Europe Medical Device Regulatory Affairs Outsourcing Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
8.31 Billion
USD
21.78 Billion
2025
2033
USD
8.31 Billion
USD
21.78 Billion
2025
2033
| 2026 –2033 | |
| USD 8.31 Billion | |
| USD 21.78 Billion | |
|
|
|
|
Mercado europeo de subcontratación de asuntos regulatorios de dispositivos médicos, por servicios (servicios de asuntos regulatorios, consultoría de calidad y redacción médica), producto (productos terminados, electrónica y materia prima), tipo de dispositivo (clase I, clase II y clase III), aplicación (cardiología, diagnóstico por imágenes, ortopedia, IVD, oftalmología, cirugía general y plástica, administración de medicamentos, odontología, endoscopia, atención de la diabetes y otros), usuario final (pequeña empresa de dispositivos médicos, mediana empresa de dispositivos médicos y gran empresa de dispositivos médicos), país (Alemania, Reino Unido, Francia, Italia, España, Países Bajos, Rusia, Suiza, Bélgica, Turquía, Irlanda, resto de Europa), tendencias de la industria y pronóstico hasta 2028
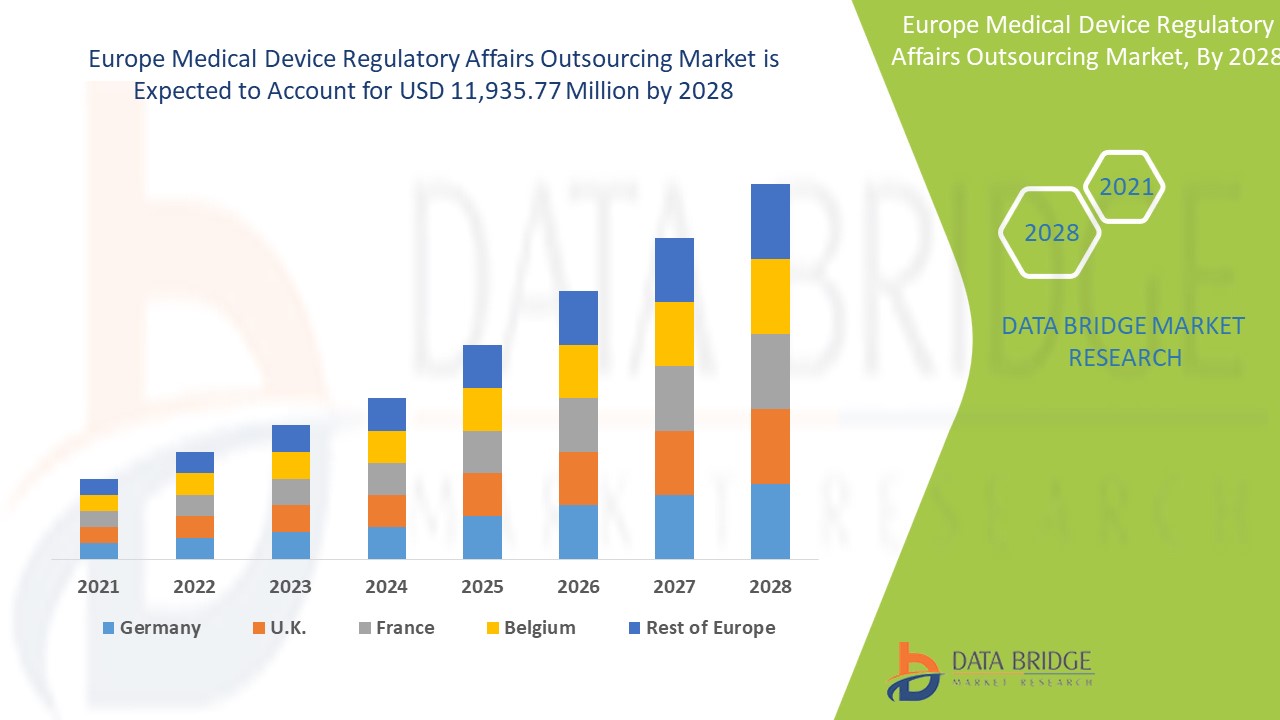 Análisis y perspectivas del mercado: mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Europa
Análisis y perspectivas del mercado: mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Europa
Se espera que el mercado de subcontratación de asuntos regulatorios de dispositivos médicos gane crecimiento de mercado en el período de pronóstico de 2021 a 2028. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 12,8% en el período de pronóstico de 2021 a 2028 y se espera que alcance los USD 11.935,77 millones para 2028. Se anticipa que la iniciativa estratégica para las expansiones geográficas impulsará el crecimiento del mercado de subcontratación de asuntos regulatorios de dispositivos médicos.
La subcontratación es una parte importante de la cadena de valor de todas las empresas farmacéuticas y biotecnológicas durante la investigación y el desarrollo (I+D). Los servicios de subcontratación de asuntos regulatorios implican la redacción médica y la publicación de documentación regulatoria por parte de autores médicos profesionales, auditores de control de calidad (QC) y editores que contribuyen a proyectos de investigación clínica de alta calidad. La demanda de subcontratación de servicios regulatorios se ha visto impulsada por un aumento sustancial de los estudios clínicos realizados en las economías emergentes, lo que proporciona una plataforma saludable para el crecimiento de esta industria.
El creciente número de vencimientos de patentes actúa como un motor para su crecimiento en el mercado de subcontratación de asuntos regulatorios de dispositivos médicos. La fluctuación en los precios de varios servicios de asuntos regulatorios de dispositivos médicos actúa como una restricción para su crecimiento en el mercado de subcontratación de asuntos regulatorios de dispositivos médicos. Los premios y el reconocimiento brindan una excelente oportunidad para el crecimiento del mercado de subcontratación de asuntos regulatorios de dispositivos médicos. El brote pandémico de COVID-19 actúa como un desafío para el crecimiento del mercado de subcontratación de asuntos regulatorios de dispositivos médicos.
El informe de mercado de subcontratación de asuntos regulatorios de dispositivos médicos proporciona detalles de la participación de mercado, nuevos desarrollos y análisis de la cartera de productos, el impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, aprobaciones de productos, decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado de subcontratación de asuntos regulatorios de dispositivos médicos, comuníquese con Data Bridge Market Research para obtener un informe de analista; nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado.
 Alcance y tamaño del mercado de subcontratación de asuntos regulatorios de dispositivos médicos
Alcance y tamaño del mercado de subcontratación de asuntos regulatorios de dispositivos médicos
El mercado de subcontratación de asuntos regulatorios de dispositivos médicos está segmentado en función de los servicios, productos, tipos de dispositivos, aplicaciones y usuarios finales. El crecimiento entre segmentos le ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
- En función de los servicios, el mercado de subcontratación de asuntos regulatorios de dispositivos médicos se segmenta en servicios de asuntos regulatorios , consultoría de calidad y redacción médica. En 2021, se espera que el segmento de servicios de asuntos regulatorios domine el mercado de subcontratación de asuntos regulatorios de dispositivos médicos debido a la mayor adopción de la subcontratación de asuntos regulatorios por parte de las principales empresas de dispositivos médicos.
- En función del producto, el mercado de subcontratación de asuntos regulatorios de dispositivos médicos se segmenta en productos terminados, productos electrónicos y materias primas. En 2021, se espera que el segmento de productos terminados domine el mercado de subcontratación de asuntos regulatorios de dispositivos médicos debido a la creciente adopción de la subcontratación de asuntos regulatorios para los productos terminados por parte de las principales empresas de dispositivos médicos.
- Según el tipo de dispositivo, el mercado de subcontratación de asuntos regulatorios de dispositivos médicos se segmenta en clase I, clase II y clase III. En 2021, se espera que el segmento de clase I domine el mercado de subcontratación de asuntos regulatorios de dispositivos médicos debido a la creciente demanda de dispositivos médicos en todo el mundo para tratar a pacientes con enfermedades crónicas.
- En función de la aplicación, el mercado de subcontratación de asuntos regulatorios de dispositivos médicos se segmenta en cardiología, diagnóstico por imágenes , ortopedia, IVD, oftalmología, cirugía general y plástica, administración de medicamentos, odontología, endoscopia, atención de la diabetes y otros. En 2021, se espera que el segmento de cardiología domine el mercado de subcontratación de asuntos regulatorios de dispositivos médicos debido a la mayor adopción de la subcontratación de asuntos regulatorios para los dispositivos médicos de clase III por parte de las principales empresas de dispositivos médicos.
- En función del usuario final, el mercado de subcontratación de asuntos regulatorios de dispositivos médicos se segmenta en pequeñas empresas de dispositivos médicos, medianas empresas de dispositivos médicos y grandes empresas de dispositivos médicos. En 2021, se espera que el segmento de empresas medianas de dispositivos médicos domine el mercado de subcontratación de asuntos regulatorios de dispositivos médicos debido a la creciente demanda de dispositivos médicos en todo el mundo.
Análisis a nivel de país del mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Europa
Se analiza el mercado de subcontratación de asuntos regulatorios de dispositivos médicos y se proporciona información sobre el tamaño del mercado por país, servicios, producto, tipo de dispositivo, aplicación y usuario final como se menciona anteriormente.
Los países cubiertos en el informe del mercado de subcontratación de asuntos regulatorios de dispositivos médicos de Europa son Alemania, Reino Unido, Francia, Italia, España, Países Bajos, Rusia, Suiza, Bélgica, Turquía, Irlanda y el resto de Europa.
Alemania domina el mercado de subcontratación de asuntos regulatorios de dispositivos médicos en Europa debido al aumento del gasto en atención médica para el segmento de servicios de asuntos regulatorios.
La sección de países del informe también proporciona factores de impacto de mercado individuales y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas globales y sus desafíos enfrentados debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
El aumento de las actividades de expansión geográfica de las empresas de dispositivos médicos está impulsando el crecimiento del mercado de subcontratación de asuntos regulatorios de dispositivos médicos
El mercado de subcontratación de asuntos regulatorios de dispositivos médicos también le proporciona un análisis detallado del mercado para el crecimiento de cada país en la industria de subcontratación de asuntos regulatorios de dispositivos médicos. Además, proporciona información detallada sobre las ventas de subcontratación de asuntos regulatorios de dispositivos médicos, el impacto de los escenarios regulatorios y los parámetros de tendencia con respecto al mercado de subcontratación de asuntos regulatorios de dispositivos médicos. Los datos están disponibles para el período histórico de 2011 a 2019.
Análisis del panorama competitivo y de la cuota de mercado de la subcontratación de asuntos regulatorios de dispositivos médicos
El panorama competitivo del mercado de externalización de asuntos regulatorios de dispositivos médicos proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y la extensión de los productos, el dominio de las aplicaciones y la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa en relación con el mercado de externalización de asuntos regulatorios de dispositivos médicos de Europa.
Las principales empresas cubiertas en el informe del mercado de subcontratación de asuntos regulatorios de dispositivos médicos de Europa son Parexel International Corporation, North American Science Associates, Inc., SGS SA, Trilogy Writing & Consulting GmbH, Creganna (una subsidiaria de TE Connectivity), Intertek Group plc, WuXi AppTec, Charles River Laboratories, Celestica Inc., Freyr, Cactus Communications, Eurofins Scientific, TÜV SÜD, Sterigenics US, LLC - A Sotera Health company, TE Connectivity, FLEX LTD., Heraeus Holding, Integer Holdings Corporation, IQVIA, Covance, Plexus Corp., Sanmina Corporation, OMICS International, Tecomet, Inc., East West Manufacturing, Omron Corporation, entre otros actores globales y nacionales. Los analistas de DBMR comprenden las fortalezas competitivas y brindan un análisis competitivo para cada competidor por separado.
Numerosas empresas de todo el mundo también inician numerosos contratos y acuerdos que también están acelerando el mercado de subcontratación de asuntos regulatorios de dispositivos médicos.
Por ejemplo,
- En enero de 2020, Charles River Laboratories anunció la adquisición de HemaCare Corporation (HemaCare), una empresa especializada en terapia celular. La adquisición estratégica de la empresa ha ampliado su cartera de productos de investigación en fase inicial y soluciones de apoyo a la fabricación, lo que ha generado un aumento de las ventas y los ingresos.
La colaboración, el lanzamiento de productos, la expansión comercial, los premios y reconocimientos, las empresas conjuntas y otras estrategias de los actores del mercado están mejorando la presencia de la empresa en el mercado de subcontratación de asuntos regulatorios de dispositivos médicos, lo que también brinda beneficios para el crecimiento de las ganancias de la organización.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.














