Global Asthma Spacers Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
1.86 Billion
USD
2.62 Billion
2024
2032
USD
1.86 Billion
USD
2.62 Billion
2024
2032
| 2025 –2032 | |
| USD 1.86 Billion | |
| USD 2.62 Billion | |
|
|
|
|
Segmentación del mercado global de espaciadores para el asma, por clase de fármaco (antiinflamatorios, broncodilatadores y terapia combinada), tipo de producto (inhaladores y nebulizadores), vía de administración (oral, inhalada, otras) y canal de distribución (farmacia minorista, farmacia hospitalaria y comercio electrónico ): tendencias y pronóstico del sector hasta 2032.
Tamaño del mercado de espaciadores para el asma
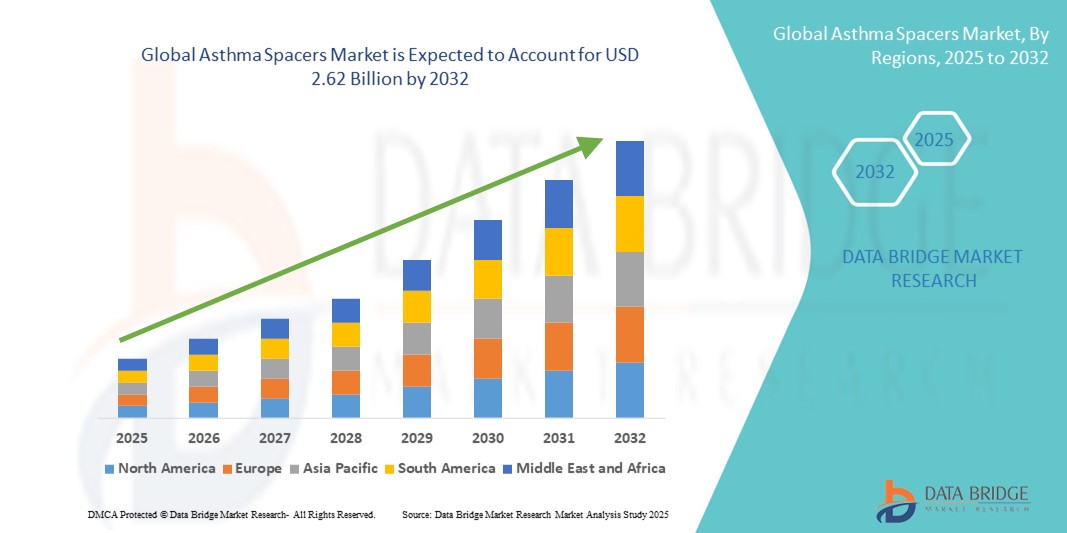
- El tamaño del mercado mundial de espaciadores para el asma se valoró en USD 1.860 millones en 2024 y se espera que alcance los USD 2.620 millones para 2032 , con una CAGR del 4,32 % durante el período de pronóstico.
- El crecimiento del mercado se ve impulsado en gran medida por la creciente adopción y el progreso tecnológico en los dispositivos de terapia de inhalación y el cuidado respiratorio, lo que conduce a una mayor digitalización e innovación de productos en el manejo del asma tanto en el hogar como en la clínica.
- Además, la creciente demanda de soluciones seguras, fáciles de usar e integradas para el manejo del asma en niños, ancianos y personas con problemas de coordinación está consolidando los espaciadores para el asma como la herramienta preferida para mejorar la administración. Estos factores convergentes están acelerando la adopción de las soluciones de espaciadores para el asma, impulsando así significativamente el crecimiento de la industria.
Análisis del mercado de espaciadores para el asma
- Los espaciadores para el asma, también conocidos como cámaras de inhalación, son dispositivos complementarios esenciales que se utilizan con los inhaladores de dosis medida (IDM) para optimizar la administración directa de medicamentos a los pulmones. Su uso está extendido tanto en entornos clínicos como domiciliarios gracias a su capacidad para reducir los errores de inhalación, mejorar la eficacia de los fármacos y mejorar el cumplimiento terapéutico del paciente, especialmente en niños y ancianos.
- La creciente prevalencia mundial del asma, que actualmente afecta a más de 339 millones de personas en todo el mundo (según la OMS), junto con el creciente énfasis clínico en la técnica correcta de inhalación, está incrementando significativamente la demanda de espaciadores para el asma. Además, las directrices de organizaciones sanitarias como GOLD y GINA recomiendan encarecidamente el uso de espaciadores para mejorar los resultados del tratamiento.
- América del Norte dominó el mercado de espaciadores para el asma con la mayor participación en los ingresos del 38,4 % en 2024, impulsada por altos niveles de conocimiento, políticas de reembolso favorables y una fuerte presencia de fabricantes líderes como Trudell Medical International, Philips Healthcare y PARI Respiratory Equipment, Inc. El mercado estadounidense está siendo testigo de un aumento constante en la adopción de espaciadores en unidades de atención pediátrica, centros de atención primaria y farmacias minoristas.
- Se proyecta que Asia-Pacífico será la región de mayor crecimiento en el mercado de espaciadores para el asma durante el período de pronóstico, registrando una tasa de crecimiento anual compuesta (TCAC) del 8,9 % entre 2025 y 2032. Este crecimiento se atribuye al rápido aumento de los diagnósticos de asma, el incremento de la contaminación urbana y la mejora del acceso a productos para el cuidado respiratorio en mercados emergentes como India, China e Indonesia. La producción local y la asequibilidad también contribuyen a una mayor penetración en el mercado.
- El segmento inhalado dominó el mercado de espaciadores para el asma con una participación del 81,3 % en 2024, ya que la terapia inhalada sigue siendo el estándar de oro en el tratamiento del asma, ofreciendo una administración dirigida de medicamentos con menos efectos secundarios sistémicos.
Alcance del informe y segmentación del mercado de espaciadores para el asma
|
Atributos |
Información clave sobre el mercado de los espaciadores para el asma |
|
Segmentos cubiertos |
|
|
Países cubiertos |
América del norte
Europa
Asia-Pacífico
Oriente Medio y África
Sudamerica
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis en profundidad de expertos, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de espaciadores para el asma
Mayor comodidad gracias a la integración de salud digital y la compatibilidad con inhaladores inteligentes
- Una tendencia significativa y en auge en el mercado global de espaciadores para el asma es la integración de tecnologías de salud digital con los sistemas de inhaladores, lo que mejora la adherencia al tratamiento, el seguimiento de la medicación y el manejo general del asma. Esta evolución está transformando los espaciadores convencionales en herramientas más inteligentes y centradas en el paciente.
- Por ejemplo, Teva Pharmaceuticals lanzó ProAir Digihaler, un inhalador digital aprobado por la FDA que se integra con una aplicación móvil para registrar los datos de uso y enviar recordatorios. Al usarse con espaciadores compatibles, esta integración permite a los pacientes y cuidadores monitorear la frecuencia del tratamiento y la técnica de inhalación en tiempo real.
- Varias soluciones compatibles con espaciadores digitales incorporan sensores con Bluetooth que rastrean las inhalaciones y sincronizan datos con aplicaciones para teléfonos inteligentes. Estas herramientas facilitan la monitorización remota de pacientes por parte de los profesionales sanitarios y permiten una intervención proactiva en casos de mala adherencia o deterioro del control.
- También se está explorando la inteligencia artificial (IA) para analizar los patrones de uso de inhaladores mediante dispositivos conectados y ofrecer información personalizada, como la identificación de desencadenantes, la señalización de tendencias de uso anormales o la predicción de exacerbaciones del asma. Esto resulta especialmente beneficioso en el manejo de casos crónicos en niños y personas mayores que pueden requerir la supervisión de un cuidador.
- Además, los recordatorios asistidos por voz y la integración de aplicaciones móviles con plataformas como Apple Health, Google Fit y otros ecosistemas de salud ayudan a los pacientes a seguir el plan de tratamiento del asma prescrito. Estas funciones son cada vez más populares entre los pacientes expertos en tecnología que buscan comodidad y control sobre su salud respiratoria.
- Esta tendencia hacia sistemas de espaciadores para el asma inteligentes, con integración de datos y fáciles de usar está transformando las expectativas de los pacientes e impulsando a los fabricantes a innovar. Empresas como Trudell Medical International, Philips Respironics y startups emergentes de salud digital están a la vanguardia de esta transformación, ofreciendo dispositivos que combinan precisión clínica con herramientas de interacción digital para lograr mejores resultados.
- Se espera que la demanda de espaciadores para el asma que ofrezcan una compatibilidad perfecta con inhaladores inteligentes, aplicaciones móviles de salud y plataformas de monitoreo digital crezca rápidamente a medida que los sistemas de atención médica cambian hacia modelos de atención respiratoria personalizados, remotos y preventivos.
Dinámica del mercado de los espaciadores para el asma
Conductor
Necesidad creciente debido a la creciente prevalencia del asma y la atención prestada a la eficiencia de los inhaladores
- La creciente carga mundial de asma, sumada a la creciente importancia de la técnica correcta de inhalación, es un factor clave para la creciente demanda de espaciadores para el asma. Estos dispositivos son esenciales para mejorar la administración de medicamentos y garantizar un manejo eficaz del asma, especialmente en la población pediátrica y geriátrica.
- Por ejemplo, en octubre de 2023, Trudell Medical International lanzó una versión actualizada de su popular espaciador AeroChamber Plus Flow-Vu, con materiales antiestáticos mejorados y un diseño de cámara optimizado para mejorar la deposición del fármaco. El dispositivo también incluye un indicador visual de flujo para ayudar a pacientes y cuidadores a confirmar el tiempo de inhalación correcto, lo que aborda uno de los problemas más comunes en el tratamiento del asma.
- Las directrices de organizaciones como NICE (Reino Unido) y GINA recomiendan cada vez más el uso de cámaras de retención con válvula (VHC) tanto en entornos clínicos como domiciliarios para reducir los errores de coordinación y mejorar los resultados del tratamiento. La demanda es especialmente alta en escuelas, clínicas pediátricas y entre los cuidadores que buscan alternativas más seguras al uso directo de inhaladores.
- Además, las crecientes campañas de concienciación sobre el cuidado del asma, especialmente durante las celebraciones mundiales de salud respiratoria, están ayudando a educar a los pacientes sobre la importancia del uso de espaciadores. Esto está impulsando su adopción tanto en países de altos ingresos como en mercados emergentes donde anteriormente los espaciadores estaban infrautilizados.
- Además, la comodidad de las opciones de espaciadores portátiles, antiestáticos y reutilizables, junto con la creciente disponibilidad a través de farmacias en línea y minoristas, está haciendo que estos dispositivos sean más accesibles que nunca, lo que respalda un crecimiento generalizado del mercado.
Restricción/Desafío
Falta de concienciación y reembolsos limitados en algunas regiones
- A pesar de su importancia clínica, los espaciadores para el asma siguen siendo infrautilizados en muchas regiones debido a la escasa concienciación entre los pacientes e incluso los profesionales sanitarios sobre los beneficios de su uso con inhaladores de dosis medida (IDM). Muchas personas creen erróneamente que los IDM por sí solos son suficientes, ignorando su papel en la mejora del depósito pulmonar y la reducción de los efectos secundarios.
- Según una encuesta de 2023 publicada en el European Respiratory Journal, menos del 50 % de los pacientes con asma en algunas partes de Europa utilizan regularmente una cámara de inhalación con su inhalador, incluso cuando se les prescribe. Esta brecha se atribuye en gran medida a la educación insuficiente del paciente y a la escasa demostración en la clínica del uso correcto de la cámara.
- Otro desafío clave es la falta de reembolso de seguros para los espaciadores en ciertos sistemas de salud, especialmente en las economías en desarrollo. Si bien el NHS del Reino Unido y los sistemas de Canadá y Australia suelen cubrir los espaciadores, los pacientes de otras regiones podrían tener que comprarlos de su bolsillo, lo que reduce el acceso en comunidades de bajos ingresos.
- Además, las alternativas de espaciadores desechables o de baja calidad que se venden en línea (sin la orientación médica adecuada) pueden comprometer la eficacia del tratamiento, lo que socava aún más la confianza del consumidor en la categoría.
- Superar estas barreras requerirá un esfuerzo coordinado entre profesionales sanitarios, legisladores y fabricantes. Las estrategias incluyen ampliar la educación del paciente, mejorar el etiquetado de los productos, promover la formación de los profesionales clínicos y abogar por una cobertura más amplia de los seguros para los espaciadores recomendados por médicos.
Alcance del mercado de los espaciadores para el asma
El mercado está segmentado según la clase de medicamento, el tipo de producto, la vía de administración y el canal de distribución.
- Por clase de fármaco
Según la clase de fármaco, el mercado de espaciadores para el asma se segmenta en antiinflamatorios, broncodilatadores y terapia combinada. El segmento de terapia combinada dominó la mayor cuota de mercado con un 44,8 % en 2024, gracias a su eficacia en el tratamiento de casos de asma de moderados a graves mediante la combinación de acciones antiinflamatorias y broncodilatadoras.
Se espera que el segmento de broncodilatadores experimente la CAGR más rápida del 8,2 % entre 2025 y 2032, impulsada por el aumento de casos de asma de emergencia y el uso cada vez mayor de medicamentos de rescate entre las poblaciones pediátricas y de edad avanzada.
- Por tipo de producto
Según el tipo de producto, el mercado se segmenta en inhaladores y nebulizadores. El segmento de inhaladores representó la mayor participación en los ingresos, con un 57,6 %, en 2024, debido principalmente a la facilidad de uso, la portabilidad y la creciente adopción de inhaladores de dosis medida (IDM) e inhaladores de polvo seco (IPS) con espaciadores.
Se anticipa que el segmento de nebulizadores crecerá a la CAGR más rápida del 7,9 % durante el período de pronóstico, impulsado por su uso creciente en hospitales y para pacientes con afecciones respiratorias graves o crónicas.
- Por vía de administración
Según la vía de administración, el mercado se segmenta en oral, inhalado y otros. El segmento inhalado captó la mayor participación, con un 81,3 %, en 2024, ya que la terapia inhalada sigue siendo el tratamiento de referencia para el asma, ofreciendo una administración dirigida del fármaco con menos efectos secundarios sistémicos.
Se proyecta que el segmento oral crecerá a una CAGR del 6,1% entre 2025 y 2032, particularmente en casos leves y entre pacientes pediátricos que enfrentan desafíos al usar dispositivos de inhalación.
- Por canal de distribución
Según el canal de distribución, el mercado de espaciadores para el asma se segmenta en farmacias minoristas, farmacias hospitalarias y comercio electrónico. El segmento de farmacias minoristas obtuvo la mayor participación en los ingresos, con un 46,7 %, en 2024, gracias a su amplia accesibilidad, la creciente disponibilidad de medicamentos sin receta (OTC) y la confianza del consumidor.
Se espera que el segmento de comercio electrónico registre la CAGR más alta del 9,5 % durante el período de pronóstico debido a la creciente penetración de Internet, la conveniencia y la expansión de las plataformas de telemedicina y salud digital.
Análisis regional del mercado de espaciadores para el asma
- América del Norte dominó el mercado de espaciadores para el asma con la mayor participación en los ingresos del 38,4 % en 2024.
- Impulsado por la alta prevalencia del asma, el uso generalizado de inhaladores de dosis medida (IDM) y un fuerte énfasis clínico en las técnicas de inhalación adecuadas.
- La región se beneficia de una infraestructura de atención médica bien establecida, una alta conciencia de salud pública y una sólida presencia de fabricantes clave como Trudell Medical International y Philips Respironics.
Perspectiva del mercado de espaciadores para el asma en EE. UU.
El mercado estadounidense de espaciadores para el asma captó la mayor participación en los ingresos, con un 79,05 %, en Norteamérica en 2024, gracias a las altas tasas de diagnóstico de asma (que afectan a más de 25 millones de estadounidenses), las favorables políticas de reembolso y la amplia adopción de las directrices de los CDC y los NIH para el manejo del asma. La mayor disponibilidad de espaciadores antiestáticos y cámaras de retención con válvula, tanto en entornos clínicos como domiciliarios, está impulsando la penetración en el mercado. El mercado también se beneficia de la reposición de recetas a través de la telesalud y de los programas educativos digitales que promueven el uso correcto de los inhaladores.
Perspectiva del mercado europeo de espaciadores para el asma
Se proyecta que el mercado europeo de espaciadores para el asma se expanda a una tasa de crecimiento anual compuesta (TCAC) sustancial durante el período de pronóstico, principalmente debido a la mayor concienciación sobre los protocolos de control del asma y las recomendaciones clínicas en todos los países. Los servicios nacionales de salud en regiones como el Reino Unido, Alemania y Francia están promoviendo el uso de espaciadores en la población pediátrica y de edad avanzada. Las iniciativas de atención del asma en las escuelas y las campañas de salud pública respaldadas por los gobiernos están fomentando aún más el uso adecuado de los espaciadores.
Análisis del mercado de espaciadores para el asma en el Reino Unido
Se prevé que el mercado británico de espaciadores para el asma crezca a una tasa de crecimiento anual compuesta (TCAC) notable entre 2025 y 2032, impulsado por las directrices del Servicio Nacional de Salud (NHS) que recomiendan su uso con inhaladores dosificadores (IDM), especialmente en niños. Este mercado también cuenta con el respaldo de las recomendaciones de la Sociedad Torácica Británica para el uso generalizado de cámaras de retención con válvula, tanto en atención primaria como en el manejo del asma en urgencias. El aumento de la educación sobre el asma en las escuelas y la creciente adopción de espaciadores antiestáticos impulsan aún más el mercado.
Análisis del mercado de espaciadores para el asma en Alemania
Se prevé un crecimiento constante del mercado alemán de espaciadores para el asma, impulsado por la cobertura de seguros médicos públicos para dispositivos contra el asma, sólidas redes de distribución farmacéutica y un enfoque proactivo hacia la atención preventiva. La mayor concienciación sobre el uso de espaciadores para el manejo de enfermedades respiratorias crónicas en la población de edad avanzada está impulsando la demanda. Las innovaciones en espaciadores ecológicos y reutilizables también están cobrando impulso gracias al énfasis de Alemania en la atención sanitaria sostenible.
Perspectiva del mercado de espaciadores para el asma en Asia-Pacífico
Se prevé que el mercado de espaciadores para el asma en Asia-Pacífico crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida, del 8,9 %, durante el período de pronóstico (2025-2032), debido al fuerte aumento de la prevalencia del asma relacionado con la contaminación urbana, el aumento de las tasas de tabaquismo y la exposición industrial. Países como India, China e Indonesia están experimentando un mayor acceso a la atención médica y esfuerzos gubernamentales para mejorar la atención respiratoria. La disponibilidad de espaciadores rentables y los programas de distribución internacionales respaldados por ONG están ayudando a mejorar el acceso en las regiones de bajos ingresos.
Análisis del mercado de espaciadores para el asma en Japón
El mercado japonés de espaciadores para el asma está experimentando un crecimiento constante, impulsado por el envejecimiento de la población, que es más propensa a enfermedades respiratorias crónicas. Los profesionales sanitarios japoneses priorizan la educación del paciente y la medicina de precisión, lo que convierte a los espaciadores con válvula en la opción preferida. Los fabricantes nacionales y las compañías farmacéuticas también están introduciendo dispositivos compactos y antiestáticos diseñados para usuarios mayores y pediátricos, lo que impulsa la expansión del mercado.
Análisis del mercado de espaciadores para el asma en China
El mercado chino de espaciadores para el asma registró la mayor participación en los ingresos de Asia-Pacífico en 2024, impulsado por el crecimiento de la clase media, los problemas de contaminación atmosférica urbana y las iniciativas gubernamentales que promueven el manejo de enfermedades crónicas. El aumento de la producción de espaciadores de bajo costo por parte de fabricantes locales y las campañas nacionales de educación sobre el asma están acelerando la adopción del producto. Las plataformas de farmacias electrónicas y las colaboraciones con hospitales están facilitando el acceso a los espaciadores en las regiones urbanas y semiurbanas.
Cuota de mercado de los espaciadores para el asma
La industria de los espaciadores para el asma está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Teva Pharmaceutical Industries Ltd (Israel)
- GSK plc (Reino Unido)
- Merck & Co., Inc. (EE. UU.)
- F. Hoffmann-La Roche Ltd (Suiza)
- AstraZeneca (Reino Unido)
- Boehringer Ingelheim International GmbH (Alemania)
- Sanofi (Francia)
- Koninklijke Philips NV (Países Bajos)
- BD (EE. UU.)
Últimos avances en el mercado mundial de espaciadores para el asma
- En enero de 2022, Monaghan Medical Corporation (MMC) recibió el Premio Zenith 2021 de la Asociación Americana de Cuidados Respiratorios (AARC), lo que marca el séptimo año consecutivo en que MMC recibe este prestigioso reconocimiento. MMC es líder en el desarrollo, la fabricación y la comercialización de dispositivos respiratorios, como AeroChamber Plus, Flow-Vu VHC, el nebulizador AeroEclipse II y el dispositivo oscilatorio de pep Aerobika.
- En octubre de 2024, Monaghan Medical Corp., conocida por dispositivos como AeroChamber Plus y Flow‑Vu VHC, recibió el Premio Zenith 2024 de la Asociación Americana de Cuidados Respiratorios (AARC). Esto demuestra su continuo liderazgo en innovación en cuidados respiratorios y la excelencia en la atención al cliente.
- En junio de 2025, la senadora Maggie Hassan inició una investigación sobre la suspensión por parte de GSK del inhalador pediátrico para el asma Flovent HFA en enero de 2024. Esta medida se ha relacionado con un aumento de las hospitalizaciones y una reducción del acceso al tratamiento para familias de bajos ingresos. La investigación también plantea la preocupación de que GSK evite recibir 367 millones de dólares en reembolsos de Medicaid.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.