Mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón, por causa (enfermedad por coronavirus 2019 (COVID-19), sepsis, inhalación de sustancias nocivas, neumonía grave y otras), tipo (diagnóstico y tratamiento), vía de administración (oral, parenteral y otras), usuario final (hospitales, clínicas especializadas, atención médica domiciliaria y otros), canal de distribución (licitación directa, farmacia hospitalaria, farmacia minorista y farmacia en línea): tendencias de la industria y pronóstico hasta 2030.
Análisis y perspectivas del mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, Estados Unidos, China y Japón
La creciente prevalencia de enfermedades infecciosas y respiratorias como la COVID-19 y el síndrome de dificultad respiratoria aguda, y el enfoque amplio en el desarrollo de vacunas y productos terapéuticos y de diagnóstico para estas afecciones han aumentado la demanda del mercado. El avance en la tecnología para facilitar el suministro de productos e instalaciones de fabricación rápidas también contribuyen al crecimiento del mercado. Los principales actores del mercado están muy centrados en los lanzamientos y aprobaciones de productos durante este período crucial. Además, el gobierno y los organismos reguladores están apoyando a los actores del mercado con la aprobación de productos debido al aumento de la aparición de nuevos productos.
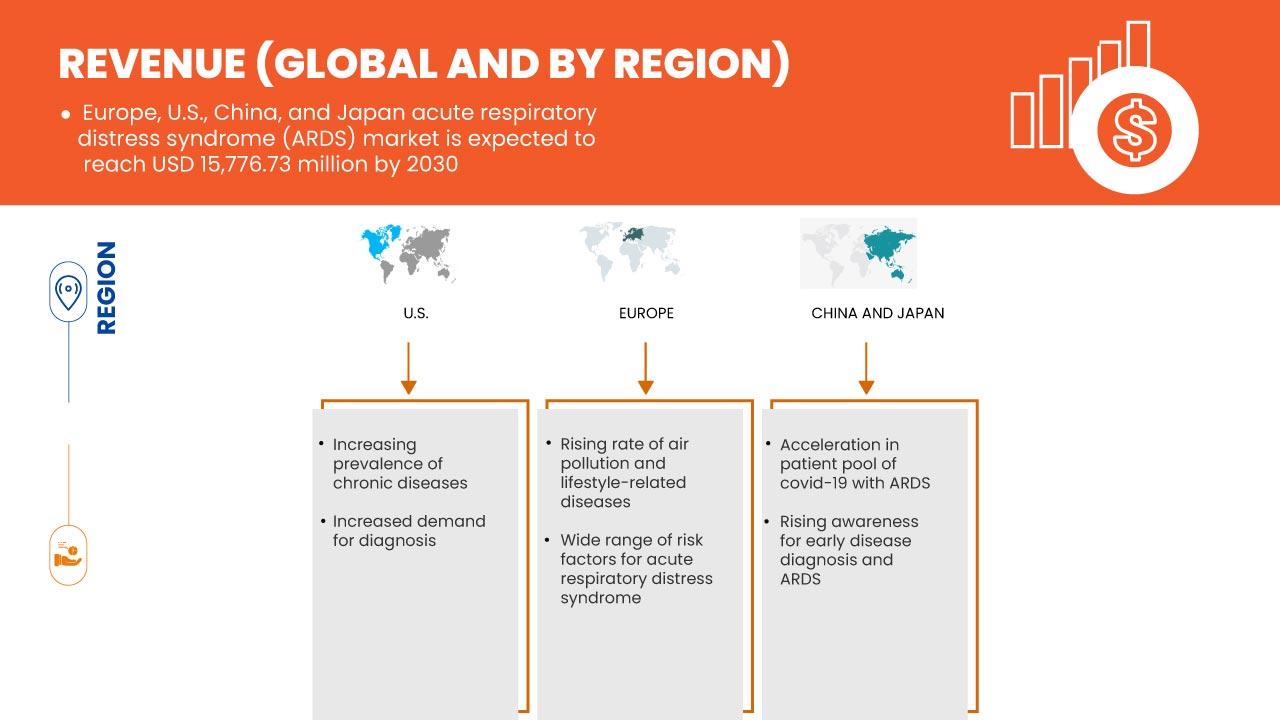
El mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón es favorable y tiene como objetivo reducir la enfermedad, mejorando así la recuperación y el rendimiento de las personas. Data Bridge Market Research analiza que el mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón crecerá a una CAGR del 9,9 % durante el período de pronóstico de 2023 a 2030.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2020-2015) |
|
Unidades cuantitativas |
Ingresos en millones, precios en USD |
|
Segmentos cubiertos |
Por causa (enfermedad por coronavirus 2019 (COVID-19), sepsis, inhalación de sustancias nocivas, neumonía grave y otras), tipo (diagnóstico y tratamiento), vía de administración (oral, parenteral y otras), usuario final (hospitales, clínicas especializadas, atención médica domiciliaria y otros), canal de distribución (licitación directa, farmacia hospitalaria, farmacia minorista y farmacia en línea) |
|
País cubierto |
EE. UU., Japón, China, Alemania, Reino Unido, Italia, Francia, España, Suiza, Rusia, Turquía, Hungría, Lituania, Austria, Irlanda, Noruega, Polonia, Países Bajos y el resto de Europa. |
|
Actores del mercado cubiertos |
Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB. y EUROSETS, entre otros. |
Definición de mercado
El síndrome de dificultad respiratoria aguda (SDRA) es una lesión pulmonar potencialmente mortal que permite que el líquido se filtre hacia los pulmones. La mayoría de las personas que padecen SDRA son hospitalizadas por traumatismos o enfermedades como la COVID-19. El síndrome suele producirse cuando se acumulan líquidos en los diminutos sacos de aire elásticos de los pulmones, llamados alvéolos. Esta acumulación de líquido hace que llegue menos oxígeno al torrente sanguíneo, lo que priva a los órganos de obtener suficiente oxígeno para su funcionamiento normal. Las personas con otras enfermedades desarrollan SDRA en unas horas o días después de la lesión o infección desencadenante. El riesgo de muerte aumenta con la edad y, según la gravedad de la enfermedad, es difícil que los pacientes sobrevivan al síndrome. Una enfermedad o lesión grave que dañe los sacos de membrana de los pulmones provoca SDRA. Las causas subyacentes más comunes de dichas enfermedades incluyen sepsis, inhalación de sustancias nocivas, neumonía grave, lesión en la cabeza, el pecho u otra lesión importante, enfermedad por coronavirus 2019 (COVID-19) y otras.
Dinámica del mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, Estados Unidos, China y Japón
En esta sección se aborda la comprensión de los factores impulsores, las oportunidades, las limitaciones y los desafíos del mercado. Todos ellos se analizan en detalle a continuación:
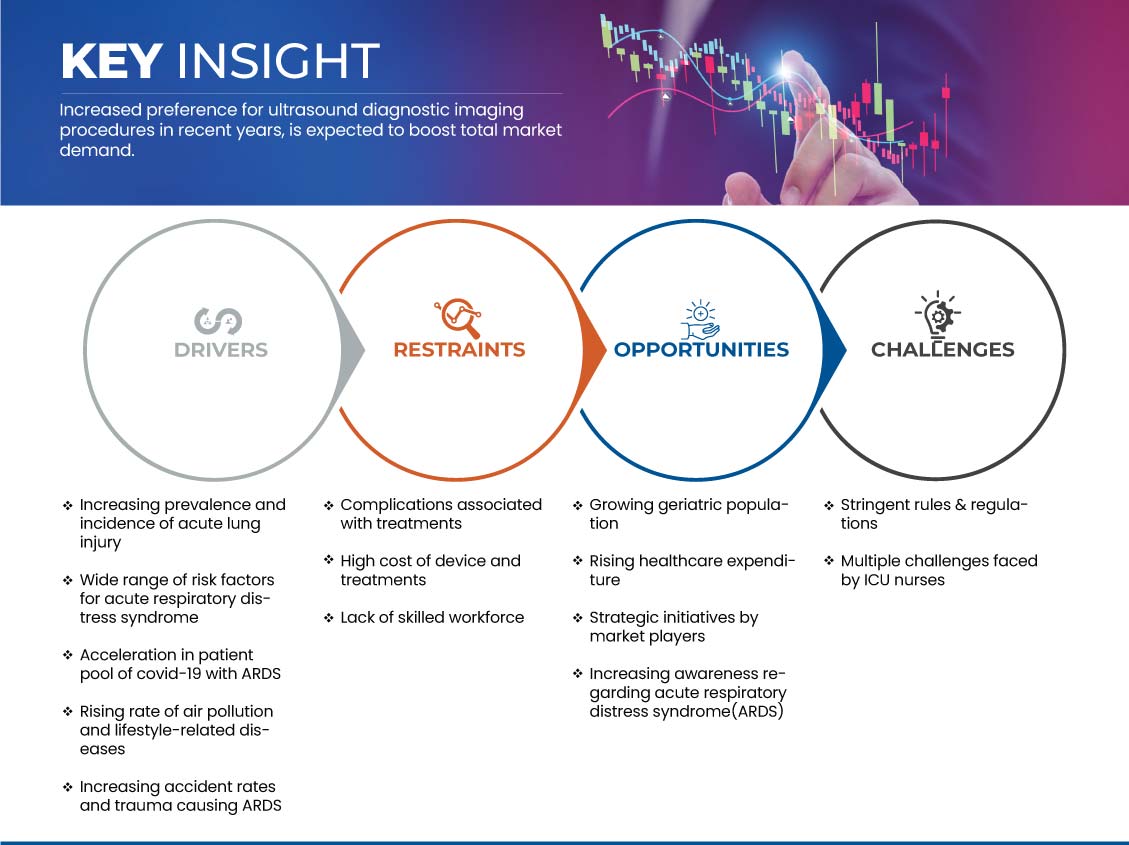
Conductores
- Aumento de la prevalencia e incidencia de lesión pulmonar aguda
Se están notificando ampliamente pacientes con lesiones pulmonares agudas debido a numerosos factores como el creciente envejecimiento de la población y el creciente número de pacientes con sepsis y neumonía. Sin embargo, a la mayoría de las personas se les diagnostica lesiones pulmonares y síndrome de dificultad respiratoria aguda solo en las últimas etapas. Dicha enfermedad es una afección de rápida progresión que se presenta en pacientes con pulmones dañados y provoca fugas de fluidos corporales. El número de casos de síndrome de dificultad respiratoria aguda y lesiones pulmonares está aumentando debido a la aparición de varios virus que causan enfermedades respiratorias en los últimos años, como COVID-19.
Por lo tanto, la incidencia y prevalencia del síndrome de dificultad respiratoria aguda siguen aumentando. La enfermedad ha sido ampliamente reconocida como un problema clínico importante en todo el mundo, con una alta carga de morbilidad y mortalidad. Por lo tanto, se espera que el aumento de la prevalencia y las tasas de incidencia de lesiones pulmonares agudas y el síndrome de dificultad respiratoria aguda que lo acompaña impulsen el mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, Estados Unidos, China y Japón.
- Amplia gama de factores de riesgo para el síndrome de dificultad respiratoria aguda (SDRA)
Se ha informado de una enorme variedad de factores de riesgo para el síndrome de dificultad respiratoria aguda. Existen factores de riesgo ambientales e individuales relacionados con el síndrome. Los pacientes con SDRA sufren diversos grados de vasoconstricción de la arteria pulmonar que dificultan la llegada de suficiente oxígeno a la sangre, por lo que suelen necesitar un respirador para respirar. El SDRA causa una alta mortalidad y mejora esta afección mortal. El síndrome de sepsis con insuficiencia orgánica múltiple es la causa más común de muerte, mientras que la insuficiencia respiratoria es la segunda. Además, la gravedad del SDRA se asocia significativamente con la tasa de mortalidad entre los pacientes con COVID-19 gravemente enfermos.
El síndrome de dificultad respiratoria aguda (SDRA) puede ser inducido por múltiples causas, incluido el traumatismo. Los factores de riesgo del SDRA después de un traumatismo múltiple incluyen traumatismo craneoencefálico y torácico, gravedad y duración del shock, número de productos sanguíneos transfundidos y cristaloides infundidos.
Oportunidad
- Aumentar la concienciación sobre el síndrome de dificultad respiratoria aguda (SDRA)
Dado que el síndrome de dificultad respiratoria aguda tiene múltiples causas diferentes, generalmente se lo ignora entre las causas más comunes de muerte. La necesidad de un tratamiento técnico avanzado y un conocimiento adecuado de la afección pueden reducir sustancialmente la incidencia del síndrome de dificultad respiratoria aguda. Como el diagnóstico y la prevención oportunos son cruciales para prevenir o recuperarse más rápidamente, la atención del público es lo más importante. Los gobiernos y las organizaciones actuales han ampliado el alcance de la investigación sobre las lesiones pulmonares para incluir la prevención primaria del síndrome de dificultad respiratoria aguda y reducir la tasa de morbilidad o mortalidad del síndrome.
Las iniciativas que se iniciaron hace tiempo siguen ayudando a prevenir infecciones pulmonares graves como el síndrome de dificultad respiratoria aguda y ayudan a las empresas biotecnológicas y farmacéuticas a innovar en sus investigaciones para lograr nuevos avances en los tratamientos. Aunque no existe una cura adecuada o específica para el síndrome de dificultad respiratoria aguda, pocas asociaciones están tratando de aumentar la concienciación sobre el síndrome y ayudar a los pacientes a recibir una curación oportuna de sus pulmones.
Estos programas de iniciativas novedosas y las unidades de cuidados paliativos puestas en marcha por diversas asociaciones de atención sanitaria y pulmonar están aumentando la conciencia entre las personas sobre la causa y el tratamiento adecuado de la enfermedad a tiempo. Por lo tanto, aumentar la conciencia sobre el síndrome de dificultad respiratoria aguda (SDRA) a través de diversas asociaciones mejora la oportunidad de crecimiento del mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón.
Restricción/Desafío
- Alto costo de dispositivos y tratamientos
Aunque el síndrome de dificultad respiratoria aguda está recibiendo una amplia gama de opciones de tratamiento avanzado, el costo de un tratamiento más prolongado es bastante difícil de afrontar para las personas de ingresos medios. El uso de servicios de cuidados críticos y de unidades de cuidados intensivos está aumentando en todo el mundo, y su elevado costo es una preocupación importante en el sistema de salud actual. Los pacientes con síndrome de dificultad respiratoria aguda suelen tener que pasar por hospitalizaciones prolongadas con monitarización frecuente y uso de ventilación, lo que consume una cantidad significativa de recursos sanitarios. Debido a esto, la mayoría de los pacientes que no pueden afrontar una estancia prolongada reciben el alta en las etapas iniciales del tratamiento. Sin embargo, esto aumenta las posibilidades y susceptibilidades de nuevas complicaciones en las infecciones, lo que exige recursos sanitarios y tratamiento adicionales.
Impacto posterior a la COVID-19 en el mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, Estados Unidos, China y Japón
El COVID-19 ha afectado positivamente el crecimiento del mercado, ya que hay un aumento en la demanda de síndrome de dificultad respiratoria aguda en la región. Durante la fase de COVID-19, se indicó que varios casos son asintomáticos, mientras que el 20% de los casos de COVID-19 siguen un curso grave, requiriendo hospitalización. Los casos graves de la enfermedad por COVID-19 finalmente conducirán al síndrome de dificultad respiratoria aguda (SDRA) y neumonía. Se ha demostrado que esto es fatal para las personas infectadas. Como el SDRA muestra el defecto pulmonar al dañar los alvéolos, que son pequeños sacos de aire en los pulmones, se ha observado el mismo nivel de defecto en los pacientes con COVID-19. Esto conduce a una entrada repentina de líquido, causando neumonía. Por lo tanto, el COVID-19 ha impactado positivamente en este mercado.
Acontecimientos recientes
- En mayo de 2021, Medtronic lanzó el sistema de monitoreo de las vías respiratorias SonarMed. El sistema utiliza tecnología acústica para detectar obstrucciones del tubo endotraqueal. Esto ha ayudado a la empresa a aumentar su cartera de productos.
- En julio de 2020, F. Hoffman-La Roche Ltd lanzó una prueba rápida de anticuerpos contra el SARS-CoV-2. La prueba se lanzó en asociación con SD Biosenseor, Inc. Esto ha ayudado a la empresa a aumentar su cartera de productos.
Alcance del mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón
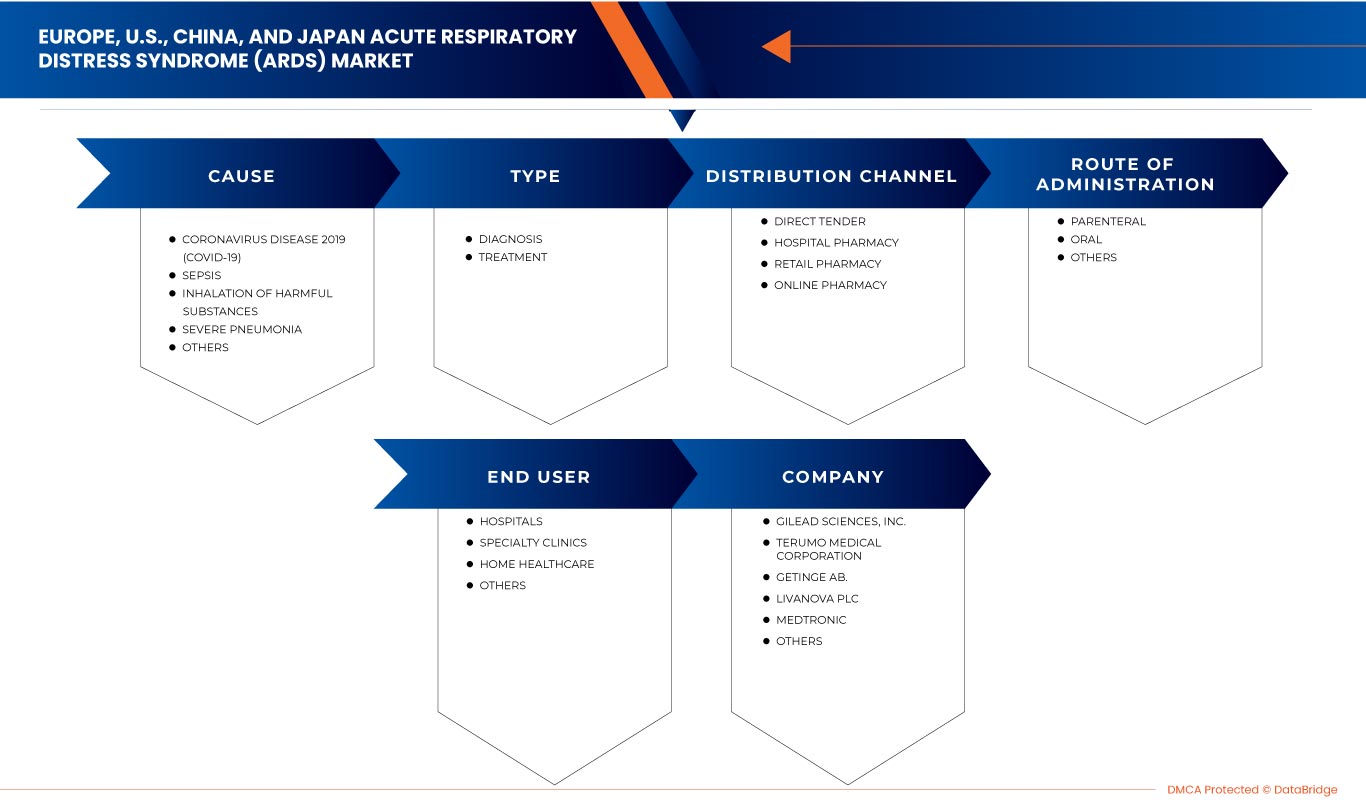
El mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón se clasifica en cinco segmentos según la causa, el tipo, la vía de administración, el usuario final y el canal de distribución. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
Causa
- Enfermedad por coronavirus 2019 (COVID-19)
- Septicemia
- Inhalación de sustancias nocivas
- Neumonía grave
- Otros
Según la causa, el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón está segmentado en enfermedad por coronavirus 2019 (COVID-19), sepsis, inhalación de sustancias nocivas, neumonía grave y otros.
Tipo
- Diagnóstico
- Tratamiento
Según el tipo, el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón está segmentado en diagnóstico y tratamiento.
Vía de administración
- Oral
- Parenteral
- Otros
Según la vía de administración, el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón está segmentado en oral, parenteral y otros.
Usuario final
- Hospitales
- Clínicas especializadas
- Atención médica domiciliaria
- Otros
Según el usuario final, el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón está segmentado en hospitales, clínicas especializadas, atención médica domiciliaria y otros.
Canal de distribución
- Licitación directa
- Farmacia hospitalaria
- Farmacia minorista
- Farmacia en línea
Según el canal de distribución, el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón está segmentado en licitación directa, farmacia hospitalaria, farmacia minorista y farmacia en línea.
Análisis y perspectivas del mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón
Se analiza el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón, y se proporcionan información y tendencias sobre el tamaño del mercado según la causa, el tipo, la vía de administración, el usuario final y el canal de distribución como se mencionó anteriormente.
Los países cubiertos en el mercado del síndrome de dificultad respiratoria aguda (SDRA) de Europa, EE. UU., China y Japón son EE. UU., Japón, China, Alemania, Reino Unido, Italia, Francia, España, Suiza, Rusia, Turquía, Hungría, Lituania, Austria, Irlanda, Noruega, Polonia, Países Bajos y el resto de Europa.
Se espera que el mercado estadounidense del síndrome de dificultad respiratoria aguda (SDRA) crezca debido a un aumento en la prevalencia de lesiones pulmonares agudas, así como a un aumento en el número de pacientes con COVID-19 con SDRA. Estos son los factores clave que se espera que impulsen el crecimiento del mercado en el país.
La sección de países del informe también proporciona factores individuales que impactan en el mercado y cambios en la regulación del mercado que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como el análisis de la cadena de valor aguas abajo y aguas arriba, las tendencias técnicas, el análisis de las cinco fuerzas de Porter y los estudios de casos son algunos de los indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales y el impacto de los aranceles nacionales y las rutas comerciales al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, Estados Unidos, China y Japón
El panorama competitivo del mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón proporciona detalles de los competidores. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento del producto, la amplitud y la extensión del producto y el dominio de la aplicación. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa en el mercado del síndrome de dificultad respiratoria aguda (SDRA) en Europa, EE. UU., China y Japón.
Algunos de los principales actores que operan en el mercado son Drägerwerk AG & Co. KGaA, Fisher & Paykel Healthcare Limited., LivaNova PLC, Gilead Sciences, Inc., Fresenius SE & Co. KGaA, Besmed Health Business Corp., Armstrong Medical, Smiths Medical, ResMed, ALung Technologies, Inc., Medtronic, F. Hoffmann-La Roche Ltd, Hamilton Medical, nice Neotech Medical Systems Pvt. Ltd., Pfizer Inc., WEINMANN Emergency Medical Technology GmbH + Co. KG, NIPRO, Terumo Medical Corporation, Getinge AB. y EUROSETS, entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CAUSE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET END USER COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PESTEL ANALYSIS
4.2 PORTER’S FIVE FORCES
4.3 ETIOLOGY BY GEOGRAPHY
4.3.1 ETIOLOGY IN U.S.
4.3.2 ETIOLOGY IN EUROPE
4.3.3 ETIOLOGY IN CHINA
4.3.4 ETIOLOGY IN JAPAN
4.4 ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) HEALTHCARE COST PER PATIENT BY GEOGRAPHY
4.5 INSURANCE REIMBURSEMENT
4.5.1 CENTER FOR MEDICARE SERVICES (CMS)–ELSO (EXTRACORPOREAL LIFE SUPPORT ORGANIZATION)
4.5.2 HEALTH RESOURCES AND SERVICES ADMINISTRATION
4.5.3 ABBOTT CODING GUIDE FOR ECMO
4.5.4 CENTRAL GOVERNMENT HEALTH SCHEME (CGHS)
4.5.5 CERN HEALTH INSURANCE SCHEME
4.5.6 AMERICAN SOCIETY OF CLINICAL ONCOLOGY (ASCO) – (MEDICARE & MEDICAID)
4.5.7 AMERICAN HOSPITAL ASSOCIATION
4.5.8 CONCLUSION
4.6 PIPELINE ANALYSIS
5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: REGULATIONS
5.1 REGULATION IN U.S.:
5.2 LABELING OF MODIFIED DEVICES
5.3 REGULATION IN EUROPE:
5.4 REGULATION IN CHINA:
5.5 REGULATION IN JAPAN:
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 INCREASING PREVALENCE AND INCIDENCE OF ACUTE LUNG INJURY
6.1.2 WIDE RANGE OF RISK FACTORS FOR ACUTE RESPIRATORY DISTRESS SYNDROME
6.1.3 ACCELERATION IN PATIENT POOL OF COVID-19 WITH ARDS
6.1.4 RISING RATE OF AIR POLLUTION AND LIFESTYLE-RELATED DISEASES
6.1.5 INCREASING ACCIDENT RATES AND TRAUMA-CAUSING ARDS
6.2 RESTRAINTS
6.2.1 COMPLICATIONS ASSOCIATED WITH TREATMENTS
6.2.2 HIGH COST OF DEVICE AND TREATMENTS
6.2.3 LACK OF SKILLED WORKFORCE
6.3 OPPORTUNITIES
6.3.1 GROWING GERIATRIC POPULATION
6.3.2 RISING HEALTHCARE EXPENDITURE
6.3.3 STRATEGIC INITIATIVES BY MARKET PLAYERS
6.3.4 INCREASING AWARENESS REGARDING ACUTE RESPIRATORY DISTRESS SYNDROME(ARDS)
6.4 CHALLENGES
6.4.1 STRINGENT RULES & REGULATIONS
6.4.2 MULTIPLE CHALLENGES FACED BY ICU NURSES
7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE
7.1 OVERVIEW
7.2 CORONAVIRUS DISEASE 2019 (COVID-19)
7.3 SEPSIS
7.4 INHALATION OF HARMFUL SUBSTANCES
7.5 SEVERE PNEUMONIA
7.6 OTHERS
8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE
8.1 OVERVIEW
8.2 DIAGNOSIS
8.2.1 IMAGING TESTS
8.2.1.1 CHEST X-RAY
8.2.1.2 CT SCAN
8.2.1.3 ULTRASOUND
8.2.1.4 OTHERS
8.2.2 BLOOD TEST
8.2.3 RESPIRATORY RATE
8.2.4 SPO2 TEST
8.2.5 OTHERS
8.3 TREATMENT
8.3.1 MECHANICAL VENTILATION
8.3.1.1 HIGH-FLOW NASAL O2
8.3.1.2 BI-LEVEL POSITIVE AIRWAY PRESSURE
8.3.1.3 CONTINUOUS POSITIVE AIRWAY PRESSURE
8.3.1.4 PRONE POSITIVE VENTILATION
8.3.1.5 OTHERS
8.3.2 CORTICOSTEROIDS
8.3.2.1 METHYLPREDNISOLONE
8.3.2.2 DEXAMETHASONE
8.3.2.3 OTHERS
8.3.3 ANTIVIRAL MEDICATION
8.3.3.1 REMDESIVIR
8.3.3.2 COMBINATION DRUGS
8.3.3.3 OTHERS
8.3.4 EXTRACORPOREAL MEMBRANE OXYGENATION (ECMO)
8.3.5 TOCILIZUMAB
8.3.6 OTHERS
9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION
9.1 OVERVIEW
9.2 PARENTERAL
9.2.1 INTRAVENOUS
9.2.2 INTRAMUSCULAR
9.3 ORAL
9.4 OTHERS
10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER
10.1 OVERVIEW
10.2 HOSPITALS
10.3 SPECIALTY CLINICS
10.4 HOME HEALTHCARE
10.5 OTHERS
11 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL
11.1 OVERVIEW
11.2 DIRECT TENDER
11.3 HOSPITAL PHARMACY
11.4 RETAIL PHARMACY
11.5 ONLINE PHARMACY
12 EUROPE
12.1 GERMANY
12.2 FRANCE
12.3 U.K.
12.4 ITALY
12.5 SPAIN
12.6 TURKEY
12.7 HUNGARY
12.8 NETHERLANDS
12.9 SWITZERLAND
12.1 AUSTRIA
12.11 LITHUANIA
12.12 POLAND
12.13 RUSSIA
12.14 IRELAND
12.15 NORWAY
12.16 REST OF EUROPE
13 EUROPE, US, CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY LANDSCAPE
13.1 COMPANY SHARE ANALYSIS: U.S.
13.2 COMPANY SHARE ANALYSIS: EUROPE
13.3 COMPANY SHARE ANALYSIS: JAPAN
13.4 COMPANY SHARE ANALYSIS: CHINA
14 SWOT ANALYSIS
15 COMPANY PROFILE
15.1 GILEAD SCIENCES INC.
15.1.1 COMPANY SNAPSHOT
15.1.2 REVENUS ANALYSIS
15.1.3 PRODUCT PORTFOLIO
15.1.4 RECENT DEVELOPMENT
15.2 TERUMO CORPORATION
15.2.1 COMPANY SNAPSHOT
15.2.2 REVENUS ANALYSIS
15.2.3 PRODUCT PORTFOLIO
15.2.4 RECENT DEVELOPMENT
15.3 GETINGE AB
15.3.1 COMPANY SNAPSHOT
15.3.2 REVENUS ANALYSIS
15.3.3 PRODUCT PORTFOLIO
15.3.4 RECENT DEVELOPMENT
15.4 LIVANOVA PLC
15.4.1 COMPANY SNAPSHOT
15.4.2 REVENUE ANALYSIS
15.4.3 PRODUCT PORTFOLIO
15.4.4 RECENT DEVELOPMENTS
15.5 MEDTRONIC
15.5.1 COMPANY SNAPSHOT
15.5.2 REVENUE ANALYSIS
15.5.3 PRODUCT PORTFOLIO
15.5.4 RECENT DEVELOPMENTS
15.6 ALUNG TECHNOLOGIES, INC
15.6.1 COMPANY SNAPSHOT
15.6.2 PRODUCT PORTFOLIO
15.6.3 RECENT DEVELOPMENT
15.7 ARMSTRONG MEDICAL
15.7.1 COMPANY SNAPSHOT
15.7.2 PRODUCT PORTFOLIO
15.7.3 RECENT DEVELOPMENT
15.8 BESMED HEALTH BUSINESS CORP.
15.8.1 COMPANY SNAPSHOT
15.8.2 PRODUCT PORTFOLIO
15.8.3 RECENT DEVELOPMENTS
15.9 DRÄGERWERK AG & CO. KGAA
15.9.1 COMPANY SNAPSHOT
15.9.2 REVENUE ANALYSIS
15.9.3 PRODUCT PORTFOLIO
15.9.4 RECENT DEVELOPMENTS
15.1 EUROSETS
15.10.1 COMPANY SNAPSHOT
15.10.2 PRODUCT PORTFOLIO
15.10.3 RECENT DEVELOPMENT
15.11 F. HOFFMANN-LA ROCHE LTD
15.11.1 COMPANY SNAPSHOT
15.11.2 RECENT ANALYSIS
15.11.3 PRODUCT PORTFOLIO
15.11.4 RECENT DEVELOPMENTS
15.12 FISHER & PAYKEL HEALTHCARE LIMITED
15.12.1 COMPANY SNAPSHOT
15.12.2 REVENUE ANALYSIS
15.12.3 PRODUCT PORTFOLIO
15.12.4 RECENT DEVELOPMENTS
15.13 FRESENIUS SE & CO. KGAA
15.13.1 COMPANY SNAPSHOT
15.13.2 REVENUS ANALYSIS
15.13.3 PRODUCT PORTFOLIO
15.13.4 RECENT DEVELOPMENTS
15.14 HAMILTON MEDICAL
15.14.1 COMPANY SNAPSHOT
15.14.2 PRODUCT PORTFOLIO
15.14.3 RECENT DEVELOPMENT
15.15 NICE NEOTECH MEDICAL SYSTEMS PVT.LTD.
15.15.1 COMPANY SNAPSHOT
15.15.2 PRODUCT PORTFOLIO
15.15.3 RECENT DEVELOPMENT
15.16 NIPRO
15.16.1 COMPANY SNAPSHOT
15.16.2 REVENUS ANALYSIS
15.16.3 PRODUCT PORTFOLIO
15.16.4 RECENT DEVELOPMENT
15.17 PFIZER INC.
15.17.1 COMPANY SNAPSHOT
15.17.2 REVENUS ANALYSIS
15.17.3 PRODUCT PORTFOLIO
15.17.4 RECENT DEVELOPMENT
15.18 RESMED
15.18.1 COMPANY SNAPSHOT
15.18.2 REVENUE ANALYSIS
15.18.3 PRODUCT PORTFOLIO
15.18.4 RECENT DEVELOPMENT
15.19 SMITHS MEDICAL
15.19.1 COMPANY SNAPSHOT
15.19.2 REVENUS ANALYSIS
15.19.3 PRODUCT PORTFOLIO
15.19.4 RECENT DEVELOPMENTS
15.2 WEINMANN EMERGENCY MEDICAL TECHNOLOGY GMBH + CO. KG
15.20.1 COMPANY SNAPSHOT
15.20.2 PRODUCT PORTFOLIO
15.20.3 RECENT DEVELOPMENT
16 QUESTIONNAIRE
17 RELATED REPORTS
Lista de Tablas
TABLE 1 HEALTHCARE COST FOR ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) ON THE BASIS OF SEVERITY BY COUNTRY IS GIVEN BELOW IN USD:
TABLE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: PIPELINE ANALYSIS
TABLE 3 REGULATION FOR VENTILATORS AND RESPIRATORY DEVICES AS PER FDA
TABLE 4 REGULATION FOR THE USE OF VENTILATOR AND ANESTHESIA GAS MACHINE BREATHING CIRCUIT DEVICES
TABLE 5 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 6 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 7 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 8 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 9 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 EUROPE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 14 U.S. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 15 CHINA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 16 JAPAN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 17 EUROPE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 18 U.S. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 19 CHINA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 20 JAPAN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 21 EUROPE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030(USD MILLION)
TABLE 22 U.S. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 23 CHINA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 24 JAPAN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 25 EUROPE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 26 U.S. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 27 CHINA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 EUROPE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 U.S. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 CHINA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 33 EUROPE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 34 U.S. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 35 CHINA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 36 JAPAN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 40 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 EUROPE PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 U.S. PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 43 CHINA PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 44 JAPAN PARENTRAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 45 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 46 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 47 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 49 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 50 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 51 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 52 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 54 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 55 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 56 GERMANY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 57 GERMANY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 58 GERMANY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 59 GERMANY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 60 GERMANY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 61 GERMANY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 62 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 63 GERMANY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 64 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 65 GERMANY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 66 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 67 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 FRANCE DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 69 FRANCE IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 70 FRANCE TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 71 FRANCE ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 72 FRANCE CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 73 FRANCE MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 74 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 FRANCE PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 76 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 77 FRANCE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 78 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 79 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 80 U.K. DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 U.K. IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 U.K. TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 U.K. ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 U.K. CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 U.K. MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 86 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 87 U.K. PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 88 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 89 U.K. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 90 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 91 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 92 ITALY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 93 ITALY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 94 ITALY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 95 ITALY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 96 ITALY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 97 ITALY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 98 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 99 ITALY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 100 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 101 ITALY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 102 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 103 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 104 SPAIN DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 105 SPAIN IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 106 SPAIN TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 107 SPAIN ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 108 SPAIN CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 109 SPAIN MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 110 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SPAIN PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SPAIN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 115 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 TURKEY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 TURKEY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 TURKEY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 TURKEY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 120 TURKEY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 121 TURKEY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 122 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 123 TURKEY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION
TABLE 124 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 125 TURKEY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 126 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 127 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 128 HUNGARY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 129 HUNGARY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 130 HUNGARY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 131 HUNGARY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 132 HUNGARY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 HUNGARY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 135 HUNGARY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 136 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 137 HUNGARY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 138 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 139 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 140 NETHERLANDS DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 141 NETHERLANDS IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 142 NETHERLANDS TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 143 NETHERLANDS ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 144 NETHERLANDS CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 145 NETHERLANDS MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 146 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 NETHERLANDS PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 148 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 149 NETHERLANDS ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 150 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 151 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 SWITZERLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 SWITZERLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 SWITZERLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 SWITZERLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 156 SWITZERLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 157 SWITZERLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 158 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 SWITZERLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 161 SWITZERLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 162 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 163 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 164 AUSTRIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 165 AUSTRIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 166 AUSTRIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 167 AUSTRIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 AUSTRIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 AUSTRIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 171 AUSTRIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 172 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 173 AUSTRIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 174 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 175 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 176 LITHUANIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 177 LITHUANIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 178 LITHUANIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 179 LITHUANIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 180 LITHUANIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 181 LITHUANIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 182 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 183 LITHUANIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 184 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 185 LITHUANIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 186 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 187 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 POLAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 POLAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 POLAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 191 POLAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 192 POLAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 193 POLAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 194 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 POLAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 197 POLAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 198 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 199 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 200 RUSSIA DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 201 RUSSIA IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 202 RUSSIA TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 203 RUSSIA ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 RUSSIA CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 RUSSIA MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 207 RUSSIA PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 208 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 209 RUSSIA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 210 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 211 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 212 IRELAND DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 213 IRELAND IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 214 IRELAND TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 215 IRELAND ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 216 IRELAND CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 217 IRELAND MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 218 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 219 IRELAND PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 220 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 221 IRELAND ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 222 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
TABLE 223 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 224 NORWAY DIAGNOSIS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 225 NORWAY IMAGING TESTS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 226 NORWAY TREATMENT IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 227 NORWAY ANTIVIRAL MEDICATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 228 NORWAY CORTICOSTEROIDS IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 229 NORWAY MECHANICAL VENTILATION IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 230 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 231 NORWAY PARENTERAL IN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 232 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 233 NORWAY ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 234 REST OF EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 2 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: GLOBAL VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: END USER COVERAGE GRID
FIGURE 9 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SEGMENTATION
FIGURE 11 ACCELERATION IN THE PATIENT POOL OF COVID-19 WITH ARDS IS EXPECTED TO DRIVE THE EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 12 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 13 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE U.S.ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 14 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 15 CORONAVIRUS DISEASE 2019 (COVID-19) SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET IN 2023 & 2030
FIGURE 16 MOST COMMON PRIMARY CAUSES OF DEATH IN ARDS PATIENTS IN U.S. COUNTRY
FIGURE 17 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE, U.S., CHINA, AND JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET
FIGURE 18 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 19 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 20 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 21 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 22 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 23 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 24 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 25 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 26 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 27 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 28 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 29 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 30 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY CAUSE, 2022
FIGURE 31 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, 2023-2030 (USD MILLION)
FIGURE 32 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, CAGR (2023-2030)
FIGURE 33 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY CAUSE, LIFELINE CURVE
FIGURE 34 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 35 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 36 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 37 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 38 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 39 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 40 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 41 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 42 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 43 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 44 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 45 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 46 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY TYPE, 2022
FIGURE 47 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 48 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 49 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 50 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 51 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 52 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 53 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 54 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 55 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 56 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 57 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 58 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 59 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 60 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 61 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 62 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY ROUTE OF ADMINISTRATION, 2022
FIGURE 63 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 64 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 65 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 66 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 67 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 68 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 69 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 70 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 71 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 72 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 73 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 74 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 75 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 76 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 77 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 78 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY END USER, 2022
FIGURE 79 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 80 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, CAGR (2023-2030)
FIGURE 81 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 82 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 83 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 84 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 85 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 86 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 87 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 88 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 89 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 90 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 91 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 92 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 93 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 94 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET, BY DISTRIBUTION CHANNEL, 2022
FIGURE 95 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 96 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 97 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 98 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: SNAPSHOT (2022)
FIGURE 99 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022)
FIGURE 100 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2023 & 2030)
FIGURE 101 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: BY COUNTRY (2022 & 2030)
FIGURE 102 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: CAUSE (2023-2030)
FIGURE 103 U.S. ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 104 EUROPE ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 105 JAPAN ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)
FIGURE 106 CHINA ACUTE RESPIRATORY DISTRESS SYNDROME (ARDS) MARKET: COMPANY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.