Global Exocrine Pancreatic Insufficiency Epi Therapeutics And Diagnostics Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
8.81 Billion
USD
15.38 Billion
2024
2032
USD
8.81 Billion
USD
15.38 Billion
2024
2032
| 2025 –2032 | |
| USD 8.81 Billion | |
| USD 15.38 Billion | |
|
|
|
|
Segmentación del mercado global de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE), por diagnóstico (pruebas de imagen y prueba de función pancreática), tratamiento (manejo nutricional, terapia de reemplazo enzimático pancreático [PERT]), tipo de fármaco (genérico y de marca), usuario final (hospitales, clínicas especializadas, atención domiciliaria, centros de diagnóstico, institutos de investigación y académicos, entre otros), canal de distribución (licitación directa, farmacias minoristas, distribuidores externos, entre otros): tendencias del sector y pronóstico hasta 2032.
Tamaño del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
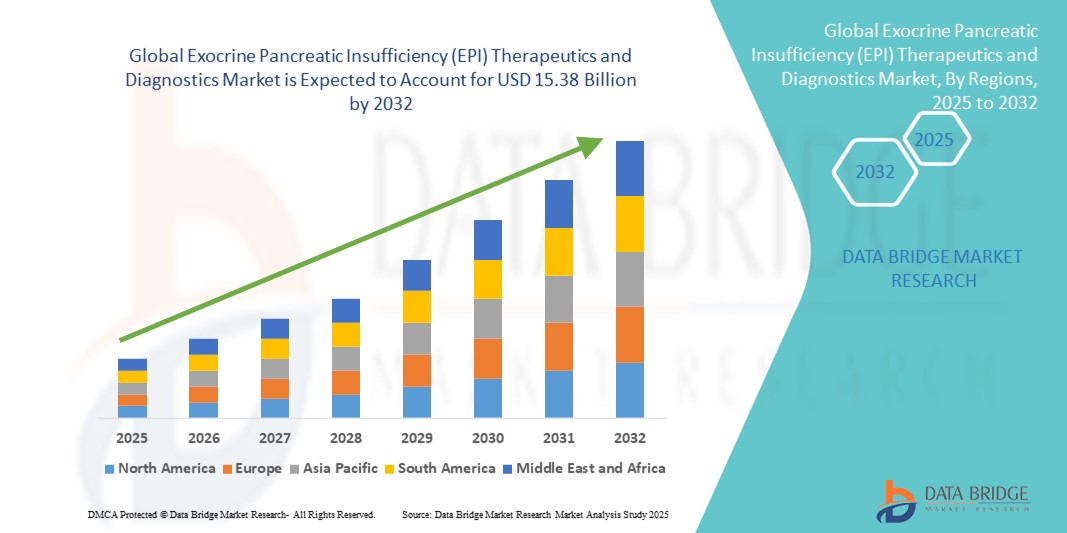
- El tamaño del mercado global de terapias y diagnósticos de insuficiencia pancreática exocrina (EPI) se valoró en USD 8.81 mil millones en 2024 y se espera que alcance los USD 15.38 mil millones para 2032 , con una CAGR de 7,20% durante el período de pronóstico.
- El crecimiento del mercado se debe en gran medida a la creciente prevalencia de trastornos pancreáticos, la mayor concienciación sobre la salud gastrointestinal y los avances en tecnologías de diagnóstico, como las pruebas de elastasa fecal y los métodos de imagen. Estos factores impulsan la detección temprana y el tratamiento oportuno de la insuficiencia pancreática exocrina (IPE).
- Además, la creciente adopción de terapias de reemplazo de enzimas pancreáticas (PERT), junto con la investigación continua sobre nuevos enfoques terapéuticos y una mayor precisión diagnóstica, está mejorando significativamente los resultados de los pacientes. Estos factores convergentes están acelerando la adopción de soluciones terapéuticas y diagnósticas para la insuficiencia pancreática exocrina (IPE), impulsando así sustancialmente el crecimiento de la industria.
Análisis del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
- Las terapias y diagnósticos de la insuficiencia pancreática exocrina (IPE), que incluyen terapias de reemplazo enzimático, soluciones de gestión nutricional y métodos de pruebas de diagnóstico, son componentes cada vez más vitales de la atención gastrointestinal moderna debido a su capacidad para mejorar la absorción de nutrientes, mejorar los resultados del paciente y reducir las complicaciones asociadas con la desnutrición.
- La creciente demanda de terapias y diagnósticos EPI se ve impulsada principalmente por la creciente prevalencia de pancreatitis crónica, fibrosis quística y cáncer de páncreas, junto con una mayor conciencia sobre el diagnóstico temprano y una creciente preferencia por las terapias de reemplazo enzimático como opción de tratamiento de primera línea.
- Norteamérica dominó el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE), con la mayor participación en los ingresos, un 39,5 % en 2024. Esta región se caracterizó por una infraestructura sanitaria avanzada, un alto nivel de conocimiento entre pacientes y médicos, y una sólida presencia de actores clave del sector. Estados Unidos aportó la mayor parte de los ingresos regionales, impulsado por la creciente adopción de terapias de reemplazo de enzimas pancreáticas (PERT) y la disponibilidad de herramientas de diagnóstico avanzadas.
- Se espera que Asia-Pacífico sea la región de más rápido crecimiento en el mercado de terapias y diagnósticos de insuficiencia pancreática exocrina (EPI) durante el período de pronóstico, con una CAGR proyectada de 8,6% de 2025 a 2032, impulsada por el aumento de las inversiones en atención médica, la creciente prevalencia de trastornos pancreáticos, la mejora del acceso a instalaciones de diagnóstico y el aumento de los ingresos disponibles en países como China e India.
- El segmento de medicamentos de marca dominó el mercado de terapias y diagnósticos de la insuficiencia pancreática exocrina (EPI) con una participación en los ingresos del mercado del 56,1 % en 2024, debido a la presencia establecida de compañías farmacéuticas líderes, una sólida evidencia clínica y una mayor confianza de los médicos en las formulaciones PERT de marca.
Alcance del informe y segmentación del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
|
Atributos |
Información clave del mercado sobre la terapia y el diagnóstico de la insuficiencia pancreática exocrina (IPE) |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
Europa
Asia-Pacífico
Oriente Medio y África
Sudamerica
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis de expertos en profundidad, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de la terapia y el diagnóstico de la insuficiencia pancreática exocrina (IPE)
Mayor comodidad gracias a terapias y diagnósticos avanzados
- Una tendencia significativa y en aceleración en el mercado global de terapias y diagnósticos de la insuficiencia pancreática exocrina (EPI) es la creciente adopción de terapias avanzadas de reemplazo enzimático (PERT) y enfoques de diagnóstico innovadores que mejoran la precisión del tratamiento, la conveniencia del paciente y el manejo a largo plazo de la EPI.
- Por ejemplo, las principales compañías farmacéuticas están introduciendo formulaciones PERT de última generación con mayor estabilidad y eficacia, lo que permite a los pacientes gestionar mejor la mala digestión y la absorción de nutrientes. De igual manera, los avances en las pruebas diagnósticas no invasivas facilitan a los médicos la confirmación de la EPI en etapas tempranas, reduciendo así los retrasos en el diagnóstico.
- La integración de datos del mundo real (RWD) y herramientas de apoyo a la toma de decisiones clínicas en la atención EPI también permite a los profesionales sanitarios adaptar los regímenes de tratamiento con mayor eficacia. Estas innovaciones favorecen la optimización de las dosis, minimizan los efectos secundarios y garantizan una mejor adherencia a largo plazo en pacientes con enfermedades pancreáticas crónicas.
- Además, las mejoras en el diseño de las cápsulas y los mecanismos de administración de enzimas están proporcionando resultados más confiables para los pacientes al garantizar que las enzimas permanezcan activas hasta que llegan al intestino delgado, mejorando la eficiencia de la absorción y el estado nutricional.
- Esta tendencia hacia terapias y diagnósticos más eficaces, intuitivos y tecnológicamente avanzados está transformando radicalmente las expectativas en la atención de la IPE. Los innovadores farmacéuticos se centran en terapias con mayor biodisponibilidad y herramientas de diagnóstico que puedan ofrecer resultados rápidos y precisos.
- La demanda de terapias y diagnósticos EPI que ofrezcan mayor comodidad, mejores resultados de tratamiento y un manejo integral de la enfermedad está creciendo rápidamente en hospitales, clínicas y centros de atención especializada, a medida que los pacientes y los proveedores priorizan cada vez más la calidad de vida y la salud nutricional a largo plazo.
Dinámica del mercado de la terapia y el diagnóstico de la insuficiencia pancreática exocrina (IPE)
Conductor
Necesidad creciente debido a la creciente conciencia sobre las enfermedades y las terapias avanzadas
- La creciente prevalencia de trastornos pancreáticos, junto con la creciente conciencia sobre la salud digestiva y las mejores capacidades de diagnóstico, es un factor importante para la mayor demanda de terapias y diagnósticos de insuficiencia pancreática exocrina (EPI).
- Por ejemplo, en abril de 2024, importantes compañías farmacéuticas anunciaron el desarrollo de formulaciones de terapia de reemplazo enzimático pancreático (PERT) de última generación, destinadas a mejorar la eficacia, la tolerabilidad y la adherencia del paciente. Se espera que estas iniciativas de actores clave impulsen el crecimiento de la industria de la terapia y el diagnóstico de la insuficiencia pancreática exocrina (IPE) durante el período de pronóstico.
- A medida que los pacientes y los proveedores de atención médica reconocen cada vez más la importancia de la detección temprana y el tratamiento eficaz, los diagnósticos avanzados, como las pruebas de diagnóstico por imágenes, las pruebas de función pancreática y los análisis de heces, están ganando terreno, lo que proporciona intervenciones oportunas y mejores resultados del tratamiento.
- Además, el creciente énfasis en la nutrición personalizada, las terapias combinadas y las soluciones de monitoreo en el hogar está mejorando la atención centrada en el paciente tanto en entornos clínicos como ambulatorios, lo que respalda una adopción más amplia de terapias y diagnósticos EPI.
- La comodidad de las terapias enzimáticas orales, el manejo nutricional específico y la facilidad de acceso a los servicios de diagnóstico son factores clave que impulsan la adopción de soluciones para la insuficiencia pancreática exocrina (IPE) en hospitales, clínicas especializadas y centros de atención domiciliaria. La creciente disponibilidad de protocolos de tratamiento estandarizados y fáciles de usar contribuye aún más al crecimiento del mercado.
Restricción/Desafío
Altos costos de tratamiento y poca concientización en los mercados emergentes
- El costo relativamente alto de las formulaciones avanzadas de PERT y las pruebas diagnósticas especializadas supone un desafío significativo para una mayor penetración en el mercado. En regiones con precios sensibles, el acceso limitado a la infraestructura sanitaria y las limitaciones financieras pueden restringir su adopción.
- Además, la falta de conocimiento sobre los síntomas de la EPI y el subdiagnóstico en ciertas poblaciones han dificultado la detección y el tratamiento tempranos, lo que podría retrasar el inicio de la terapia.
- Abordar estos desafíos mediante campañas de educación de los pacientes, la expansión de la cobertura de seguros y el desarrollo de diagnósticos y terapias rentables es crucial para ampliar el acceso al mercado.
- Además, la investigación y la innovación continuas destinadas a producir formulaciones de reemplazo de enzimas asequibles y eficientes serán vitales para sostener el crecimiento del mercado a nivel mundial.
- Si bien los precios de algunas terapias enzimáticas genéricas están disminuyendo gradualmente, la percepción de un precio superior para las soluciones de diagnóstico de marca o avanzadas aún puede dificultar su adopción, especialmente en las regiones en desarrollo. Superar estos desafíos mediante una mayor cobertura sanitaria, la optimización de costes y la estandarización de las pautas de tratamiento será clave para la expansión a largo plazo de la industria.
Alcance del mercado de la terapéutica y el diagnóstico de la insuficiencia pancreática exocrina (IPE)
El mercado está segmentado en función del diagnóstico, el tratamiento, el tipo de fármaco, el usuario final y el canal de distribución.
- Por diagnóstico
En función del diagnóstico, el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se segmenta en pruebas de imagen y pruebas de función pancreática. El segmento de pruebas de función pancreática dominó la mayor cuota de mercado en ingresos, con un 52,3 % en 2024, gracias a su alta precisión en la evaluación de la deficiencia enzimática, su amplia adopción en la práctica clínica y su papel crucial en la orientación de los planes de tratamiento para pacientes con pancreatitis crónica y fibrosis quística. Las pruebas de función pancreática se consideran esenciales para la detección temprana y la monitorización continua, ofreciendo información práctica tanto para médicos como para pacientes. Proporcionan resultados fiables para el ajuste del tratamiento y el manejo a largo plazo. El segmento también se beneficia de la creciente cobertura de reembolsos en mercados desarrollados clave. Además, los profesionales sanitarios prefieren las pruebas de función pancreática por su precisión diagnóstica y su capacidad para complementar otras evaluaciones clínicas.
Se espera que el segmento de pruebas de imagen experimente la tasa de crecimiento anual compuesta (TCAC) más rápida, del 7,9 %, entre 2025 y 2032, impulsada por los avances en tecnologías de imagen no invasivas, como la resonancia magnética y la tomografía computarizada, la mayor concienciación sobre el diagnóstico precoz y su creciente adopción en clínicas especializadas y centros de diagnóstico. Las pruebas de imagen proporcionan información complementaria a los ensayos funcionales, lo que facilita la evaluación integral de la morfología pancreática y sus complicaciones. La expansión de la infraestructura sanitaria en Asia-Pacífico y Latinoamérica impulsa su rápida adopción. Las innovaciones tecnológicas han mejorado la resolución de las imágenes, facilitando la detección de cambios pancreáticos sutiles. La reducción de costes en equipos de imagen también está impulsando su adopción. En general, las pruebas de imagen se están convirtiendo en la opción preferida por los médicos que buscan un perfil diagnóstico completo.
- Por tratamiento
En función del tratamiento, el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se segmenta en gestión nutricional y terapia de reemplazo enzimático pancreático (TRE). El segmento de TRE obtuvo la mayor cuota de mercado en ingresos, con un 48,7 % en 2024, gracias a su eficacia demostrada para mejorar la absorción de nutrientes, reducir la desnutrición y mejorar la calidad de vida de los pacientes con IPE. La TRE es ampliamente recomendada por gastroenterólogos y goza de una sólida preferencia entre médicos y pacientes en Norteamérica y Europa. Garantiza la administración estandarizada de enzimas y resultados terapéuticos consistentes. Su adopción está respaldada por extensas guías clínicas. El segmento se beneficia de las continuas innovaciones en productos que mejoran la estabilidad y la biodisponibilidad enzimática. Los programas de adherencia al tratamiento también fortalecen la posición de mercado de las terapias TRE.
Se prevé que el segmento de gestión nutricional registre la tasa de crecimiento anual compuesta (TCAC) más rápida, del 8,4 %, entre 2025 y 2032, impulsada por la creciente adopción de dietas especializadas, suplementos nutricionales ricos en proteínas y planes de nutrición personalizados que complementan la terapia enzimática, especialmente en mercados emergentes con una mayor concienciación sobre las estrategias de gestión de la EPI. La gestión nutricional complementa la terapia PERT al abordar la desnutrición general y apoyar la salud del paciente. El creciente enfoque en las intervenciones sobre el estilo de vida y los programas dirigidos por dietistas impulsa el crecimiento. La disponibilidad de fórmulas nutricionales listas para usar acelera el cumplimiento terapéutico del paciente. La expansión de los servicios de atención domiciliaria para el apoyo nutricional está impulsando la adopción del mercado. Los mercados emergentes invierten cada vez más en programas de educación para pacientes que priorizan la gestión dietética.
- Por tipo de fármaco
Según el tipo de fármaco, el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se segmenta en formulaciones genéricas y de marca. El segmento de medicamentos de marca dominó la mayor cuota de mercado, con un 56,1%, en 2024, gracias a la presencia consolidada de compañías farmacéuticas líderes, la sólida evidencia clínica y la mayor confianza de los médicos en las formulaciones PERT de marca. Los medicamentos de marca suelen ofrecer mayor estabilidad, un contenido enzimático estandarizado y aprobaciones regulatorias que respaldan su adopción generalizada. Se benefician de sólidas campañas de marketing y educación. El segmento cuenta con el respaldo de redes de distribución exclusivas en los mercados desarrollados. Los medicamentos de marca mantienen la confianza del paciente gracias a su calidad constante. Los ensayos clínicos refuerzan aún más la eficacia de las formulaciones de marca.
Se prevé que el segmento de medicamentos genéricos experimente la tasa de crecimiento anual compuesta (TCAC) más rápida, del 9,2 %, entre 2025 y 2032, impulsada por la rentabilidad, el aumento de la cobertura sanitaria y la mayor disponibilidad en las regiones en desarrollo. Las formulaciones genéricas hacen que las terapias EPI sean más accesibles a una base de pacientes más amplia. El aumento de las aprobaciones regulatorias para genéricos está impulsando la entrada de los fabricantes en el mercado. La aceptación por parte de pacientes y proveedores está en aumento debido a una eficacia comparable a la de los medicamentos de marca. La adquisición a granel por parte de hospitales y farmacias minoristas impulsa el crecimiento. La asequibilidad impulsa la adopción en los mercados emergentes. Los programas educativos para profesionales clínicos sobre la eficacia de los genéricos están impulsando aún más su adopción.
- Por el usuario final
En función del usuario final, el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se segmenta en hospitales, clínicas especializadas, atención domiciliaria, centros de diagnóstico, institutos de investigación y académicos, entre otros. El segmento hospitalario dominó la mayor cuota de mercado en ingresos, con un 50,4 % en 2024, gracias a la alta afluencia de pacientes, las instalaciones de atención integrada y las sólidas redes de médicos que gestionan terapias y diagnósticos para la IPE. Los hospitales actúan como centros primarios tanto para el diagnóstico como para la administración de la TEP, garantizando la adherencia y el seguimiento continuo. Ofrecen atención integral, que incluye asesoramiento dietético y servicios de seguimiento. Los canales de distribución y adquisición establecidos en los hospitales fortalecen su cuota de mercado. Los hospitales también impulsan ensayos clínicos y colaboraciones de investigación para el manejo de la IPE. La preferencia institucional por las terapias estandarizadas respalda su dominio.
Se espera que el segmento de atención domiciliaria experimente la tasa de crecimiento anual compuesta (TCAC) más rápida, del 10,1 %, entre 2025 y 2032, impulsada por la creciente preferencia de los pacientes por la terapia enzimática domiciliaria, el apoyo a la monitorización remota y la creciente adopción de plataformas de telemedicina para el manejo de enfermedades. La atención domiciliaria mejora la comodidad y la adherencia del paciente. La expansión de proveedores especializados de atención domiciliaria facilita la administración de la terapia. La educación y la monitorización remotas mejoran la eficacia del tratamiento. El crecimiento del segmento se ve acelerado por la cobertura de seguros de apoyo para la atención domiciliaria. Las campañas de concienciación dirigidas a pacientes y cuidadores fomentan la atención domiciliaria. La administración tecnológica de terapia percutánea (PERT) y el apoyo nutricional impulsan la adopción.
- Por canal de distribución
Según el canal de distribución, el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se segmenta en licitación directa, farmacias minoristas, distribuidores externos y otros. El segmento de licitación directa representó la mayor cuota de mercado en ingresos, con un 45,6 % en 2024, impulsado por la adquisición a gran escala por parte de hospitales, clínicas especializadas y programas gubernamentales, lo que garantiza un suministro constante y ventajas en costos para los compradores de alto volumen. Los acuerdos de licitación directa suelen incluir precios preferenciales y contratos de suministro. Los hospitales y las grandes instituciones prefieren este canal por su fiabilidad. Las sólidas redes logísticas respaldan la eficiencia de la distribución. Este segmento garantiza el acceso a formulaciones de marca a gran escala. Las iniciativas gubernamentales para la concienciación sobre la IPE y la cobertura del tratamiento refuerzan aún más este canal. Los contratos a largo plazo mantienen la estabilidad del mercado y reducen la escasez de existencias.
Se prevé que el segmento de farmacias minoristas experimente la tasa de crecimiento anual compuesta (TCAC) más rápida, del 9,5 %, entre 2025 y 2032, impulsada por la creciente concienciación de los pacientes, la disponibilidad sin receta de suplementos nutricionales y productos PERT, y la expansión de las redes minoristas en zonas urbanas y semiurbanas. Las farmacias minoristas ofrecen a los pacientes acceso cómodo e inmediato a las terapias. Una mayor educación de los pacientes sobre el manejo de la EPI fomenta su adopción. Las alianzas con fabricantes mejoran la disponibilidad de existencias. La expansión minorista en mercados emergentes mejora la accesibilidad. El segmento se beneficia de las campañas de marketing dirigidas a cuidadores y pacientes. La creciente preferencia por la terapia autogestionada en casa impulsa el crecimiento del canal minorista.
Análisis regional del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
- América del Norte dominó el mercado de terapias y diagnósticos de la insuficiencia pancreática exocrina (EPI) con la mayor participación en los ingresos del 39,5 % en 2024, caracterizada por una infraestructura de atención médica avanzada, altos niveles de concienciación entre pacientes y médicos, y la fuerte presencia de actores clave de la industria.
- El crecimiento del mercado está impulsado por la creciente adopción de terapias de reemplazo de enzimas pancreáticas (PERT), mejores soluciones de gestión nutricional y la disponibilidad de herramientas de diagnóstico avanzadas para la detección temprana y el monitoreo de la insuficiencia pancreática.
- Las sólidas actividades de investigación, el creciente enfoque en los trastornos digestivos centrados en el cuerpo y la amplia cobertura de atención médica respaldan aún más la expansión del mercado en la región.
Análisis del mercado estadounidense de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
El mercado estadounidense de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) acaparó la mayor parte de los ingresos de Norteamérica en 2024, impulsado por la alta concienciación de los pacientes, la extensa investigación clínica y la rápida adopción de opciones de tratamiento innovadoras. La creciente prevalencia de trastornos pancreáticos crónicos, sumada a la disponibilidad de plataformas diagnósticas integrales, como las pruebas de imagen y de función pancreática, está impulsando una demanda significativa. Además, la creciente cartera de formulaciones avanzadas de PERT y la especialización en enfoques terapéuticos centrados en el paciente están impulsando el crecimiento del mercado.
Análisis del mercado europeo de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
Se proyecta que el mercado europeo de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se expanda a una tasa de crecimiento anual compuesta (TCAC) sustancial durante el período de pronóstico, impulsado principalmente por la creciente prevalencia de trastornos pancreáticos, el aumento del gasto sanitario y los marcos regulatorios favorables para el diagnóstico y la terapia. La creciente concienciación entre los profesionales sanitarios sobre el diagnóstico precoz y las estrategias óptimas de tratamiento está fomentando la adopción de enfoques farmacológicos y nutricionales. Los principales países europeos están realizando inversiones considerables en infraestructura sanitaria, lo que mejora el acceso a clínicas especializadas y centros de diagnóstico para los pacientes con IPE.
Análisis del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) en el Reino Unido
Se prevé que el mercado británico de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) crezca a una tasa de crecimiento anual compuesta (TCAC) notable durante el período de pronóstico, impulsado por una mayor concienciación sobre la salud digestiva, el aumento de las tasas de diagnóstico y la adopción tanto de la TPER como del tratamiento nutricional de apoyo. Además, la expansión de clínicas especializadas y centros de investigación especializados en trastornos pancreáticos está contribuyendo al desarrollo del mercado, mientras que los programas de atención médica que promueven la intervención temprana fomentan una mayor adopción del tratamiento.
Análisis del mercado alemán de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
Se prevé que el mercado alemán de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) se expanda a una tasa de crecimiento anual compuesta (TCAC) considerable, impulsado por un fuerte énfasis en la atención médica de precisión, la creciente inversión en tecnologías de diagnóstico y la demanda de soluciones terapéuticas tecnológicamente avanzadas. La creciente prevalencia de la insuficiencia pancreática, junto con una infraestructura hospitalaria bien desarrollada y un alto nivel de educación del paciente, respalda la adopción de la terapia de reemplazo enzimático y las pruebas diagnósticas avanzadas.
Análisis del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) en Asia-Pacífico
Se prevé que el mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) en Asia-Pacífico sea la región de mayor crecimiento durante el período de pronóstico, con una tasa de crecimiento anual compuesta (TCAC) del 8,6 % entre 2025 y 2032. Este crecimiento se ve impulsado por el aumento de la inversión en atención médica, la creciente prevalencia de trastornos pancreáticos, la mejora del acceso a centros de diagnóstico y el aumento de la renta disponible en países como China e India. La expansión de las campañas de concienciación sobre salud pública, las iniciativas gubernamentales de apoyo a la salud digestiva y el desarrollo de la capacidad local de fabricación de productos farmacéuticos están acelerando la penetración en el mercado.
Análisis del mercado japonés de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
El mercado japonés de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) está cobrando impulso debido a la rápida urbanización, el envejecimiento de la población y la creciente atención a la salud digestiva y pancreática. La adopción de terapias PERT avanzadas y soluciones diagnósticas integrales se ve respaldada por un sistema de salud tecnológicamente avanzado y una creciente concienciación de los pacientes.
Análisis del mercado de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) en China
El mercado chino de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE) representó una importante participación en los ingresos de Asia-Pacífico en 2024, gracias al desarrollo de la infraestructura sanitaria, el crecimiento de la clase media y las iniciativas gubernamentales para mejorar el diagnóstico y el tratamiento tempranos de los trastornos pancreáticos. La creciente prevalencia de problemas digestivos y la expansión del acceso a la atención médica en las zonas urbanas y semiurbanas son factores clave que impulsan el crecimiento del mercado.
Cuota de mercado de la terapéutica y el diagnóstico de la insuficiencia pancreática exocrina (IPE)
La industria de la terapéutica y el diagnóstico de la insuficiencia pancreática exocrina (EPI) está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- EagleBio (EE. UU.)
- AbbVie Inc. (EE. UU.)
- Nordmark Pharma GmbH (Alemania)
- Digestive Care, Inc. (EE. UU.)
- LUMITOS AG (Alemania)
- Alcresta Therapeutics, Inc. (EE. UU.)
- ChiRhoClin, Inc. (EE. UU.)
- Abbott (EE. UU.)
- Bioserv Analytics and Medical Devices GmbH (Alemania)
- LabCorp (EE. UU.)
- Grupo de empresas Organon (EE. UU.)
- Metagenics LLC (EE. UU.)
- Johnson & Johnson y sus filiales (EE. UU.)
- Nestlé Health Science (Suiza)
- VIVUS LLC (EE. UU.)
- ScheBo Biotech AG (Alemania)
Últimos avances en el mercado global de terapias y diagnósticos para la insuficiencia pancreática exocrina (IPE)
- En mayo de 2025, un estudio publicado en el Pharmaceutical Journal destacó que la insuficiencia exocrina pancreática (IEP) ocurre cuando el páncreas no puede producir suficientes enzimas para ayudar a la digestión, lo que conduce a la desnutrición debido a la absorción inadecuada de nutrientes.
- En agosto de 2021, AzurRx anunció que había participado en el desarrollo de la lipasa derivada de levadura, MS1819, que ha sido diseñada para tener una actividad enzimática superior en comparación con los tratamientos actuales.
- En febrero de 2023, Codexis, Inc. y Nestlé Health Science anunciaron los resultados provisionales de un ensayo de fase 1 que examina la seguridad, la tolerabilidad, la farmacocinética (FC) y la farmacodinamia del CDX-7108. Una variante de la lipasa, llamada CDX-7108, se creó expresamente para solucionar las desventajas del actual tratamiento de reemplazo de enzimas pancreáticas (PERT). Esto ha ayudado a la empresa a comercializar el producto.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.