Global Electronic Clinical Outcome Assessment Ecoa Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
1.70 Billion
USD
5.52 Billion
2024
2032
USD
1.70 Billion
USD
5.52 Billion
2024
2032
| 2025 –2032 | |
| USD 1.70 Billion | |
| USD 5.52 Billion | |
|
|
|
|
Segmentación del mercado global de evaluación electrónica de resultados clínicos (eCOA), por tipo (evaluación de resultados informada por el médico [CLINRO], evaluación de resultados informada por el paciente [PRO], evaluación de resultados informada por el observador [OBSRO] y evaluación de resultados de rendimiento [PERFO]), modalidad (soluciones in situ, soluciones web y dispositivos portátiles), usuario final (organizaciones de investigación por contrato [CRO], empresas farmacéuticas y biotecnológicas, empresas de dispositivos médicos, hospitales/proveedores de atención médica, empresas de servicios de consultoría, institutos académicos y de investigación, entre otros), modo de entrega (en la nube y alojado en la web): tendencias del sector y pronóstico hasta 2032
Tamaño del mercado de evaluación electrónica de resultados clínicos (eCOA)
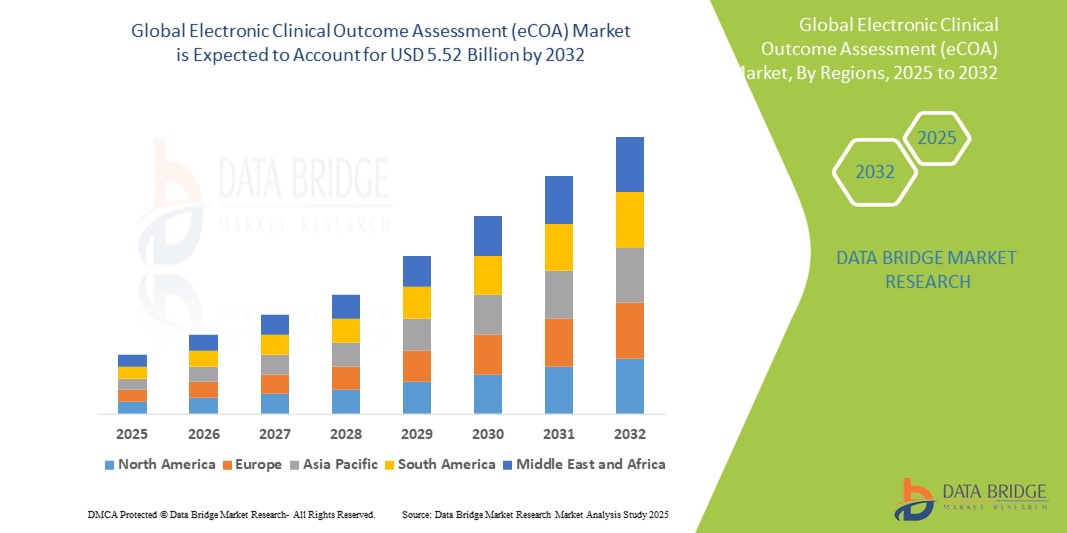
- El tamaño del mercado global de evaluación electrónica de resultados clínicos (eCOA) se valoró en USD 1.70 mil millones en 2024 y se espera que alcance los USD 5.52 mil millones para 2032 , con una CAGR del 15,80% durante el período de pronóstico.
- El crecimiento del mercado está impulsado principalmente por la creciente adopción de tecnologías digitales en ensayos clínicos e investigación sanitaria, lo que facilita una recopilación y un seguimiento más precisos y eficientes de datos de los pacientes.
- Además, la creciente demanda de información de los pacientes en tiempo real, un mejor cumplimiento normativo y una mayor integridad de los datos está impulsando la adopción de soluciones eCOA en empresas farmacéuticas, organizaciones de investigación por contrato (CRO) y proveedores de atención médica.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA)
- Las soluciones eCOA, que permiten la captura electrónica de datos de resultados clínicos directamente de pacientes, cuidadores o médicos, son componentes cada vez más vitales de los ensayos clínicos modernos y la investigación en atención médica debido a su mayor precisión de datos, capacidades de monitoreo en tiempo real e integración perfecta con los ecosistemas de salud digital.
- La creciente demanda de eCOA se ve impulsada principalmente por la adopción generalizada de tecnologías de salud digital, el énfasis creciente en los ensayos centrados en el paciente y una creciente preferencia por métodos de recopilación de datos remotos y fáciles de usar que mejoran la eficiencia y el cumplimiento de los ensayos.
- América del Norte domina el mercado de evaluación electrónica de resultados clínicos (eCOA) con la mayor participación en los ingresos del 43,5 % en 2024, caracterizada por la adopción temprana de soluciones de ensayos clínicos digitales, sectores farmacéuticos y biotecnológicos sólidos y marcos regulatorios que respaldan la captura electrónica de datos, mientras que Estados Unidos experimenta un crecimiento sustancial impulsado por innovaciones de proveedores establecidos y proveedores de tecnología emergentes centrados en plataformas móviles y basadas en la nube.
- Se espera que Asia-Pacífico sea la región de más rápido crecimiento en el mercado de evaluación electrónica de resultados clínicos (eCOA) durante el período de pronóstico debido al aumento de las actividades de ensayos clínicos, el aumento de las inversiones en atención médica y la creciente conciencia de los beneficios de las herramientas digitales en mercados emergentes como China e India.
- El segmento de evaluación de resultados informados por el paciente (PRO) domina el mercado de evaluación electrónica de resultados clínicos (eCOA) con una participación de mercado del 48,5 % en 2024, impulsado por su papel fundamental en la captura de las perspectivas de los pacientes sobre la eficacia del tratamiento y la calidad de vida, que son cada vez más priorizadas por los patrocinadores y las agencias reguladoras.
Alcance del informe y segmentación del mercado de evaluación electrónica de resultados clínicos (eCOA)
|
Atributos |
Análisis clave del mercado de la evaluación electrónica de resultados clínicos (eCOA) |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
Europa
Asia-Pacífico
Oriente Medio y África
Sudamerica
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis en profundidad de expertos, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de la evaluación electrónica de resultados clínicos (eCOA)
Mayor eficiencia en ensayos clínicos mediante IA y monitorización remota de pacientes
- Una tendencia significativa y en auge en el mercado global de eCOA es la creciente integración de la inteligencia artificial (IA) y las tecnologías de monitorización remota de pacientes en las plataformas de recopilación de datos de ensayos clínicos. Esta fusión de tecnologías está mejorando significativamente la precisión, la puntualidad y la orientación al paciente de las evaluaciones de resultados clínicos.
- Por ejemplo, proveedores líderes de eCOA, como Medidata y ERT, incorporan análisis basados en IA para identificar patrones en los datos notificados por los pacientes, lo que permite la detección temprana de eventos adversos y una mejor toma de decisiones en los ensayos clínicos. De igual manera, los dispositivos portátiles, combinados con plataformas de eCOA, facilitan la monitorización continua en tiempo real de las métricas de salud del paciente, más allá de las visitas clínicas tradicionales.
- La integración de IA en eCOA permite funciones como análisis predictivos para la adherencia del paciente, controles automatizados de la calidad de los datos y alertas inteligentes para respuestas inusuales del paciente. Además, las funciones de monitorización remota ofrecen a los pacientes interfaces prácticas e intuitivas para informar los resultados desde casa, lo que mejora la integridad de los datos y la participación.
- La integración fluida de los sistemas eCOA con plataformas más amplias de salud digital y gestión de ensayos clínicos permite a los patrocinadores centralizar la gestión de datos y optimizar los flujos de trabajo de los ensayos. Mediante paneles de control unificados, los equipos clínicos pueden supervisar los datos de los pacientes, el rendimiento del centro y el cumplimiento normativo en tiempo real.
- Esta tendencia hacia soluciones de resultados clínicos más inteligentes, conectadas y fáciles de usar para el paciente está transformando radicalmente las expectativas sobre la captura de datos de ensayos clínicos. En consecuencia, empresas como Oracle Health y CRF Health están desarrollando plataformas de eCOA basadas en IA con capacidades predictivas mejoradas y funcionalidades de captura remota de datos.
- La demanda de soluciones eCOA con integración de IA y monitorización remota de pacientes está creciendo rápidamente en los sectores farmacéutico, biotecnológico y de dispositivos médicos, a medida que las partes interesadas priorizan cada vez más la eficiencia de los ensayos, la precisión de los datos y la experiencia del paciente.
Dinámica del mercado de la evaluación electrónica de resultados clínicos (eCOA)
Conductor
Creciente demanda de ensayos centrados en el paciente y precisión de datos digitales
- El creciente enfoque en los ensayos clínicos centrados en el paciente, combinado con la creciente necesidad de una recopilación de datos digitales precisa y en tiempo real, es un impulsor importante de la mayor demanda de soluciones de evaluación electrónica de resultados clínicos (eCOA).
- Por ejemplo, en enero de 2024, Medidata, una empresa de Dassault Systèmes, introdujo nuevas mejoras basadas en IA en su plataforma eCOA para optimizar el cumplimiento del paciente y la calidad de los datos en ensayos clínicos descentralizados. Se espera que estas innovaciones, realizadas por actores clave del sector, impulsen el crecimiento del mercado de eCOA durante el período de pronóstico.
- A medida que las empresas farmacéuticas y de biotecnología buscan optimizar los procesos de ensayos clínicos y reducir el tiempo de comercialización, las plataformas eCOA ofrecen funciones avanzadas como captura de datos en tiempo real, informes remotos de pacientes y validación automatizada, lo que proporciona una mejora sustancial con respecto a los métodos tradicionales basados en papel.
- Además, la creciente adopción de modelos de ensayos clínicos descentralizados e híbridos está posicionando a eCOA como un componente esencial para la recopilación remota de datos, mejorando la participación del paciente y manteniendo altos estándares de cumplimiento normativo.
- La capacidad de las plataformas eCOA para mejorar la eficiencia de los ensayos mediante la recopilación electrónica de datos, el soporte multilingüe y la integración con dispositivos portátiles o aplicaciones móviles es un factor clave que impulsa su adopción por parte de las CRO, compañías farmacéuticas e instituciones de investigación. El creciente énfasis en la reducción de las tasas de abandono de los ensayos clínicos y la mejora de la integridad de los datos refuerza la integración generalizada de las soluciones eCOA en la investigación clínica moderna.
Restricción/Desafío
Preocupaciones sobre la privacidad de datos, el cumplimiento normativo y los altos costos de implementación
- Las preocupaciones en torno a la privacidad de los datos, el cumplimiento normativo y los altos costos iniciales de implementación de las plataformas de evaluación electrónica de resultados clínicos (eCOA) plantean desafíos importantes para una adopción más amplia en el mercado.
- Como los sistemas eCOA implican la captura y transmisión electrónica de datos confidenciales de salud de los pacientes, están sujetos a estrictas regulaciones de protección de datos como HIPAA, GDPR y 21 CFR Parte 11, lo que hace que el cumplimiento sea complejo y requiera muchos recursos para los patrocinadores y las CRO.
- Por ejemplo, varios patrocinadores de ensayos clínicos han expresado cautela a la hora de realizar la transición completa a sistemas eCOA debido a las incertidumbres en torno a las normas de localización de datos y la complejidad de garantizar el cumplimiento de los datos transfronterizos, especialmente en ensayos multirregionales.
- Abordar estos desafíos requiere una infraestructura robusta de seguridad de datos, auditorías periódicas y el cumplimiento de los estándares globales de cumplimiento. Proveedores líderes de eCOA, como Oracle Health y Signant Health, invierten significativamente en plataformas cifradas y capacitación regulatoria para mitigar el riesgo y mantener la confianza de las partes interesadas en los ensayos.
- Además, el elevado coste inicial asociado a la implementación de sistemas eCOA —incluyendo las tasas de licencia, la adquisición de hardware, la formación del personal y la integración de sistemas— puede ser una barrera de entrada, especialmente para organizaciones de investigación pequeñas y medianas. Si bien los beneficios a largo plazo, como la mejora de la precisión de los datos y la reducción de la duración de los ensayos, son ampliamente reconocidos, la carga financiera inicial puede limitar su adopción en instituciones con recursos limitados.
- Superar estos desafíos a través de modelos de precios escalables, entrega basada en la nube e innovación continua en plataformas seguras y fáciles de usar será esencial para impulsar una adopción más amplia y sostenida de soluciones eCOA en todo el panorama de la investigación clínica.
Alcance del mercado de la evaluación electrónica de resultados clínicos (eCOA)
El mercado está segmentado según tipo, modalidad, usuario final y modo de entrega.
- Por tipo
Según el tipo, el mercado de la evaluación electrónica de resultados clínicos (eCOA) se segmenta en resultados informados por el paciente (PRO), resultados informados por el clínico (ClinRO), resultados informados por el observador (ObsRO) y resultados de rendimiento (PerfO). El segmento de resultados informados por el paciente (PRO) obtuvo la mayor cuota de mercado, con un 48,5 % en 2024, gracias a su enfoque centrado en el paciente, que permite obtener información directa sobre sus experiencias, síntomas y resultados del tratamiento. Las herramientas PRO permiten a los pacientes informar directamente sobre sus datos de salud en tiempo real a través de plataformas electrónicas, lo que mejora la precisión de los datos y la participación del paciente, mejorando así la calidad de los estudios clínicos.
Se prevé un crecimiento sustancial del segmento de resultados informados por el médico (ClinRO) durante el período de pronóstico, debido a la creciente complejidad de los ensayos clínicos y a la necesidad de métodos de recopilación de datos precisos y estandarizados. ClinRO implica evaluaciones realizadas por profesionales sanitarios capacitados, que proporcionan datos objetivos y fiables para evaluar las intervenciones clínicas, especialmente en casos en los que la autodeclaración del paciente no es viable.
- Por modalidad
Según la modalidad, el mercado de la evaluación electrónica de resultados clínicos (eCOA) se segmenta en soluciones in situ, soluciones web y dispositivos portátiles. El segmento de soluciones web obtuvo la mayor cuota de mercado en 2024, gracias a sus interfaces intuitivas, su fácil acceso y la menor inversión requerida. Las soluciones alojadas en la web almacenan los datos de los clientes en servidores en la nube, accesibles a través de la web con hardware informático básico y conexión a internet, lo que ofrece flexibilidad de personalización y permite adaptarlos a las necesidades específicas del cliente.
Se espera que el segmento de dispositivos portátiles experimente un crecimiento significativo durante el período de pronóstico, impulsado por la creciente adopción de tecnologías móviles en ensayos clínicos. Los dispositivos portátiles facilitan la captura de datos en tiempo real y mejoran el cumplimiento del paciente, lo que los convierte en una opción atractiva para estudios clínicos descentralizados y remotos.
- Por el usuario final
En función del usuario final, el mercado de la evaluación electrónica de resultados clínicos (eCOA) se segmenta en empresas farmacéuticas y biotecnológicas, organizaciones de investigación por contrato (CRO), empresas de dispositivos médicos, hospitales/proveedores de servicios de salud, empresas de servicios de consultoría, institutos académicos y de investigación, entre otros. El segmento de empresas farmacéuticas y biotecnológicas domina el mercado, representando el 50,66 % de la cuota de mercado en 2024. Este predominio se debe al papel fundamental que desempeñan las soluciones de eCOA para optimizar la recopilación y el análisis de datos durante los procesos de desarrollo de fármacos, garantizar el cumplimiento de las normas regulatorias y mejorar la precisión de los datos de los ensayos clínicos.
Se prevé un crecimiento sustancial del segmento de las organizaciones de investigación por contrato (CRO) durante el período de pronóstico, impulsado por la creciente tendencia de las principales empresas biofarmacéuticas y de dispositivos médicos a externalizar la gestión de la investigación clínica. Las CRO ofrecen servicios integrales que abarcan el diseño de estudios, el reclutamiento de pacientes, la recopilación y el análisis de datos, lo que las convierte en actores clave en el panorama de los eCOA.
Por modo de entrega
Según el modo de entrega, el mercado de la evaluación electrónica de resultados clínicos (eCOA) se segmenta en soluciones en la nube y alojadas en la web. El segmento de soluciones alojadas en la web tuvo la mayor cuota de mercado, con un 58,9 %, en 2025, gracias a su rentabilidad en comparación con las soluciones en la nube. Las plataformas alojadas en la web implican una menor inversión inicial en infraestructura para los usuarios finales, lo que reduce los gastos de capital para las compañías farmacéuticas, las CRO y los proveedores de atención médica.
Se espera que el segmento de soluciones en la nube experimente un crecimiento significativo durante el período de pronóstico, impulsado por su escalabilidad, flexibilidad y rentabilidad. Las plataformas en la nube facilitan y agilizan el acceso a los datos para las partes interesadas en ensayos clínicos, independientemente de su ubicación, lo cual es crucial para los ensayos multicéntricos.
Análisis regional del mercado de la evaluación electrónica de resultados clínicos (eCOA)
- América del Norte domina el mercado de evaluación electrónica de resultados clínicos (eCOA) con la mayor participación en los ingresos del 43,5 % en 2024, impulsada por la adopción temprana de soluciones de ensayos clínicos digitales, sectores farmacéuticos y biotecnológicos sólidos y marcos regulatorios que respaldan la captura electrónica de datos.
- La región se beneficia de un marco regulatorio sólido que apoya la transformación digital de la investigación clínica, alentando a las compañías farmacéuticas y organizaciones de investigación por contrato a adoptar plataformas eCOA para mejorar la precisión de los datos y el cumplimiento normativo.
- Además, las fuertes inversiones en I+D, la sólida infraestructura sanitaria y la adopción temprana de ensayos clínicos descentralizados y centrados en el paciente contribuyen significativamente al crecimiento del mercado. La presencia de proveedores líderes de soluciones eCOA y CRO acelera aún más la expansión regional de las herramientas de evaluación electrónica de resultados clínicos.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA) en EE. UU.
El mercado estadounidense de evaluación electrónica de resultados clínicos (eCOA) capturó la mayor participación en los ingresos, con un 79,6 %, en 2024 en Norteamérica, impulsado por el liderazgo del país en ensayos clínicos y la rápida digitalización de las prácticas de investigación clínica. Agencias reguladoras como la FDA abogan firmemente por el uso de herramientas digitales para mejorar la calidad de los datos y la participación del paciente, lo que contribuye a la adopción generalizada de los sistemas eCOA. Además, la creciente necesidad de modelos de ensayos clínicos descentralizados e híbridos está impulsando la demanda de plataformas remotas de recopilación de datos de pacientes en tiempo real. El mercado estadounidense también se beneficia de una sólida financiación en I+D, una amplia presencia de gigantes farmacéuticos y una sofisticada infraestructura de TI para la salud.
Análisis del mercado europeo de la evaluación electrónica de resultados clínicos (eCOA)
Se prevé que el mercado europeo de evaluación electrónica de resultados clínicos (eCOA) se expanda a una tasa de crecimiento anual compuesta (TCAC) sustancial durante el período de pronóstico, impulsado por el creciente énfasis regulatorio en la evidencia del mundo real, la atención centrada en el paciente y la estandarización de datos en los ensayos clínicos. La creciente necesidad de soluciones digitales multilingües y adaptadas culturalmente en toda la UE está acelerando la adopción de plataformas de eCOA flexibles y escalables. Además, el aumento de las colaboraciones en investigación académica, junto con políticas favorables para la transformación digital de la salud, impulsa el crecimiento regional. Países como Alemania, el Reino Unido y Francia lideran la adopción de tecnología en sus respectivos ecosistemas de ensayos.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA) en el Reino Unido
Se prevé que el mercado británico de evaluación electrónica de resultados clínicos (eCOA) crezca a una tasa de crecimiento anual compuesta (TCAC) notable durante el período de pronóstico, impulsado por su sólido sector de I+D biofarmacéutico y las estrategias proactivas de salud digital del NHS. El creciente número de ensayos descentralizados y la claridad regulatoria en torno a la captura electrónica de datos están impulsando su adopción en el mercado. Con un panorama avanzado de investigación clínica y una inversión significativa en informática sanitaria, el Reino Unido está experimentando una rápida adopción de las tecnologías de eCOA para garantizar el cumplimiento normativo, mejorar la participación del paciente y permitir un seguimiento eficiente de los resultados.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA) en Alemania
Se espera que el mercado alemán de evaluación electrónica de resultados clínicos (eCOA) crezca a una tasa de crecimiento anual compuesta (TCAC) considerable durante el período de pronóstico, impulsado por la reputación del país en cuanto a la excelencia en ensayos clínicos y sus estrictas leyes de protección de datos. Los organismos reguladores alemanes priorizan la fiabilidad y seguridad de los datos clínicos, lo que impulsa a los patrocinadores y a las CRO a invertir en soluciones de eCOA seguras y validadas. Además, la creciente demanda alemana de captura de datos en tiempo real en ensayos de fase I a IV y su sólida infraestructura de TI sanitaria promueven una mayor integración de las tecnologías de eCOA en la investigación de dispositivos médicos y farmacéutica.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA) en Asia-Pacífico
Se prevé que el mercado de evaluación electrónica de resultados clínicos (eCOA) en Asia-Pacífico crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida durante el período de pronóstico de 2025 a 2032, impulsado por el aumento de la actividad de investigación clínica y la transformación digital de la atención médica en países como China, India, Corea del Sur y Japón. La expansión de los ensayos multinacionales y la disponibilidad de diversas poblaciones de pacientes impulsan el crecimiento regional. Los incentivos gubernamentales para la adopción de plataformas de salud digital y la creciente demanda de soluciones móviles hacen que la adopción de eCOA sea más viable y generalizada en las regiones urbanas y semiurbanas. Las alianzas locales entre CRO y farmacéuticas globales están impulsando aún más la implementación de eCOA.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA) en Japón
El mercado japonés de evaluación electrónica de resultados clínicos (eCOA) está cobrando impulso debido a la sofisticación tecnológica del país, el envejecimiento de la población y la importancia de la calidad de los datos en los ensayos clínicos. El organismo regulador japonés, la PMDA, se muestra cada vez más receptivo a los puntos finales digitales y a las herramientas de captura remota de datos. El mercado también se ve influenciado por el aumento de los ensayos clínicos domiciliarios y ambulatorios, lo que impulsa la necesidad de sistemas de eCOA precisos y fáciles de usar para el paciente. Se espera que la integración con ecosistemas eClínicos más amplios y las herramientas de interacción con el paciente basadas en IA impulsen aún más el crecimiento.
Análisis del mercado de la evaluación electrónica de resultados clínicos (eCOA) en India
El mercado indio de evaluación electrónica de resultados clínicos (eCOA) representó la mayor cuota de mercado en ingresos en Asia Pacífico en 2024, impulsado por un aumento en la actividad de ensayos clínicos, una población con conocimientos tecnológicos y la expansión de la capacidad de fabricación farmacéutica. El panorama rentable de CRO de India y las políticas gubernamentales favorables a la digitalización de la salud están animando a los patrocinadores globales a implementar herramientas de eCOA en ensayos nacionales. La creciente penetración de teléfonos inteligentes, el auge de la telemedicina y la mejora de la conectividad a internet en zonas urbanas y semiurbanas están haciendo que las plataformas de eCOA basadas en dispositivos móviles y alojadas en la nube sean más accesibles y ampliamente adoptadas.
Cuota de mercado de la evaluación electrónica de resultados clínicos (eCOA)
La industria de evaluación electrónica de resultados clínicos (eCOA) está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- IQVIA (EE. UU.)
- Clario (EE. UU.)
- Medidata (EE. UU.)
- Veeva Systems (EE. UU.)
- Tecnología de Recursos Terrestres (EE. UU.)
- Oracle Health Sciences (EE. UU.)
- YPrime, LLC (EE. UU.)
- ArisGlobal LLC (EE. UU.)
- Castor EDC (Países Bajos)
- eClinicalWorks (EE. UU.)
- Medrio, Inc. (EE. UU.)
- ClinOne (EE. UU.)
- Signant Health (EE. UU.)
- Clinical Ink, Inc. (EE. UU.)
- Curebase, Inc. (EE. UU.)
- Kayentis (Francia)
- Calyx (Reino Unido)
- Datacubed Health (EE. UU.)
- HealthDiary, Inc. (EE. UU.)
Últimos avances en el mercado global de evaluación electrónica de resultados clínicos (eCOA)
- En mayo de 2025, Clario (EE. UU.) adquirió el negocio de eCOA de WCG Clinical (EE. UU.), una decisión estratégica para reforzar su liderazgo en soluciones digitales de datos de puntos finales, en particular para ensayos clínicos en neurociencia. Esta adquisición amplía la plataforma integral de datos de puntos finales de Clario, lo que permite un mejor soporte para entornos de ensayos complejos y consolida aún más su posición en el cambiante panorama de eCOA.
- En mayo de 2025, el Critical Path Institute (EE. UU.) continuó su iniciativa "eCOA: Mejorando Juntos", cuyo objetivo es unificar a patrocinadores, proveedores de tecnología y organismos reguladores. Este esfuerzo colaborativo, que se extenderá hasta marzo de 2025, se centra en establecer las mejores prácticas precompetitivas y un léxico común para la captura de datos de eCOA, promoviendo la estandarización y acelerando su adopción en diversas regiones.
- En noviembre de 2023, Clinical Ink mejoró su plataforma de interacción con el paciente al incorporar la herramienta de diagnóstico conductual SPUR de Observia. Esta integración combina la evaluación conductual con la modificación del estilo de vida, eCOA, eSource y biomarcadores digitales, con el objetivo de proporcionar una comprensión más integral del comportamiento del paciente y mejorar los resultados de los ensayos clínicos.
- En octubre de 2023, Clario estableció una alianza estratégica con Trial Data, proveedor de servicios de ensayos clínicos descentralizados (ECD). Esta colaboración fortalece la presencia de Clario en el sector de ensayos clínicos de China, combinando su experiencia para ofrecer soluciones de ensayos descentralizados de vanguardia y promover enfoques centrados en el paciente en la región.
- En diciembre de 2022, Suvoda LLC, empresa de tecnología de eCOA para ensayos clínicos, presentó su kit de herramientas para el diseño de evaluaciones electrónicas de resultados clínicos (eCOA). Este kit se creó para integrarse fluidamente con Suvoda IRT y eConsent, solucionando deficiencias históricas en la implementación de eCOA y optimizando el proceso de diseño.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.