Global Influenza Drug Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
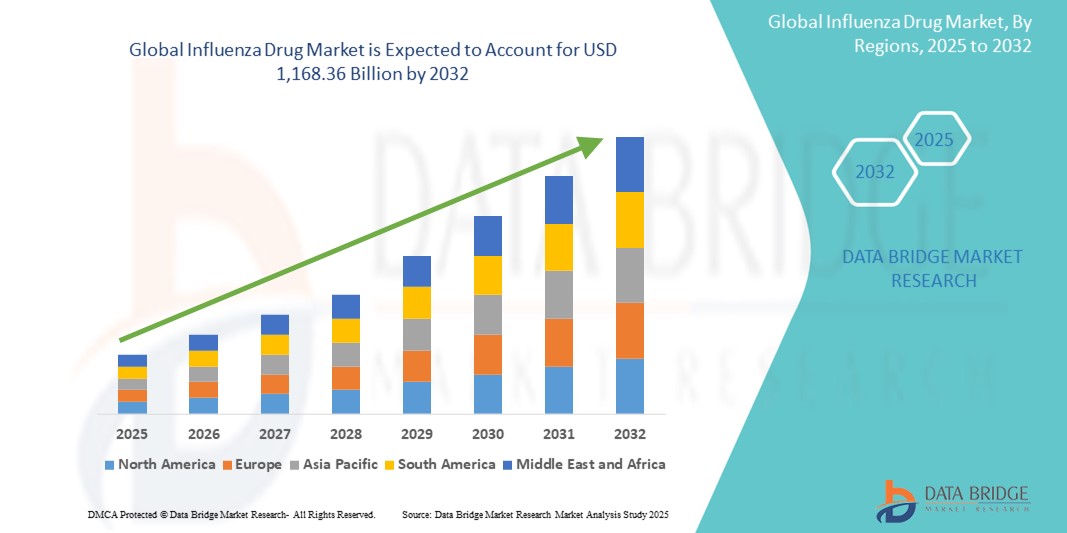
981.68 Billion
USD
1,168.36 Billion
2024
2032
USD
981.68 Billion
USD
1,168.36 Billion
2024
2032
| 2025 –2032 | |
| USD 981.68 Billion | |
| USD 1,168.36 Billion | |
|
|
|
|
Segmentación del mercado global de medicamentos contra la influenza, por tipo (influenza A, influenza B y influenza C), tratamiento (vacunas y medicamentos), vía de administración (oral, intramuscular, intradérmica, intranasal e intravenosa), edad (pediatría y adultos), usuario final (hospitales y atención domiciliaria), canal de distribución (licitaciones directas y ventas minoristas): tendencias y pronóstico del sector hasta 2032.
Tamaño del mercado de medicamentos contra la gripe
- El tamaño del mercado mundial de medicamentos contra la influenza se valoró en USD 981,68 mil millones en 2024 y se espera que alcance los USD 1,168,36 mil millones para 2032 , con una CAGR del 2,20% durante el período de pronóstico.
- El crecimiento del mercado se ve impulsado en gran medida por la creciente incidencia de la gripe estacional y otras enfermedades respiratorias contagiosas, junto con una creciente población de pacientes en riesgo de desarrollar complicaciones relacionadas con la gripe.
- Además, las crecientes inversiones en actividades de investigación y desarrollo por parte de empresas farmacéuticas e instituciones de investigación para desarrollar medicamentos avanzados y mejorados, incluidos nuevos medicamentos antivirales y vacunas recombinantes , están acelerando la adopción de soluciones farmacológicas contra la gripe, impulsando así significativamente el crecimiento de la industria.
Análisis del mercado de medicamentos contra la gripe
- El mercado mundial de medicamentos contra la gripe abarca una gama de medicamentos antivirales y vacunas diseñadas para prevenir, tratar y aliviar los síntomas de las infecciones de gripe, que plantean un importante desafío para la salud pública debido a su naturaleza recurrente y estacional, mutaciones virales constantes y potencial de pandemias.
- La creciente demanda de medicamentos contra la gripe se ve impulsada principalmente por la prevalencia mundial constante de infecciones de gripe, la creciente conciencia sobre los beneficios de la vacunación y los avances continuos en la investigación farmacéutica que conducen a terapias antivirales más eficaces y vacunas de próxima generación.
- Norteamérica domina el mercado de medicamentos contra la gripe, con la mayor cuota de ingresos, un 60,5 % en 2024. Se caracteriza por una sólida infraestructura sanitaria, altas tasas de vacunación y la presencia de importantes compañías farmacéuticas. Estados Unidos experimenta un fuerte crecimiento del mercado impulsado por extensas campañas de salud pública, iniciativas gubernamentales de preparación ante pandemias y la continua I+D en nuevos tratamientos y vacunas contra la gripe.
- Se espera que Asia-Pacífico sea la región de más rápido crecimiento en el mercado de medicamentos contra la influenza durante el período de pronóstico debido a la creciente conciencia sobre la influenza, el aumento del gasto en atención médica, la creciente urbanización y un gran grupo de pacientes susceptibles a infecciones en países densamente poblados.
- El segmento de influenza A domina el mercado de medicamentos contra la influenza con una participación de mercado del 47,78 % en 2024, impulsado por su mayor tasa de mutación, rango de huéspedes más amplio, potencial pandémico significativo y mayor gravedad de la enfermedad, lo que requiere estrategias de vacunación más complejas y actualizaciones frecuentes de los tratamientos.
Alcance del informe y segmentación del mercado de medicamentos contra la influenza
|
Atributos |
Perspectivas clave del mercado de medicamentos contra la gripe |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
Europa
Asia-Pacífico
Oriente Medio y África
Sudamerica
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis en profundidad de expertos, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de medicamentos contra la gripe
Creciente adopción de herramientas de telemedicina y salud digital
- Una tendencia significativa y en auge en el mercado mundial de medicamentos contra la gripe es la creciente integración de la telemedicina y las herramientas de salud digital para el manejo, diagnóstico y prescripción de la gripe. Esta fusión de tecnologías está mejorando significativamente la accesibilidad a los servicios de salud, especialmente para los pacientes con gripe.
- Por ejemplo, las consultas virtuales permiten a las personas con síntomas gripales conectarse con profesionales sanitarios desde la comodidad de sus hogares, lo que reduce el riesgo de transmisión viral en clínicas y hospitales. Las plataformas de telemedicina pueden facilitar un diagnóstico rápido basado en la evaluación de los síntomas y orientar a los pacientes sobre el autocuidado adecuado o la prescripción de medicamentos antivirales como el oseltamivir o el baloxavir. Aproximadamente el 33 % de los sistemas de salud integran el tratamiento de la gripe en los servicios de telemedicina, lo que mejora la accesibilidad en las zonas rurales.
- Las herramientas de salud digital, como las aplicaciones móviles y los dispositivos portátiles, permiten la monitorización continua de parámetros fisiológicos que pueden indicar la aparición o la progresión de la gripe. Algunos termómetros inteligentes , como los de KINSA, pueden registrar datos agregados de temperatura para identificar posibles focos de gripe. Estas tecnologías pueden proporcionar alertas tempranas, incitando a las personas a buscar atención médica con mayor rapidez, lo cual es crucial para la eficacia de los tratamientos antivirales.
- La integración fluida de las herramientas de salud digital con los sistemas de salud también respalda iniciativas más amplias de vigilancia de la salud pública. Los datos de los dispositivos portátiles pueden contribuir a la vigilancia de la gripe en tiempo real, ayudando a las autoridades sanitarias a rastrear brotes y asignar recursos de forma más eficaz. Esta vigilancia mejorada puede fundamentar campañas de vacunación específicas y la distribución oportuna de medicamentos antivirales.
- Esta tendencia hacia una gestión de la gripe más accesible, proactiva y basada en datos está transformando radicalmente la participación del paciente y la prestación de servicios de salud. En consecuencia, las compañías farmacéuticas y los profesionales sanitarios están explorando colaboraciones y desarrollando plataformas que aprovechen estas capacidades digitales para mejorar los resultados de los pacientes y la adherencia a la medicación.
- La demanda de medicamentos contra la gripe se ve impulsada indirectamente por estas tendencias, ya que la telemedicina facilita que los pacientes reciban recetas oportunas y el monitoreo digital puede conducir a un diagnóstico e intervención más tempranos, maximizando la efectividad de los tratamientos disponibles.
Dinámica del mercado de medicamentos contra la gripe
Conductor
Creciente necesidad debido a la constante prevalencia mundial de la gripe y a las iniciativas de salud pública.
- La prevalencia mundial creciente y constante de epidemias de gripe estacional, junto con la amenaza constante de cepas pandémicas y sólidas iniciativas de salud pública, es un factor importante que impulsa la mayor demanda de medicamentos y vacunas contra la gripe.
- Por ejemplo, gobiernos de todo el mundo, incluyendo el de Estados Unidos, Europa y Asia, lanzan continuamente extensas campañas de vacunación e invierten en programas nacionales de inmunización para mitigar el impacto de las temporadas anuales de gripe y prepararse para posibles pandemias. Estas iniciativas, junto con una mayor vigilancia y avances en el diagnóstico rápido, impulsan un mayor impulso a la vacunación y al tratamiento temprano con antivirales.
- A medida que la población general y los profesionales sanitarios son cada vez más conscientes de la gravedad de la gripe y sus posibles complicaciones, aumenta la demanda de soluciones tanto preventivas como terapéuticas. Esto es especialmente cierto para las poblaciones de alto riesgo, como los ancianos, los niños pequeños y las personas con enfermedades preexistentes.
- Además, los continuos esfuerzos de investigación y desarrollo de las compañías farmacéuticas para crear medicamentos antivirales más eficaces y de espectro más amplio, así como vacunas de próxima generación, están ampliando el panorama de tratamientos disponibles y mejorando la eficacia de las intervenciones existentes, impulsando así el crecimiento del mercado.
- La necesidad de actualizar anualmente las formulaciones de vacunas, debido a la constante evolución de los virus de la gripe, garantiza una demanda sostenida y recurrente de productos novedosos. Este ciclo continuo de desarrollo, producción y distribución es un factor clave que impulsa el mercado de medicamentos contra la gripe tanto en las economías desarrolladas como en las emergentes.
Restricción/Desafío
“Desafíos de la mutabilidad viral y la resistencia a los fármacos, y altos costos de desarrollo”
- Un desafío importante para el mercado mundial de medicamentos contra la gripe es la mutabilidad inherente de los virus de la gripe, que experimentan constantes variaciones antigénicas. Esta rápida evolución puede reducir la eficacia de las vacunas existentes y provocar la aparición de resistencia a los antivirales, lo que representa una amenaza continua para la salud pública.
- Por ejemplo, la frecuente necesidad de actualizar anualmente las cepas de vacunas exige un ciclo continuo de investigación, desarrollo y producción, lo cual es costoso y requiere mucho tiempo. Además, la aparición de cepas resistentes a los medicamentos, como las resistentes a antivirales más antiguos como la amantadina o, más recientemente, algunos virus H1N1 resistentes al oseltamivir (Tamiflu), limita las opciones de tratamiento y complica el manejo clínico.
- Abordar estos desafíos biológicos requiere una inversión significativa y sostenida en I+D para nuevos agentes antivirales con nuevos mecanismos de acción y un espectro de actividad más amplio, así como vacunas universales contra la gripe que puedan ofrecer protección duradera contra múltiples cepas. Sin embargo, el desarrollo de vacunas y fármacos es un proceso excepcionalmente largo y costoso, que suele durar entre 10 y 15 años y costar entre cientos de millones y más de mil millones de dólares, con una alta tasa de fracaso.
- Además, los desafíos logísticos, en particular el mantenimiento de la cadena de frío para la distribución de vacunas desde los centros de fabricación hasta zonas remotas, pueden provocar desperdicios y reducir la eficacia. Factores como la infraestructura inadecuada, los errores humanos y los cortes de electricidad en los países de bajos ingresos plantean obstáculos importantes.
- El elevado coste inicial de desarrollar y comercializar nuevos medicamentos y vacunas contra la gripe, sumado a los rigurosos procesos de aprobación regulatoria, crea importantes obstáculos para los fabricantes. Esto a menudo se traduce en precios más altos para tratamientos avanzados, lo que podría limitar el acceso en mercados sensibles a los precios o para poblaciones con seguro insuficiente.
- Superar estos desafíos mediante colaboraciones internacionales, mayor financiación pública y privada para I+D, vías regulatorias simplificadas e inversión en infraestructuras de distribución global sólidas será vital para el crecimiento sostenido del mercado y una preparación eficaz ante pandemias.
Alcance del mercado de medicamentos contra la gripe
El mercado está segmentado según tipo, tratamiento, vía de administración, edad, usuario final y canal de distribución.
- Por tipo
Según el tipo, el mercado de medicamentos contra la gripe se segmenta en influenza A, influenza B y influenza C. El segmento de influenza A domina el mercado con una participación del 47,78 % en 2024, impulsado por su mayor tasa de mutación, mayor rango de hospedadores, un potencial pandémico significativo y una mayor gravedad de la enfermedad, lo que requiere estrategias de vacunación más complejas para controlar los brotes. La amenaza constante de cepas de influenza A, como H1N1 y H3N2, garantiza una demanda sostenida de medicamentos y vacunas relacionados.
Se espera que la influenza B alcance la mayor tasa de crecimiento anual compuesta (TCAC) del mercado debido a su inclusión en las vacunas tetravalentes, que ofrecen una protección más amplia contra las cepas A y B. La creciente conciencia sobre el papel de la influenza B en las epidemias estacionales y el creciente énfasis en las estrategias integrales de prevención de la gripe impulsan la demanda de vacunas más eficaces que cubran estas cepas, especialmente en poblaciones vulnerables.
- Por tratamiento
En función del tratamiento, el mercado de medicamentos contra la gripe se segmenta en vacunas y medicamentos. El segmento de vacunas domina el mercado con una cuota de mercado del 87,23 % en 2024, debido a su papel fundamental en la prevención de enfermedades, la reducción de la transmisión y la atención a las preocupaciones de salud pública asociadas con brotes estacionales y posibles pandemias. Los continuos avances en la tecnología de vacunas, incluido el desarrollo de vacunas tetravalentes que ofrecen una protección más amplia, consolidan aún más su liderazgo en el mercado.
El segmento de medicamentos, que incluye antivirales como oseltamivir y baloxavir, desempeña un papel crucial en el tratamiento de infecciones establecidas, y el baloxavir marboxil (Xofluza) se destaca como el tipo de medicamento de más rápido crecimiento debido a su administración de dosis única y su eficacia.
- Por vía de administración
Según la vía de administración, el mercado de medicamentos contra la gripe se segmenta en oral, intramuscular, intradérmica, intranasal e intravenosa. Se prevé que el segmento oral domine el mercado con una cuota de mercado del 45,04 % en 2024, impulsado principalmente por su comodidad, facilidad de autoadministración y amplia accesibilidad para pacientes, tanto en consultas externas como en el hogar. Los antivirales orales suelen ser el tratamiento de primera línea para la gripe.
Se prevé que la vía intranasal sea la de mayor crecimiento durante el período de pronóstico. Esto se debe a su administración no invasiva y sin aguja, que mejora la adherencia al tratamiento, especialmente en la población pediátrica, y a su capacidad para inducir inmunidad sistémica y mucosa.
- Por edad
En función de la edad, el mercado de medicamentos contra la gripe se segmenta en pediátricos y adultos. El segmento de adultos es el más grande en el mercado de la gripe, con una participación del 66,7 % en 2024, impulsado por las altas tasas de vacunación entre las personas mayores y las personas con enfermedades crónicas, quienes tienen un mayor riesgo de sufrir complicaciones graves por la gripe.
Se prevé que el segmento pediátrico crezca a una CAGR notable, ya que los niños pequeños, en particular los menores de 5 años, tienen mayor riesgo de sufrir complicaciones graves por influenza, lo que acelera el alcance del mercado para las formulaciones adaptadas a los niños y los esfuerzos de vacunación.
- Por uso final
En función del usuario final, el mercado de medicamentos contra la gripe se segmenta en hospitales y atención domiciliaria. El segmento hospitalario domina el mercado mundial de medicamentos contra la gripe con una cuota de mercado del 72,62 % en 2024, debido principalmente a su papel en el manejo de casos graves de gripe que requieren monitorización intensiva, antivirales intravenosos y cuidados intensivos para pacientes de alto riesgo. Los hospitales también son puntos clave para las campañas de vacunación.
Se prevé que el segmento de atención domiciliaria experimente la tasa de crecimiento anual compuesta (TCAC) más rápida entre 2025 y 2032 en el mercado mundial de medicamentos contra la gripe, impulsado por la creciente preferencia por las opciones de tratamiento en casa, los avances en telemedicina y la creciente adopción de servicios de atención médica domiciliaria. Estos factores permiten a los pacientes controlar eficazmente los síntomas de la gripe desde la comodidad de sus hogares, reduciendo la necesidad de hospitalización y minimizando la exposición a centros de salud.
- Por canal de distribución
Según el canal de distribución, el mercado mundial de medicamentos contra la gripe se segmenta en licitaciones directas y ventas minoristas. Se proyecta que el segmento de licitaciones directas domine el mercado mundial de medicamentos contra la gripe, con la mayor cuota de mercado, un 56,34 %, en 2024. Esto se debe principalmente a la adquisición a gran escala de vacunas y antivirales por parte de gobiernos y organizaciones de salud pública para programas nacionales de inmunización y el acopio estratégico. Este canal garantiza la compra a granel y una distribución eficiente para las campañas de vacunación masiva.
El segmento de ventas minoristas también es un canal dominante, que actúa como punto de acceso clave tanto para vacunas como para tratamientos de venta libre y con receta para el público en general, y se espera que experimente un crecimiento significativo, particularmente a través de farmacias en línea debido a la conveniencia y la accesibilidad.
Análisis regional del mercado de medicamentos contra la gripe
- América del Norte domina el mercado de medicamentos contra la gripe con la mayor participación en los ingresos del 60,5 % en 2024, impulsada por una sólida infraestructura de atención médica, altas tasas de cobertura de vacunación y la presencia de importantes compañías farmacéuticas.
- Los consumidores de la región priorizan enormemente la atención médica preventiva y tienen fácil acceso a vacunas y tratamientos antivirales.
- Esta adopción generalizada se ve respaldada además por políticas de reembolso favorables, una población tecnológicamente avanzada y el creciente énfasis en las iniciativas de salud pública, que establecen los medicamentos contra la gripe como un componente crucial del manejo de las enfermedades estacionales.
Perspectiva del mercado de medicamentos contra la gripe en EE. UU.
El mercado estadounidense de medicamentos contra la gripe captó la mayor participación en los ingresos, con un 56,2 %, en 2024 en Norteamérica, lo que refleja el avanzado sistema de salud del país y sus estrategias proactivas de salud pública. Estados Unidos es un importante impulsor del mercado de la gripe debido a su considerable carga de gripe estacional, sus sólidos programas de vacunación y la continua inversión en I+D para nuevos tratamientos y vacunas. Los consumidores priorizan cada vez más las medidas preventivas y acceden fácilmente a los medicamentos contra la gripe. La sólida integración de las recomendaciones de salud pública, la amplia disponibilidad de vacunas y antivirales, y una importante población de pacientes contribuyen a la demanda sostenida.
Perspectivas del mercado europeo de medicamentos contra la gripe
Se proyecta que el mercado europeo de medicamentos contra la gripe se expandirá a una tasa de crecimiento anual compuesta (TCAC) del 2,5 % durante el período de pronóstico, impulsado principalmente por las iniciativas gubernamentales establecidas para la vacunación y el tratamiento de la gripe, las estrictas directrices de salud pública y la alta incidencia de la gripe estacional. El enfoque de la región en la atención preventiva, sumado a una infraestructura sanitaria avanzada y una mayor concienciación sobre las complicaciones de la gripe, está impulsando la adopción de medicamentos contra la gripe. Los países europeos experimentan una demanda constante de vacunas y terapias antivirales en respuesta a los brotes anuales.
Perspectivas del mercado de medicamentos contra la gripe en el Reino Unido
Se prevé que el mercado británico de medicamentos contra la gripe crezca a una tasa de crecimiento anual compuesta (TCAC) notable durante el período de pronóstico, impulsado por los sólidos programas gubernamentales de vacunación, una mayor atención a la salud pública y el deseo de una mayor protección contra la gripe estacional. El consolidado sistema sanitario británico y las firmes recomendaciones de vacunación anual contra la gripe están fomentando una alta tasa de vacunación y tratamiento antiviral entre la población. El compromiso del país con las iniciativas de salud pública impulsa aún más el crecimiento del mercado.
Análisis del mercado alemán de medicamentos contra la gripe
Se prevé que el mercado alemán de medicamentos contra la gripe se expanda a una tasa de crecimiento anual compuesta (TCAC) considerable durante el período de pronóstico, impulsado por una mayor concienciación sobre la seguridad sanitaria pública, un fuerte énfasis en la medicina preventiva y la demanda de soluciones tecnológicamente avanzadas. La sólida infraestructura sanitaria alemana, sumada a su enfoque en altas tasas de vacunación y una gestión eficiente de la enfermedad, promueve la adopción de medicamentos contra la gripe, especialmente tanto en la práctica médica general como en el ámbito hospitalario. La integración de diagnósticos avanzados y la preferencia por tratamientos basados en la evidencia se alinean con las expectativas de los consumidores y la comunidad médica local.
Perspectivas del mercado de medicamentos contra la gripe en Asia-Pacífico
Se prevé que el mercado de medicamentos contra la gripe en Asia-Pacífico crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida durante el período de pronóstico, impulsado por la creciente urbanización, el aumento de la renta disponible y los avances tecnológicos en la atención médica en países como China, Japón e India. La numerosa y creciente población de la región es muy susceptible a los brotes de gripe, lo que genera una mayor concienciación y demanda de tratamientos y vacunas eficaces. Además, a medida que Asia-Pacífico amplía su infraestructura sanitaria y las iniciativas gubernamentales promueven un mayor acceso a medicamentos esenciales, la asequibilidad y la accesibilidad de los medicamentos contra la gripe se están extendiendo a una base de consumidores más amplia.
Perspectivas del mercado de medicamentos contra la gripe en Japón
El mercado japonés de medicamentos antigripales está cobrando impulso en el sector de los antivirales debido a la alta incidencia de gripe estacional, la rápida urbanización y la fuerte demanda de tratamientos eficaces. El mercado japonés prioriza la salud pública y la cobertura de vacunación, y la adopción de medicamentos antigripales se debe a la creciente concienciación sobre las complicaciones, especialmente en el envejecimiento de la población. La integración de medicamentos antivirales avanzados y los continuos esfuerzos de I+D impulsan el crecimiento, ya que Japón prioriza la reducción de la incidencia de la gripe.
Perspectivas del mercado de medicamentos contra la gripe en India
El mercado indio de medicamentos contra la gripe representó una cuota de mercado significativa en Asia Pacífico en 2024, debido a la expansión de la clase media, la rápida urbanización y el mayor acceso a la atención médica. India es un mercado grande con una alta incidencia de enfermedades infecciosas, y los medicamentos contra la gripe están adquiriendo cada vez mayor importancia tanto en los programas de salud pública como en la atención médica privada. El impulso hacia la mejora de la infraestructura sanitaria y la disponibilidad de medicamentos contra la gripe, tanto de fabricación nacional como importados, son factores clave que impulsan el mercado en India, con una tasa de crecimiento anual compuesta (TCAC) proyectada del 2,6 %.
Cuota de mercado de medicamentos contra la gripe
La industria de medicamentos contra la gripe está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- AbbVie Inc. (EE. UU.)
- AstraZeneca (Reino Unido)
- BioNTech SE (Alemania)
- Bristol-Myers Squibb Company (EE. UU.)
- Cipla (India)
- COCRYSTAL PHARMA, INC. (EE. UU.)
- CSL (Reino Unido)
- Daiichi Sankyo Company, Limited (Japón)
- Laboratorios Dr. Reddy Ltd. (India)
- F. Hoffmann-La Roche AG (Suiza)
- Gilead Sciences, Inc. (EE. UU.)
- GSK plc. (Reino Unido)
- Johnson & Johnson Services, Inc. (EE. UU.)
- Merck & Co., Inc. (EE. UU.)
- Moderna, Inc. (EE. UU.)
- Novartis AG (Suiza)
- Novavax (EE. UU.)
- Osivax (Francia)
- Sanofi SA (Francia)
Últimos avances en el mercado mundial de medicamentos contra la gripe
- En septiembre de 2024, FluMist de AstraZeneca fue aprobado para autoadministración en EE. UU. Esto representa un avance significativo, ya que es la primera vacuna contra la gripe que no requiere la administración de un profesional de la salud, lo que mejora la accesibilidad y la comodidad para quienes buscan prevenir la gripe. FluMist fue aprobado por la FDA de EE. UU. para autoadministración en adultos de hasta 49 años o para administración por un padre/cuidador en personas de 2 a 17 años.
- En agosto de 2024, científicos de la Facultad de Medicina de Harvard desarrollaron un espray nasal llamado Espray de Captura y Neutralización de Patógenos (PCANS), o Profi. Este espray afirma proteger contra la gripe, los resfriados y la COVID-19 con una eficacia superior al 99,99 %, al formar una barrera en la cavidad nasal que captura y neutraliza virus y bacterias.
- En mayo de 2024, Sanofi y Novavax anunciaron un acuerdo de licencia coexclusiva para comercializar conjuntamente la vacuna independiente con adyuvante contra la COVID-19 de Novavax en todo el mundo y desarrollar una nueva combinación de vacunas contra la influenza y la COVID-19. Esta colaboración busca acelerar el desarrollo de vacunas combinadas, ofreciendo mayor comodidad y protección a los pacientes.
- En abril de 2023, Sinovac Biotech Co., Ltd. anunció la reciente puesta en marcha de su nueva planta de fabricación de vacunas contra la influenza en Pekín. Esta expansión aumenta la capacidad de producción global de vacunas contra la influenza, lo que contribuye a una mayor disponibilidad y suministro.
- En marzo de 2023, el Comité Asesor de Vacunas y Productos Biológicos Relacionados (VRBPAC) de la FDA se reunió para determinar la composición de la vacuna para la temporada de influenza 2023-2024 en EE. UU. Este proceso anual es crucial para garantizar la eficacia de las vacunas contra las cepas circulantes de los virus de la influenza.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.