Mercado europeo de células madre pluripotentes inducidas (iPSC), por fuente celular (células de la piel y células sanguíneas), tipo (IPSC humanas e IPSC de ratón), producto (instrumentos, consumibles, kits y servicios), aplicaciones (investigación académica, medicina regenerativa, terapia celular, detección toxicológica, descubrimiento y desarrollo de fármacos, modelado de enfermedades, bancos de células madre, bioimpresión 3D y otros), usuario final (empresas biotecnológicas y farmacéuticas, laboratorios de investigación, laboratorios de diagnóstico y otros), canal de distribución (licitación directa y ventas minoristas), país (Alemania, Reino Unido, Italia, Francia, España, Suiza, Rusia, Turquía, Bélgica, Países Bajos y resto de Europa): tendencias de la industria y pronóstico hasta 2029.
Análisis y perspectivas del mercado: mercado europeo de células madre pluripotentes inducidas (iPSC)
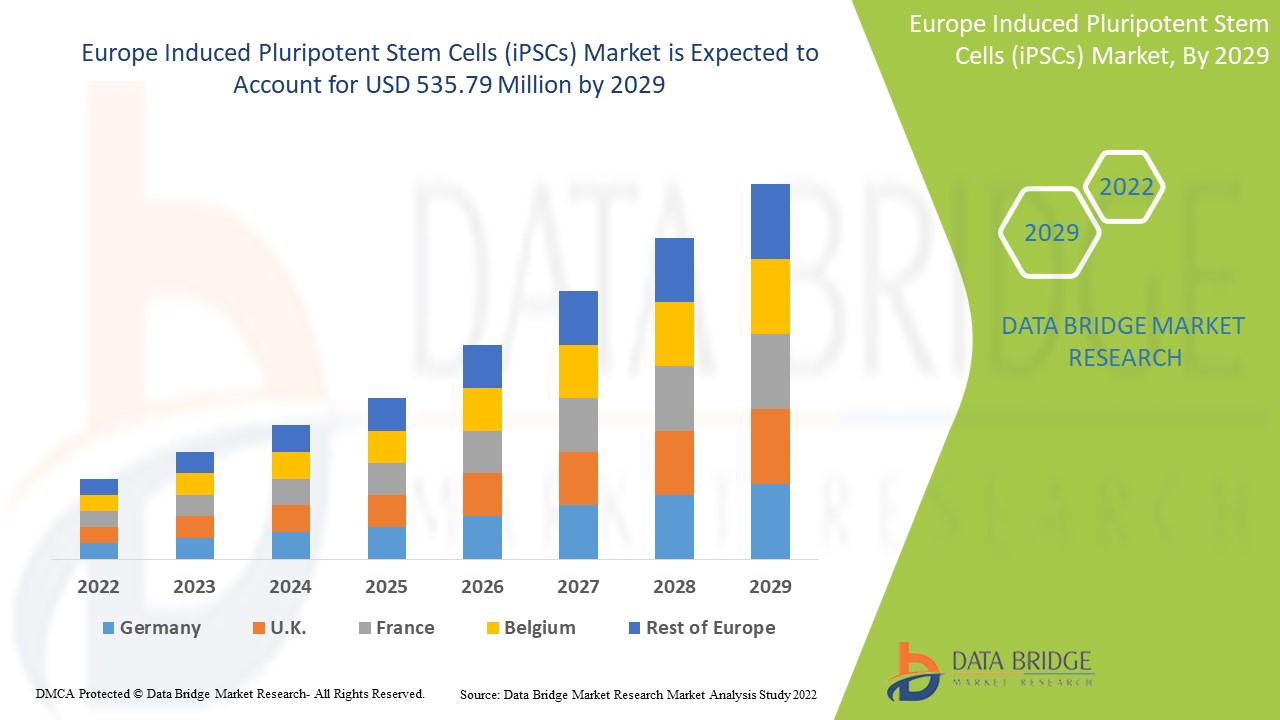
Se espera que el mercado europeo de células madre pluripotentes inducidas (iPSC) gane crecimiento de mercado en el período de pronóstico de 2022 a 2029. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 8,9% en el período de pronóstico de 2022 a 2029 y se espera que alcance los USD 535,79 millones para 2029. El aumento de las actividades de investigación sobre terapias con células madre actúa como impulsor del crecimiento del mercado de células madre pluripotentes inducidas (iPSC).
Las células madre pluripotentes inducidas son un tipo de células derivadas de los tejidos somáticos adultos y reprogramadas con un conjunto de genes y factores para obtener la naturaleza pluripotente. Se añaden ciertos genes y factores para lograr propiedades definidas de las células madre embrionarias. Las células pluripotentes inducidas son casi idénticas a las células donantes y ayudan en el modelado de enfermedades. Los retrovirus se utilizan comúnmente como vectores para reprogramar las células madre pluripotentes inducidas. Las principales aplicaciones de las células madre pluripotentes inducidas son el modelado de enfermedades, el descubrimiento y desarrollo de fármacos, los estudios de toxicidad y las terapias génicas. Se utilizan ampliamente en tratamientos para enfermedades cardiovasculares, diabetes mellitus y varios tipos de cáncer. Las células madre pluripotentes inducidas humanas muestran las propiedades relevantes de la enfermedad, ya que llevan el genotipo específico de la enfermedad, lo que permite nuevas opciones terapéuticas de forma específica para el paciente.
La creciente adopción de terapias con células madre, el crecimiento del sector de la biotecnología con una mayor inversión y la creciente prevalencia de enfermedades crónicas actúan como impulsores del mercado de células madre pluripotentes inducidas (iPSC). Otros factores que se prevé que impulsen el crecimiento del mercado de células madre pluripotentes inducidas (iPSC) en Europa incluyen la amplia gama de aplicaciones clínicas de las células madre pluripotentes inducidas y los avances tecnológicos emergentes de las iPSC.
Sin embargo, factores como el alto costo asociado con las terapias con células madre y la disponibilidad de alternativas para el tratamiento de tumores están obstaculizando el crecimiento del mercado europeo de células madre pluripotentes inducidas (iPSC). Por otro lado, el creciente número de productos en desarrollo, el mayor interés en la medicina personalizada y el aumento en el gasto en atención médica actúan como una oportunidad para el crecimiento del mercado europeo de células madre pluripotentes inducidas (iPSC). Las estrictas normas y regulaciones y la inestabilidad genómica de las IPSC son el principal desafío del mercado al que se enfrenta el mercado europeo de células madre pluripotentes inducidas (iPSC).
El informe de mercado de células madre pluripotentes inducidas (iPSC) proporciona detalles de la participación de mercado, nuevos desarrollos y análisis de la cartera de productos, el impacto de los actores del mercado nacional y localizado, analiza las oportunidades en términos de bolsillos de ingresos emergentes, cambios en las regulaciones del mercado, aprobaciones de productos, decisiones estratégicas, lanzamientos de productos, expansiones geográficas e innovaciones tecnológicas en el mercado. Para comprender el análisis y el escenario del mercado de células madre pluripotentes inducidas (iPSC), comuníquese con Data Bridge Market Research para obtener un informe de analista; nuestro equipo lo ayudará a crear una solución de impacto en los ingresos para lograr su objetivo deseado.
Alcance y tamaño del mercado de células madre pluripotentes inducidas (iPSC)
El mercado de células madre pluripotentes inducidas (iPSC) está segmentado en función de la fuente de células, el tipo, el producto, las aplicaciones, los usuarios finales y el canal de distribución. El crecimiento entre segmentos lo ayuda a analizar nichos de crecimiento y estrategias para abordar el mercado y determinar sus áreas de aplicación principales y la diferencia en sus mercados objetivo.
El mercado europeo de células madre pluripotentes inducidas (iPSC) se clasifica en seis segmentos notables según la fuente de la célula, el tipo, el producto, las aplicaciones, los usuarios finales y el canal de distribución.
- En función de la fuente de células, el mercado europeo de células madre pluripotentes inducidas (iPSC) se segmenta en células cutáneas y células sanguíneas. En 2022, se espera que el segmento de células cutáneas domine el mercado debido a la amplia disponibilidad de fuentes de células cutáneas y al alto conocimiento de las mismas.
- En función del tipo, el mercado europeo de células madre pluripotentes inducidas (iPSC) se segmenta en IPSC humanas e IPSC de ratón. En 2022, se espera que el segmento de IPSC humanas domine el mercado debido a la expansión de la población geriátrica y al aumento del número de ensayos clínicos en Francia.
- En función del producto, el mercado europeo de células madre pluripotentes inducidas (iPSC) se segmenta en instrumentos, consumibles y kits, y servicios. En 2022, se espera que el segmento de consumibles y kits domine el mercado debido al aumento de la I+D en el Reino Unido gracias a la presencia de expertos en biología del desarrollo y la reproducción.
- En función de la aplicación, el mercado europeo de células madre pluripotentes inducidas (iPSC) se segmenta en investigación académica, medicina regenerativa , terapia celular, detección toxicológica, descubrimiento y desarrollo de fármacos, modelado de enfermedades, bancos de células madre, bioimpresión 3D y otros. En 2022, se espera que el segmento de descubrimiento y desarrollo de fármacos domine el mercado, ya que la subcontratación de ensayos de iPSC ha reforzado finalmente el proceso de aprobación de fármacos.
- En función de los usuarios finales, el mercado europeo de células madre pluripotentes inducidas (iPSC) está segmentado en empresas biotecnológicas y farmacéuticas, laboratorios de investigación, laboratorios de diagnóstico y otros. En 2022, se espera que el segmento de empresas biotecnológicas y farmacéuticas domine el mercado, ya que la región se ha convertido recientemente en el centro de las empresas biofarmacéuticas de pequeña y gran escala y se ha vuelto dependiente del sector de las organizaciones de investigación por contrato y otros servicios clínicos para las operaciones de investigación y desarrollo.
- En función del canal de distribución, el mercado europeo de células madre pluripotentes inducidas (iPSC) se segmenta en licitación directa y ventas minoristas. En 2022, se espera que el segmento de licitación directa domine el mercado debido al elevado número de fuentes de suministro.
Análisis a nivel de país del mercado de células madre pluripotentes inducidas (iPSC)
Se analiza el mercado de células madre pluripotentes inducidas (iPSC) y se proporciona información sobre el tamaño del mercado por país, fuente de células, tipo, producto, aplicaciones, usuarios finales y canal de distribución como se menciona anteriormente.
Los países cubiertos en el informe del mercado de células madre pluripotentes inducidas (iPSC) son el Reino Unido, Alemania, Francia, España, Italia, Países Bajos, Suiza, Rusia, Turquía, Austria, Irlanda y el resto de Europa.
Se espera que el segmento de células madre inducidas humanas en Alemania de la región europea crezca a la tasa más alta en el período de pronóstico de 2022 a 2029 debido al uso creciente de la tecnología de células madre. El segmento de células madre inducidas humanas en Francia domina el mercado europeo debido al aumento de casos de enfermedades crónicas y la alta adopción de fuentes de células madre para mejores terapias. El Reino Unido lidera el crecimiento del mercado y el segmento de células madre inducidas humanas domina en este país debido al creciente número de centros de biotecnología y actividades de investigación.
La sección de países del informe también proporciona factores de impacto individuales en el mercado y cambios en la regulación en el mercado a nivel nacional que afectan las tendencias actuales y futuras del mercado. Los puntos de datos como nuevas ventas, ventas de reemplazo, demografía del país, leyes regulatorias y aranceles de importación y exportación son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, se considera la presencia y disponibilidad de marcas europeas y los desafíos que enfrentan debido a la competencia grande o escasa de las marcas locales y nacionales, el impacto de los canales de venta al proporcionar un análisis de pronóstico de los datos del país.
Las crecientes actividades estratégicas de los principales actores del mercado para mejorar el conocimiento sobre el tratamiento con células madre pluripotentes inducidas (iPSC) están impulsando el crecimiento del mercado de células madre pluripotentes inducidas (iPSC).
El mercado de células madre pluripotentes inducidas (iPSC) también le proporciona un análisis detallado del mercado para el crecimiento de cada país en un mercado en particular. Además, proporciona información detallada sobre la estrategia de los actores del mercado y su presencia geográfica. Los datos están disponibles para el período histórico de 2011 a 2020.
Análisis del panorama competitivo y de la cuota de mercado de las células madre pluripotentes inducidas (iPSC)
El panorama competitivo del mercado de células madre pluripotentes inducidas (iPSC) proporciona detalles por competidor. Los detalles incluidos son una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, los sitios e instalaciones de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, las líneas de prueba de productos, las aprobaciones de productos, las patentes, la amplitud y la extensión de los productos, el dominio de las aplicaciones, la curva de la línea de vida de la tecnología. Los puntos de datos anteriores proporcionados solo están relacionados con el enfoque de la empresa relacionado con el mercado de células madre pluripotentes inducidas (iPSC).
Las principales empresas que se dedican al desarrollo de células madre pluripotentes inducidas (iPSC) son Thermo Fisher Scientific Inc., FUJIFILM Corporation, LumaCyte, Horizon Discovery Ltd., Takara Bio Inc., Lonza., Evotec SE., Axol Bioscience Ltd., R & D Systems, Inc., Charles River Laboratories International, Inc., Corning Incorporated, REPROCELL Inc., Merck KGaA y otras empresas nacionales. Los analistas de DBMR comprenden las fortalezas competitivas y brindan un análisis competitivo para cada competidor por separado.
Numerosos contratos y acuerdos son también iniciados por empresas de todo el mundo que están acelerando también el mercado de células madre pluripotentes inducidas (iPSC).
Por ejemplo,
- En febrero de 2021, Thermo Fisher Scientific Inc. anunció que había ganado seis premios en los premios anuales CMO Leadership Awards. Los premios, presentados por Life Science Leader y Outsourced Pharma, reconocen a los principales socios de fabricación por contrato según la evaluación de las empresas biofarmacéuticas y biotecnológicas. Se estima que este reconocimiento fortalecerá la presencia de la empresa en el mercado europeo y conducirá a un aumento del crecimiento de la empresa en los próximos años.
- En junio de 2020, LumaCyte colaboró con Catalent, proveedor europeo de tecnologías avanzadas de administración, desarrollo y fabricación de soluciones para medicamentos, productos biológicos, terapias celulares y genéticas y productos de salud para el consumidor. Esta colaboración ayudó a expandir el producto de tecnología de células madre de la empresa, Radiance, y su aplicación.
La colaboración, el lanzamiento de productos, la expansión comercial, los premios y reconocimientos, las empresas conjuntas y otras estrategias de los actores del mercado están mejorando la presencia de la empresa en el mercado de bombas de infusión veterinaria, lo que también brinda beneficios para el crecimiento de las ganancias de la organización.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 CELL SOURCE LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 MARKET APPLICATION COVERAGE GRID
2.11 VENDOR SHARE ANALYSIS
2.12 SECONDARY SOURCES
2.13 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
5 EUROPE MEDICAL CARTS MARKET: REGULATIONS
5.1 REGULATION IN U.S.
5.2 REGULATION IN CANADA
5.3 REGULATION IN EUROPE
5.4 REGULATION IN INDIA
5.5 REGUALTION IN JAPAN
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 WIDE RANGE OF CLINICAL APPLICATION OF INDUCED PLURIPOTENT STEM CELLS
6.1.2 EMERGING TECHNOLOGICAL ADVANTAGES OF IPSCS
6.1.3 RISING PREVALENCE OF SEVERAL CHRONIC DISEASES
6.1.4 INCREASING ADOPTION OF STEM CELL THERAPY
6.1.5 GROWING BIOTECHNOLOGY SECTOR WITH BETTER INVESTMENT
6.2 RESTRAINT
6.2.1 HIGH COST ASSOCIATED WITH STEM CELL THERAPIES AND LARGE-SCALE APPLICATIONS OF IPSCS
6.2.2 AVAILABILITY OF ALTERNATIVES FOR TUMOR TREATMENT
6.2.3 ADVERSE EFFECTS OF STEM CELL TRANSPLANTS
6.3 OPPORTUNITIES
6.3.1 INCREASING NUMBER OF PIPELINE PRODUCTS
6.3.2 INCREASING INTEREST OF PERSONALIZED MEDICINE
6.3.3 SURGE IN HEALTHCARE EXPENDITURE
6.3.4 STRATEGIC INITIATIVES BY KEY MARKET PLAYERS
6.4 CHALLENGES
6.4.1 GENOMIC INSTABILITY OF IPSCS IS THE KEY MARKET CHALLENGE
6.4.2 LACK OF SKILLED PROFESSIONALS
6.4.3 STRINGENT REGULATORY FRAMEWORK
7 IMPACT OF COVID-19 ON THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
7.1 IMPACT ON PRICE
7.2 IMPACT ON DEMAND
7.3 IMPACT ON SUPPLY CHAIN
7.4 STRATEGIC DECISIONS BY MANUFACTURERS
7.5 CONCLUSION
8 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE
8.1 OVERVIEW
8.2 SKIN CELLS
8.2.1 FIBROBLAST
8.2.2 KERATINOCYTES
8.2.3 ADIPOSE DERIVED STEM CELLS
8.2.4 HEPATOCYTES
8.2.5 MELANOCYTES
8.2.6 NEURAL STEM CELLS
8.2.7 OTHERS
8.3 BLOOD CELLS
8.3.1 PERIPHERAL BLOOD
8.3.2 CORD BLOOD ENDOTHELIAL CELLS
8.3.3 CORD BLOOD STEM CELLS
8.3.4 OTHERS
9 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE
9.1 OVERVIEW
9.2 HUMAN IPSCS
9.3 MOUSE IPSCS
10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT
10.1 OVERVIEW
10.2 CONSUMABLES & KITS
10.2.1 REPROGRAMMING KITS
10.2.2 MEDIA
10.2.3 TRANSFECTION KITS
10.2.4 CELL IDENTIFICATION KITS
10.2.5 ACCESSORIES
10.2.6 OTHERS
10.3 SERVICES
10.4 INSTRUMENTS
10.4.1 IMAGING SYSTEMS
10.4.2 ELECTROPORATION DEVICE
10.4.3 INCUBATORS
10.4.4 OTHERS
11 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION
11.1 OVERVIEW
11.2 DRUG DISCOVERY AND DEVELOPMENT
11.3 ACADEMIC RESEARCH
11.4 DISEASE MODELLING
11.5 CELLULAR THERAPY
11.6 REGENERATIVE MEDICINE
11.7 TOXICOLOGY SCREENING
11.8 STEM CELL BANKING
11.9 3D BIOPRINTING
11.1 OTHERS
12 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER
12.1 OVERVIEW
12.2 BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES
12.3 RESEARCH LABORATORIES
12.4 DIAGNOSTIC LABORATORIES
12.5 OTHERS
13 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL
13.1 OVERVIEW
13.2 DIRECT TENDER
13.3 RETAIL SALES
14 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION
14.1 EUROPE
14.1.1 GERMANY
14.1.2 FRANCE
14.1.3 U.K.
14.1.4 ITALY
14.1.5 RUSSIA
14.1.6 SPAIN
14.1.7 TURKEY
14.1.8 NETHERLANDS
14.1.9 SWITZERLAND
14.1.10 BELGIUM
14.1.11 REST OF EUROPE
15 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY LANDSCAPE
15.1 COMPANY SHARE ANALYSIS: EUROPE
16 SWOT ANALYSIS
17 COMPANY PROFILE
17.1 FUJIFILM CORPORATION
17.1.1 COMPANY SNAPSHOT
17.1.2 REVENUE ANALYSIS
17.1.3 COMPANY SHARE ANALYSIS
17.1.4 PRODUCT PORTFOLIO
17.1.5 RECENT DEVELOPMENT
17.1.5.1 ACQUISITION
17.2 THERMO FISHER SCIENTIFIC INC.
17.2.1 COMPANY SNAPSHOT
17.2.2 REVENUE ANALYSIS
17.2.3 COMPANY SHARE ANALYSIS
17.2.4 PRODUCT PORTFOLIO
17.2.5 RECENT DEVELOPMENTS
17.2.5.1 EVENT
17.2.5.2 ACQUISITION
17.3 LONZA.
17.3.1 COMPANY SNAPSHOT
17.3.2 REVENUE ANALYSIS
17.3.3 COMPANY SHARE ANALYSIS
17.3.4 PRODUCT PORTFOLIO
17.3.5 RECENT DEVELOPMENTS
17.3.5.1 EXPANSION
17.4 MERCK KGAA
17.4.1 COMPANY SNAPSHOT
17.4.2 REVENUE ANALYSIS
17.4.3 COMPANY SHARE ANALYSIS
17.4.4 PRODUCT PORTFOLIO
17.4.5 RECENT DEVELOPMENT
17.4.5.1 AGREEMENT
17.5 EVOTEC SE.
17.5.1 COMPANY SNAPSHOT
17.5.2 REVENUE ANALYSIS
17.5.3 COMPANY SHARE ANALYSIS
17.5.4 PRODUCT PORTFOLIO
17.5.5 RECENT DEVELOPMENTS
17.5.5.1 AGREEMENT
17.5.5.2 COLLABORATION
17.6 APPLIED STEMCELL.
17.6.1 COMPANY SNAPSHOT
17.6.2 PRODUCT PORTFOLIO
17.6.3 RECENT DEVELOPMENTS
17.6.3.1 PRODUCT LAUNCH
17.7 AXOL BIOSCIENCE LTD.
17.7.1 COMPANY SNAPSHOT
17.7.2 PRODUCT PORTFOLIO
17.7.3 RECENT DEVELOPMENTS
17.7.3.1 MERGER
17.7.3.2 PRODUCT LAUNCH
17.8 CELL APPLICATIONS, INC.
17.8.1 COMPANY SNAPSHOT
17.8.2 PRODUCT PORTFOLIO
17.8.3 RECENT DEVELOPMENT
17.8.3.1 PARTNERSHIP
17.9 CHARLES RIVER LABORATORIES INTERNATIONAL, INC.
17.9.1 COMPANY SNAPSHOT
17.9.2 REVENUE ANALYSIS
17.9.3 PRODUCT PORTFOLIO
17.9.4 RECENT DEVELOPMENT
17.9.4.1 ACQUISITION
17.1 CITIUS PHARMACEUTICALS, INC.
17.10.1 COMPANY SNAPSHOT
17.10.2 PRODUCT PORTFOLIO
17.10.3 RECENT DEVELOPMENT
17.10.3.1 AGREEMENT
17.11 CORNING INCORPORATED
17.11.1 COMPANY SNAPSHOT
17.11.2 REVENUE ANALYSIS
17.11.3 PRODUCT PORTFOLIO
17.11.4 RECENT DEVELOPMENT
17.11.4.1 AGREEMENT
17.12 FATE THERAPEUTICS
17.12.1 COMPANY SNAPSHOT
17.12.2 PRODUCT PORTFOLIO
17.12.3 RECENT DEVELOPMENT
17.12.3.1 CLINICAL TRIAL
17.13 GENECOPOEIA, INC.
17.13.1 COMPANY SNAPSHOT
17.13.2 PRODUCT PORTFOLIO
17.13.3 RECENT DEVELOPMENT
17.14 HOPSTEM BIOTECHNOLOGY LLC.
17.14.1 COMPANY SNAPSHOT
17.14.2 PRODUCT PORTFOLIO
17.14.3 RECENT DEVELOPMENT
17.14.3.1 PARTNERSHIP
17.15 HORIZON DISCOVERY LTD.
17.15.1 COMPANY SNAPSHOT
17.15.2 PRODUCT PORTFOLIO
17.15.3 RECENT DEVELOPMENT
17.16 LUMACYTE
17.16.1 COMPANY SNAPSHOT
17.16.2 PRODUCT PORTFOLIO
17.16.3 RECENT DEVELOPMENT
17.16.3.1 COLLABORATION
17.17 R & D SYSTEMS, INC.
17.17.1 COMPANY SNAPSHOT
17.17.2 REVENUE ANALYSIS
17.17.3 PRODUCT PORTFOLIO
17.17.4 RECENT DEVELOPMENT
17.18 REPROCELL INC.
17.18.1 COMPANY SNAPSHOT
17.18.2 PRODUCT PORTFOLIO
17.18.3 RECENT DEVELOPMENTS
17.18.3.1 COLLABORATION
17.18.3.2 FACILITY EXPANSION
17.18.3.3 SERVICE LAUNCH
17.19 TAKARA BIO INC.
17.19.1 COMPANY SNAPSHOT
17.19.2 REVENUE ANALYSIS
17.19.3 PRODUCT PORTFOLIO
17.19.4 RECENT DEVELOPMENTS
17.19.4.1 NEW FACILITY LAUNCH
17.2 UNIVERSAL CELLS INC. (AN ASTELLAS COMPANY)
17.20.1 COMPANY SNAPSHOT
17.20.2 REVENUE ANALYSIS
17.20.3 PRODUCT PORTFOLIO
17.20.4 RECENT DEVELOPMENT
17.20.4.1 ACQUISITION
18 QUESTIONNAIRE
19 RELATED REPORTS
Lista de Tablas
TABLE 1 NEW CANCER CASES, AGES 85+, IN THE U.S.
TABLE 2 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 3 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 4 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 5 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 6 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 7 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 8 EUROPE HUMAN IPSCS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 9 EUROPE MOUSE IPSCS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 11 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 12 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 13 EUROPE SERVICES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 14 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 15 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 16 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 17 EUROPE DRUG DISCOVERY AND DEVELOPMENT IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 18 EUROPE ACADEMIC RESEARCH IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 19 EUROPE DISEASE MODELLING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 20 EUROPE CELLULAR THERAPY IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 21 EUROPE REGENERATIVE MEDICINE IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 22 EUROPE TOXICOLOGY SCREENING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 23 EUROPE STEM CELL BANKING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 24 EUROPE 3D BIOPRINTING IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 25 EUROPE OTHERS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 26 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 27 EUROPE BIOTECHNOLOGY & PHARMACEUTICAL COMPANIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 28 EUROPE RESEARCH LABORATORIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 29 EUROPE DIAGNOSTIC LABORATORIES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 30 EUROPE OTHERS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 31 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 32 EUROPE DIRECT TENDER IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 33 EUROPE RETAIL SALES IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY REGION, 2020-2029 (USD MILLION)
TABLE 34 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY COUNTRY, 2020-2029 (USD MILLION)
TABLE 35 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 36 EUROPE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 37 EUROPE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 38 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 39 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 40 EUROPE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 41 EUROPE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 42 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 43 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 44 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 45 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 46 GERMANY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 47 GERMANY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 48 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 49 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 50 GERMANY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 51 GERMANY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 52 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 53 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 54 GERMANY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 55 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 56 FRANCE SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 57 FRANCE BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 58 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 59 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 60 FRANCE INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 61 FRANCE CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 62 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 63 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 64 FRANCE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 65 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 66 U.K. SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 67 U.K. BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 68 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 69 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 70 U.K. INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 71 U.K. CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 72 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 73 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 74 U.K. INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 75 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 76 ITALY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 77 ITALY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 78 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 79 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 80 ITALY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 81 ITALY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 82 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 83 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 84 ITALY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 85 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 86 RUSSIA SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 87 RUSSIA BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 88 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 89 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 90 RUSSIA INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 91 RUSSIA CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 92 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 93 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 94 RUSSIA INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 95 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 96 SPAIN SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 97 SPAIN BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 98 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 99 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 100 SPAIN INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 101 SPAIN CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 102 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 103 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 104 SPAIN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 105 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 106 TURKEY SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 107 TURKEY BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 108 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 109 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 110 TURKEY INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 111 TURKEY CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 112 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 113 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 114 TURKEY INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 115 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 116 NETHERLANDS SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 117 NETHERLANDS BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 118 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 119 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 120 NETHERLANDS INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 121 NETHERLANDS CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 122 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 123 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 124 NETHERLANDS INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 125 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 126 SWITZERLAND SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 127 SWITZERLAND BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 128 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 129 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 130 SWITZERLAND INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 131 SWITZERLAND CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 132 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 133 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 134 SWITZERLAND INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 135 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 136 BELGIUM SKIN CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 137 BELGIUM BLOOD CELLS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
TABLE 138 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY TYPE, 2020-2029 (USD MILLION)
TABLE 139 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 140 BELGIUM INSTRUMENTS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 141 BELGIUM CONSUMABLES & KITS IN INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY PRODUCT, 2020-2029 (USD MILLION)
TABLE 142 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY APPLICATION, 2020-2029 (USD MILLION)
TABLE 143 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY END USER, 2020-2029 (USD MILLION)
TABLE 144 BELGIUM INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
TABLE 145 REST OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, BY CELL SOURCE, 2020-2029 (USD MILLION)
Lista de figuras
FIGURE 1 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SEGMENTATION
FIGURE 2 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DATA TRIANGULATION
FIGURE 3 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DROC ANALYSIS
FIGURE 4 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: EUROPE VS REGIONAL MARKET ANALYSIS
FIGURE 5 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: VENDOR SHARE ANALYSIS
FIGURE 10 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SEGMENTATION
FIGURE 11 THE WIDE RANGE OF CLINICAL APPLICATION OF INDUCED PLURIPOTENT STEM CELLS (IPSC) ARE EXPECTED TO DRIVE THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 12 SKIN CELLS SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET IN 2022 & 2029
FIGURE 13 NORTH AMERICA IS EXPECTED TO DOMINATE THE EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET, AND ASIA-PACIFIC IS EXPECTED TO GROW WITH THE HIGHEST CAGR IN THE FORECAST PERIOD OF 2022 TO 2029
FIGURE 14 DRIVERS, RESTRAINTS, OPPORTUNITIES, AND CHALLENGES OF EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET
FIGURE 15 PREVALENCE OF CHRONIC DISEASES
FIGURE 16 NUMBER OF PEOPLE WITH DIABETES (MILLION) AMONG AGES 20–79 YEARS
FIGURE 17 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, 2021
FIGURE 18 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, 2020-2029 (USD MILLION)
FIGURE 19 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, CAGR (2022-2029)
FIGURE 20 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE, LIFELINE CURVE
FIGURE 21 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, 2021
FIGURE 22 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, 2020-2029 (USD MILLION)
FIGURE 23 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, CAGR (2022-2029)
FIGURE 24 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY TYPE, LIFELINE CURVE
FIGURE 25 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, 2021
FIGURE 26 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, 2020-2029 (USD MILLION)
FIGURE 27 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, CAGR (2022-2029)
FIGURE 28 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY PRODUCT, LIFELINE CURVE
FIGURE 29 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, 2021
FIGURE 30 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, 2020-2029 (USD MILLION)
FIGURE 31 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, CAGR (2022-2029)
FIGURE 32 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY APPLICATION, LIFELINE CURVE
FIGURE 33 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, 2021
FIGURE 34 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, 2020-2029 (USD MILLION)
FIGURE 35 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, CAGR (2022-2029)
FIGURE 36 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY END USER, LIFELINE CURVE
FIGURE 37 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, 2021
FIGURE 38 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, 2020-2029 (USD MILLION)
FIGURE 39 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, CAGR (2022-2029)
FIGURE 40 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 41 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: SNAPSHOT (2021)
FIGURE 42 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2021)
FIGURE 43 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2022 & 2029)
FIGURE 44 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY COUNTRY (2021 & 2029)
FIGURE 45 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: BY CELL SOURCE (2022-2029)
FIGURE 46 EUROPE INDUCED PLURIPOTENT STEM CELLS (IPSCS) MARKET: COMPANY SHARE 2021 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.