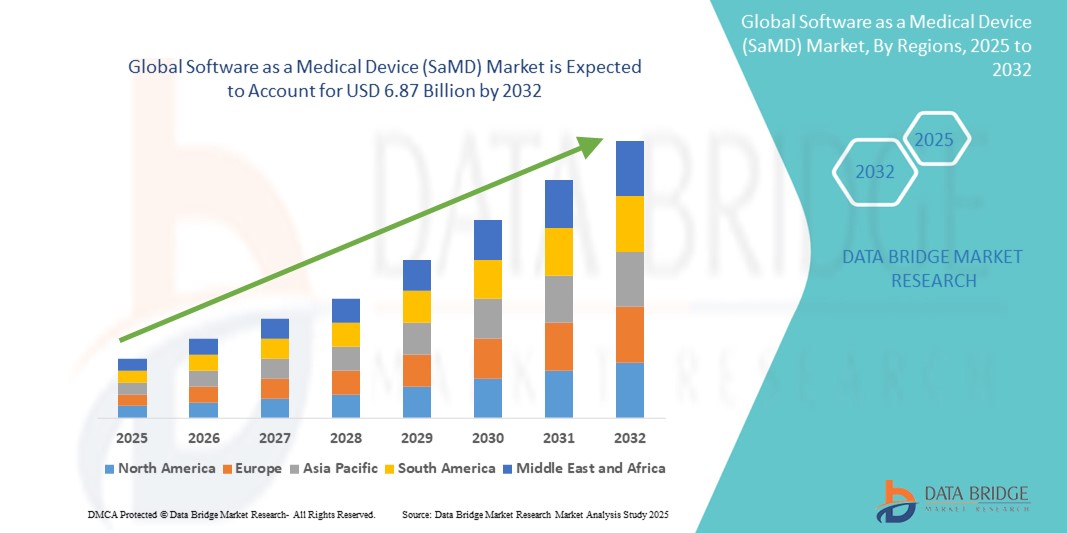
- El tamaño del mercado global de software como dispositivo médico (SaMD) se valoró en USD 1.580 millones en 2024 y se espera que alcance los USD 6.870 millones para 2032 , con una CAGR del 20,13 % durante el período de pronóstico.
- El crecimiento del mercado está impulsado en gran medida por la creciente adopción y el progreso tecnológico en inteligencia artificial (IA), computación en la nube y plataformas de salud conectadas, lo que lleva a una mayor digitalización en áreas de diagnóstico, monitoreo y terapéuticas.
- Además, la creciente demanda de soluciones de salud digital seguras, fáciles de usar y que cumplan con la normativa por parte de los consumidores está consolidando el Software como Dispositivo Médico (SaMD) como la opción preferida para una atención médica moderna y centrada en el paciente. Estos factores convergentes están acelerando la adopción de este tipo de soluciones, impulsando así significativamente el crecimiento del sector.
Análisis del mercado de software como dispositivo médico (SaMD)
- El software como dispositivo médico (SaMD), que se refiere al software diseñado para fines médicos sin formar parte de un dispositivo médico físico, se está volviendo cada vez más esencial en los sistemas de salud modernos debido a sus capacidades de diagnóstico, monitorización y gestión de enfermedades, tanto en entornos clínicos como remotos. Estas soluciones ofrecen mayor accesibilidad, información en tiempo real e integración con los ecosistemas de salud digital.
- La creciente demanda de SaMD se debe principalmente al aumento global de enfermedades crónicas, la proliferación de plataformas de salud conectadas, las vías regulatorias favorables y la creciente necesidad de soluciones de atención centradas en el paciente. Los avances en IA, computación en la nube y tecnologías de salud móvil están acelerando aún más su adopción entre profesionales de la salud y pacientes.
- Norteamérica dominó el mercado de software como dispositivo médico (SaMD), con la mayor participación en los ingresos, un 44,8 % en 2024, gracias a la temprana adopción de la salud digital , los marcos regulatorios favorables de la FDA y la sólida inversión en startups de tecnología sanitaria. Estados Unidos lidera el mercado regional, impulsado por la amplia integración de sistemas de apoyo a la toma de decisiones clínicas basados en IA, herramientas de monitorización remota de pacientes y aplicaciones de diagnóstico.
- Se proyecta que Asia-Pacífico sea la región de más rápido crecimiento en el mercado de software como dispositivo médico (SaMD), registrando una CAGR del 21,6 % entre 2025 y 2032, debido a la creciente penetración de teléfonos inteligentes, las iniciativas de salud digital lideradas por el gobierno, la creciente adopción de la telesalud y la creciente conciencia sobre la atención médica en países como China, India y Japón.
- El segmento basado en la nube dominó el mercado de software como dispositivo médico (SaMD), con la mayor participación en los ingresos, un 65,4 % en 2024, impulsado por la creciente adopción de plataformas de atención médica digital escalables e interoperables y la creciente demanda de atención remota al paciente. SaMD, basado en la nube, ofrece actualizaciones en tiempo real, accesibilidad global e integración con la telemedicina, lo que lo convierte en el modelo de implementación preferido por los profesionales de la salud.
Alcance del informe y segmentación del mercado de software como dispositivo médico (SaMD)
|
Atributos |
Perspectivas clave del mercado del software como dispositivo médico (SaMD) |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
Europa
Asia-Pacífico
Oriente Medio y África
Sudamerica
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis en profundidad de expertos, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de software como dispositivo médico (SaMD)
Mayor comodidad gracias al apoyo a la toma de decisiones clínicas y la interoperabilidad .
- Una tendencia significativa y en auge en el mercado global de software como dispositivo médico (SaMD) es el creciente enfoque en las herramientas de apoyo a la toma de decisiones clínicas y la interoperabilidad fluida con las historias clínicas electrónicas (HCE) y los dispositivos médicos conectados. Esta integración permite una toma de decisiones más rápida y basada en la evidencia, y mejora los flujos de trabajo clínicos en todos los entornos asistenciales.
- Por ejemplo, las principales plataformas SaMD ofrecen asistencia diagnóstica en tiempo real para afecciones como la retinopatía diabética, el riesgo cardiovascular y la detección de accidentes cerebrovasculares. Estas plataformas analizan los datos de los pacientes al instante y brindan a los profesionales clínicos información práctica, lo que reduce los retrasos en el diagnóstico y mejora los resultados del tratamiento.
- Además, las soluciones SaMD se diseñan cada vez más para funcionar en diversas plataformas digitales, incluidas aplicaciones móviles, sistemas basados en la nube y terminales hospitalarias, lo que garantiza la accesibilidad y la facilidad de uso tanto para los pacientes como para los proveedores de atención médica.
- La integración fluida de las herramientas de SaMD con la infraestructura de TI sanitaria existente permite a los proveedores sincronizar el historial del paciente, monitorizar las constantes vitales de forma remota y optimizar la recopilación de datos. Esta interoperabilidad es especialmente valiosa para la gestión de enfermedades crónicas, donde el flujo continuo de datos entre el paciente y el proveedor es fundamental.
- Esta tendencia hacia sistemas de diagnóstico y monitorización inteligentes, en tiempo real y conectados, basados en software, está transformando radicalmente la forma en que se presta la atención médica. Empresas como Biofourmis, BrightInsight y Digital Diagnostics están desarrollando activamente soluciones SaMD que ofrecen resultados clínicos medibles y cumplimiento normativo.
- La demanda de soluciones SaMD que ofrecen monitoreo remoto, análisis predictivo y alertas en tiempo real está creciendo rápidamente en hospitales, clínicas especializadas y entornos de atención domiciliaria, a medida que las partes interesadas buscan soluciones de atención médica más eficientes, escalables y centradas en el paciente.
Dinámica del mercado del software como dispositivo médico (SaMD)
Conductor
Creciente necesidad debido al avance de las tecnologías de salud digital y la monitorización remota.
- La creciente demanda de soluciones de atención médica remota, acelerada por el aumento de enfermedades crónicas y el envejecimiento de la población, impulsa significativamente la creciente adopción del software como dispositivo médico (SaMD). Estas soluciones digitales permiten la monitorización, el diagnóstico y el apoyo terapéutico de pacientes en tiempo real sin necesidad de integrar hardware tradicional.
- Por ejemplo, en abril de 2024, Roche amplió su cartera de salud digital al anunciar planes para desarrollar nuevas soluciones SaMD basadas en IA para el apoyo personalizado a la toma de decisiones sobre terapias oncológicas, integrando datos genómicos con los historiales clínicos. Se espera que estas iniciativas de actores clave de la industria impulsen el crecimiento del mercado global de SaMD durante el período de pronóstico.
- Las plataformas SaMD ofrecen varias ventajas, entre ellas, el monitoreo continuo de la salud, diagnósticos rápidos, detección temprana de enfermedades y una mejor participación del paciente, características que se están volviendo cada vez más esenciales en el contexto del aumento de la atención virtual y los servicios médicos domiciliarios.
- Además, la transición global hacia ecosistemas de salud inteligentes e interoperables, impulsados por dispositivos portátiles, plataformas en la nube y aplicaciones móviles de salud, está convirtiendo a SaMD en una parte integral de la infraestructura de atención médica digital. Estas herramientas de software optimizan los flujos de trabajo clínicos y permiten a los profesionales de la salud ofrecer una atención escalable y personalizada.
- La comodidad del acceso mediante aplicaciones, el intercambio de datos entre profesionales sanitarios y pacientes, y la compatibilidad con dispositivos portátiles de salud (como relojes inteligentes y biosensores) son factores clave que impulsan su adopción. Las soluciones SaMD gozan de una creciente popularidad tanto en entornos hospitalarios como ambulatorios debido a su capacidad para mejorar los resultados clínicos y reducir los costes sanitarios.
Restricción/Desafío
Preocupaciones sobre la privacidad de datos y marcos regulatorios estrictos
- A pesar de sus beneficios, el mercado de SaMD enfrenta importantes desafíos relacionados con la privacidad de los datos y el cumplimiento normativo. Dado que estas soluciones manejan información sanitaria confidencial y dependen en gran medida de la infraestructura en la nube, son vulnerables a ciberamenazas, accesos no autorizados y filtraciones de datos.
- Por ejemplo, los informes sobre violaciones de la privacidad relacionadas con aplicaciones de salud y plataformas integradas en wearables han generado preocupación tanto entre los profesionales sanitarios como entre los pacientes, lo que ha ralentizado su adopción en algunas regiones. Garantizar el almacenamiento, el cifrado y la transmisión de datos seguros es fundamental para salvaguardar la confianza del paciente.
- Las empresas deben gestionar procesos regulatorios complejos y en constante evolución, como el Programa de Precertificación SaMD de la FDA de EE. UU. o el Reglamento de Dispositivos Médicos (MDR) de la UE, que exigen una rigurosa validación clínica, evidencia real y vigilancia poscomercialización para garantizar el cumplimiento. Esto puede aumentar el tiempo y el coste del desarrollo de productos.
- Además, el costo relativamente alto asociado con el desarrollo y mantenimiento de los productos SaMD, especialmente aquellos que utilizan tecnologías avanzadas como IA y aprendizaje automático, puede representar una barrera para las empresas emergentes de salud digital más pequeñas o los sistemas de atención médica en entornos de bajos recursos.
- Superar estos desafíos requerirá inversión en arquitecturas seguras, prácticas de datos transparentes y colaboración con los organismos reguladores para agilizar los procesos de aprobación. Educar a los profesionales sanitarios y a los pacientes sobre la seguridad de los datos, junto con el desarrollo de ofertas rentables de SaMD, será vital para una mayor penetración en el mercado.
Alcance del mercado del software como dispositivo médico (SaMD)
El mercado está segmentado según el tipo y la aplicación.
- Por tipo
Según el tipo, el mercado de software como dispositivo médico (SaMD) se segmenta en soluciones en la nube y locales. El segmento en la nube dominó el mercado con la mayor cuota de ingresos, un 65,4 %, en 2024, impulsado por la creciente adopción de plataformas de atención médica digital escalables e interoperables y la creciente demanda de atención remota al paciente. SaMD, basado en la nube, ofrece actualizaciones en tiempo real, accesibilidad global e integración con la telemedicina, lo que lo convierte en el modelo de implementación preferido por los profesionales sanitarios.
Se proyecta que el segmento local crecerá a una CAGR máxima del 13,2 % entre 2025 y 2032, en particular en las organizaciones de atención médica que priorizan el control total sobre el almacenamiento y la privacidad de los datos, especialmente en regiones con marcos de cumplimiento estrictos.
- Por aplicación
En función de su aplicación, el mercado de software como dispositivo médico (SaMD) se segmenta en cribado y diagnóstico, monitorización y alertas, y gestión de enfermedades crónicas. El segmento de cribado y diagnóstico registró la mayor cuota de ingresos, con un 47,8 %, en 2024, gracias al auge de las plataformas de diagnóstico basadas en IA para la detección del cáncer, las evaluaciones cardiovasculares y el análisis de imágenes. Las herramientas SaMD en este ámbito mejoran la precisión y la velocidad del diagnóstico en entornos clínicos. Se prevé que el segmento de monitorización y alertas registre la tasa de crecimiento anual compuesta (TCAC) más rápida, del 25,1 %, entre 2025 y 2032, impulsada por la creciente adopción de sistemas de monitorización remota de pacientes y plataformas integradas en wearables que proporcionan alertas de salud en tiempo real, especialmente para pacientes de edad avanzada y de alto riesgo.
Análisis regional del mercado de software como dispositivo médico (SaMD)
- América del Norte dominó el mercado de software como dispositivo médico (SaMD) con la mayor participación en los ingresos del 44,8 % en 2024, impulsada por una creciente demanda de herramientas de atención médica digital y apoyo regulatorio para soluciones clínicas basadas en software.
- La sólida infraestructura de TI de salud de la región, el alto gasto en atención médica y la creciente dependencia de herramientas de monitoreo y diagnóstico remotos contribuyen significativamente a este liderazgo.
- Estados Unidos lidera el mercado norteamericano debido a un entorno regulatorio favorable de la FDA, una rápida transformación digital en los hospitales y la presencia de desarrolladores clave de SaMD.
Perspectiva del mercado estadounidense de software como dispositivo médico (SaMD)
El mercado estadounidense de software como dispositivo médico representó la mayor participación en Norteamérica, aportando el 81 % de los ingresos de mercado de la región en 2024. El crecimiento se debe a la integración generalizada de la IA en el diagnóstico, la expansión de la telesalud y la transición hacia una atención basada en el valor. Las empresas se están centrando en las autorizaciones 510(k) de la FDA y en la ampliación de las indicaciones de los productos SaMD para el manejo de enfermedades crónicas y la monitorización de la salud mental.
Perspectiva del mercado europeo de software como dispositivo médico (SaMD)
Se proyecta que el mercado europeo de software como dispositivo médico crecerá a una notable tasa de crecimiento anual compuesta (TCAC) del 21,3 % entre 2025 y 2032, gracias a la implementación del Reglamento sobre Dispositivos Médicos (MDR) de la UE y a un fuerte enfoque en la seguridad de los datos de los pacientes y la innovación. Países como Alemania, Francia y el Reino Unido están impulsando la transformación digital de la salud mediante modelos de reembolso e inversión en plataformas de telemedicina.
Perspectiva del mercado de software como dispositivo médico (SaMD) en el Reino Unido
Se prevé que el mercado británico de software como dispositivo médico crezca a una sólida tasa de crecimiento anual compuesta (TCAC) del 20,7 % durante el período de pronóstico. Entre los factores clave se incluyen las iniciativas de NHS Digital, la creciente alfabetización digital entre los profesionales sanitarios y la confianza pública en las herramientas basadas en IA para la autogestión de la salud mental y las enfermedades crónicas.
Análisis del mercado alemán de software como dispositivo médico (SaMD)
Se proyecta que el mercado alemán de software como dispositivo médico se expandirá a una CAGR del 20,9 % entre 2025 y 2032. La Ley de Atención Sanitaria Digital (DVG) y el proceso de vía rápida DiGA son catalizadores clave que permiten que las aplicaciones de salud digital reciban reembolsos, fomentando así la innovación y la adopción.
Perspectiva del mercado de software como dispositivo médico (SaMD) en Asia-Pacífico
Se espera que el mercado de software como dispositivo médico de Asia-Pacífico registre la tasa de crecimiento anual compuesta (TCAC) más rápida, del 21,6 %, entre 2025 y 2032, gracias a las campañas gubernamentales de salud digital, el creciente uso de la telemedicina y la mayor penetración de los teléfonos inteligentes. Países como China, Japón e India invierten activamente en diagnósticos basados en IA, aplicaciones de salud móvil y plataformas SaMD basadas en la nube.
Análisis del mercado japonés de software como dispositivo médico (SaMD)
El mercado japonés de software como dispositivo médico está en constante crecimiento, influenciado por el envejecimiento de su población y las sólidas reformas sanitarias impulsadas por la tecnología. La iniciativa Sociedad 5.0 del gobierno japonés apoya la innovación en software médico, y las aseguradoras están empezando a reembolsar las terapias digitales.
Análisis del mercado de software como dispositivo médico (SaMD) en China
El mercado chino de software como dispositivo médico registró la mayor participación en ingresos en Asia-Pacífico en 2024 gracias al sólido apoyo gubernamental a la IA en la atención médica, la amplia base de pacientes y el mayor uso de herramientas de salud basadas en teléfonos inteligentes. Las directrices de la Comisión Nacional de Salud sobre IA y dispositivos médicos digitales han impulsado la claridad regulatoria, impulsando el rápido desarrollo y la adopción nacional de SaMD.
Cuota de mercado del software como dispositivo médico (SaMD)
La industria del software como dispositivo médico (SaMD) está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- MindMaze (Suiza)
- Siemens Healthineers AG (Alemania)
- Biofourmis (EE. UU.)
- Digital Diagnostics Inc. (EE. UU.)
- Silicon & Software Systems Ltd. (Irlanda)
- BrightInsight, Inc. (EE. UU.)
- Arterias (EE. UU.)
- Medtronic (Irlanda)
- Viz.ai, Inc. (EE. UU.)
- iSchemaView, Inc. (EE. UU.)
- Abbott (EE. UU.)
- Oracle (EE. UU.)
- 4S Information Systems Ltd. (India)
- Axis Clinical Software, Inc. (EE. UU.)
- Software médico para CV (EE. UU.)
Últimos avances en el mercado global de software como dispositivo médico (SaMD)
- En mayo de 2025, la Administración de Alimentos y Medicamentos de EE. UU. (FDA) publicó un borrador actualizado de la guía sobre las Funciones de Software de Dispositivos con Inteligencia Artificial (IA), que impacta directamente al sector de los SaMD. Esta guía introduce un Plan Predeterminado de Control de Cambios (PCCP), que permite a los fabricantes gestionar proactivamente las actualizaciones de algoritmos tras su comercialización. Esta medida implica adaptabilidad regulatoria, acorde con la naturaleza dinámica de los SaMD con IA, y busca acelerar la innovación, manteniendo al mismo tiempo los estándares de seguridad y eficacia.
- En febrero de 2025, Biofourmis, empresa estadounidense de tecnología sanitaria, anunció la expansión de su plataforma SaMD, autorizada por la FDA para la monitorización remota de la insuficiencia cardíaca, a los mercados europeos tras obtener la certificación CE. La solución de la empresa, basada en IA, permite obtener información del paciente en tiempo real y ha demostrado una reducción en los reingresos hospitalarios, lo que ofrece un valor significativo en la atención postaguda y crónica.
- En enero de 2025, Digital Diagnostics Inc. lanzó su nueva plataforma de diagnóstico autónomo basada en SaMD para la detección de retinopatía diabética en centros de atención primaria. Aprobada por la FDA y ahora en expansión en Asia-Pacífico, esta herramienta elimina la necesidad de intervención especializada en el diagnóstico inicial, abordando así las deficiencias de accesibilidad en la atención oftalmológica.
- En marzo de 2024, Siemens Healthineers anunció una alianza estratégica con redes hospitalarias estadounidenses para implementar su plataforma de imágenes SaMD, basada en IA, en todos los departamentos de radiología. Esta colaboración se centra en el apoyo a la toma de decisiones en tiempo real para la detección de tumores y el tratamiento de accidentes cerebrovasculares, mejorando la precisión diagnóstica y la eficiencia del flujo de trabajo.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.