Asia Pacific Vaccines Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
USD
9,048.51 Million
USD
18,031.69 Million
2022
2030
| 2023 –2030 | |
| USD 9,048.51 Million | |
| USD 18,031.69 Million | |
|
|
|
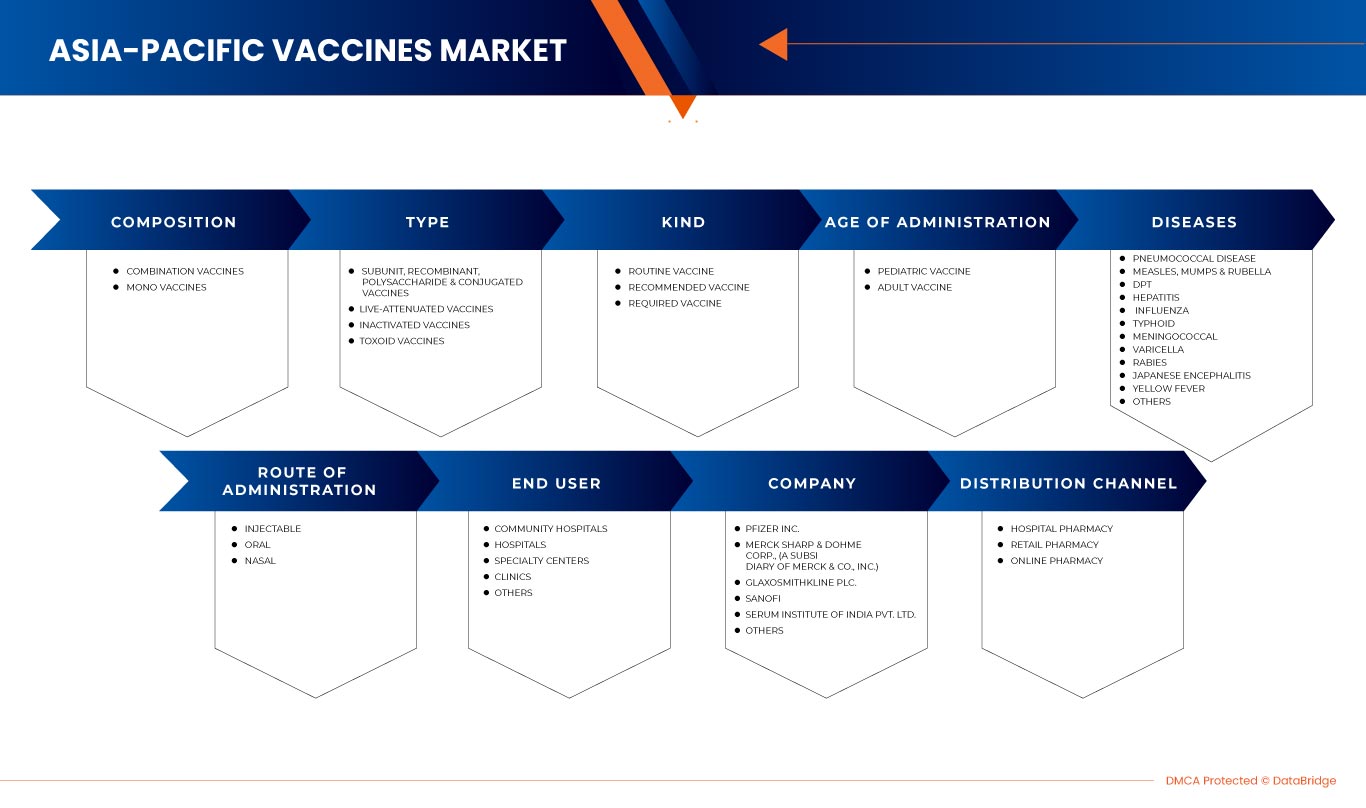
Mercado de vacunas de Asia y el Pacífico, por composición (vacunas combinadas y monovacunas), tipo (vacunas de subunidades, recombinantes, polisacáridas y conjugadas, vacunas vivas atenuadas, vacunas inactivadas y vacunas toxoides), tipo (vacuna de rutina, vacuna recomendada y vacuna requerida), edad de administración (vacuna pediátrica y vacuna para adultos), enfermedades (enfermedad neumocócica, sarampión, paperas y varicela, DPT, hepatitis, influenza, fiebre tifoidea, meningococo, rabia, encefalitis japonesa, fiebre amarilla y otras), vía de administración (inyectable, oral y nasal), usuario final (hospitales comunitarios, hospitales, centros especializados, clínicas y otros), canal de distribución (farmacia hospitalaria, farmacia minorista y farmacia en línea) - Tendencias de la industria y pronóstico hasta 2030.
Análisis y perspectivas del mercado de vacunas en Asia y el Pacífico
La creciente prevalencia de enfermedades infecciosas, incluidas las enfermedades bacterianas y virales, proporciona al mercado un crecimiento lucrativo. Junto con esto, el aumento del apoyo gubernamental y el lanzamiento de nuevas vacunas también están impulsando el mercado de vacunas. Otro factor que impulsa el crecimiento del mercado de vacunas es el aumento de la concienciación sobre la vacunación y la demanda de vacunas efectivas contra la COVID-19.
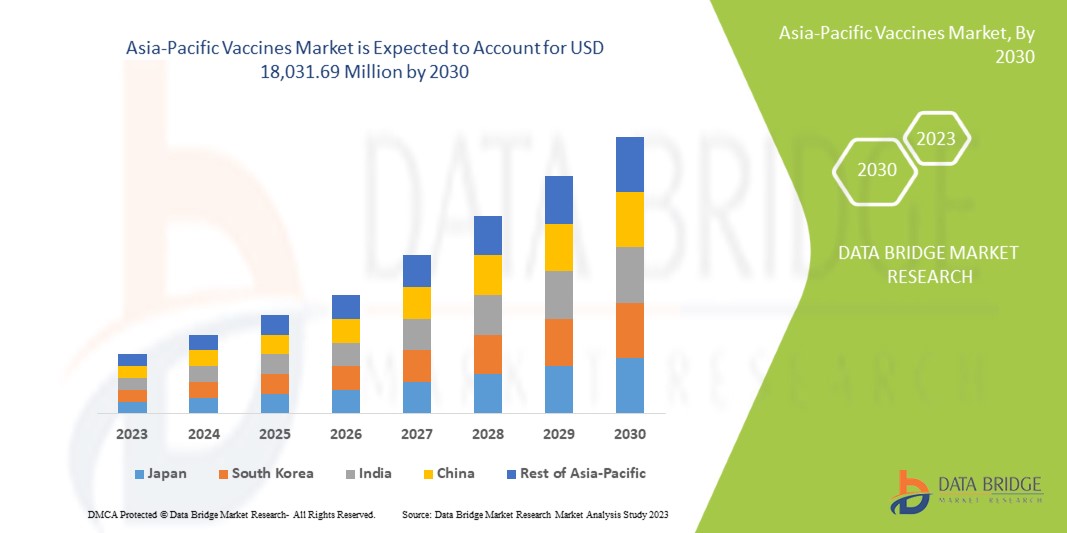
Se espera que el mercado de vacunas de Asia y el Pacífico crezca en el período de pronóstico de 2023 a 2030. Data Bridge Market Research analiza que el mercado está creciendo con una CAGR del 9,4% en el período de pronóstico de 2023 a 2030 y se espera que alcance los USD 18.031,69 millones para 2030 desde USD 9.048,51 millones en 2022.
|
Métrica del informe |
Detalles |
|
Período de pronóstico |
2023 a 2030 |
|
Año base |
2022 |
|
Años históricos |
2021 (Personalizable para 2015-2020) |
|
Unidades cuantitativas |
Ingresos en millones de USD |
|
Segmentos cubiertos |
Por composición (vacunas combinadas y monovacunas), tipo (vacunas de subunidades, recombinantes, polisacáridas y conjugadas, vacunas vivas atenuadas, vacunas inactivadas y vacunas toxoides), tipo (vacuna de rutina, vacuna recomendada y vacuna obligatoria), edad de administración (vacuna pediátrica y vacuna para adultos), enfermedades (enfermedad neumocócica, sarampión, paperas y varicela, DPT, hepatitis, influenza, fiebre tifoidea, meningococo, rabia, encefalitis japonesa, fiebre amarilla y otras), vía de administración (inyectable, oral y nasal), usuario final (hospitales comunitarios, hospitales, centros especializados, clínicas y otros), canal de distribución (farmacia hospitalaria, farmacia minorista y farmacia en línea) |
|
Países cubiertos |
Japón, China, Australia, India, Corea del Sur, Singapur, Indonesia, Tailandia, Malasia, Filipinas, Vietnam y resto de Asia-Pacífico |
|
Actores del mercado cubiertos |
Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Pvt. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (una subsidiaria de Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (una subsidiaria de Johnson & Johnson Services, Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd y BAXTER VACCINES (una subsidiaria de Baxter), entre otras. |
Definición del mercado de vacunas de Asia y el Pacífico
Las vacunas son productos que estimulan el sistema inmunológico de una persona para inducir inmunidad contra una enfermedad en particular. Las vacunas funcionan según el principio de memoria y reconocimiento. Cuando se inyectan microbios debilitados o muertos en un cuerpo, estos microbios hacen que las células B, células de memoria del sistema inmunológico, reconozcan el patógeno. En el futuro, si el mismo patógeno ataca al cuerpo, actuará contra ellos. Se han descubierto vacunas para enfermedades infecciosas, incluidas la enfermedad neumocócica, el sarampión, las paperas, la rubéola, la hepatitis, la gripe, la fiebre tifoidea, la varicela y la rabia.
Las vacunas son de dos tipos: vacunas combinadas (que contienen diferentes cepas del patógeno) y monovacunas (que contienen una sola cepa del patógeno). Se han desarrollado diferentes tipos de vacunas en función del material extraído del patógeno, que puede ser la cubierta polisacárida, el ADN, el ARN y el organismo completo, ya sea inactivado o vivo.
Estas son las vacunas que han permitido erradicar enfermedades como la polio. Según la preferencia y la eficacia de las vacunas, estas pueden inyectarse por varias vías de administración, que pueden ser inyectables, orales o nasales. Sin embargo, la vía inyectable de administración de la vacuna es la más preferida, ya que induce una respuesta sistémica. La vacunación se puede realizar en hospitales, clínicas comunitarias y clínicas especializadas, entre otros, por personal capacitado que tenga los conocimientos adecuados sobre los dispositivos de administración de vacunas.
La creciente prevalencia de enfermedades infecciosas, incluidas las bacterianas y virales, proporciona al mercado un crecimiento lucrativo. Junto con esto, el creciente apoyo gubernamental y el lanzamiento de vacunas más nuevas también impulsan el mercado de vacunas. Otro factor que impulsa el crecimiento del mercado de vacunas es el aumento de la concienciación sobre la vacunación y la demanda de vacunas efectivas contra la COVID-19.
Dinámica del mercado de vacunas en Asia y el Pacífico
En esta sección se aborda la comprensión de los factores impulsores, las ventajas, las oportunidades, las limitaciones y los desafíos del mercado. Todo esto se analiza en detalle a continuación:
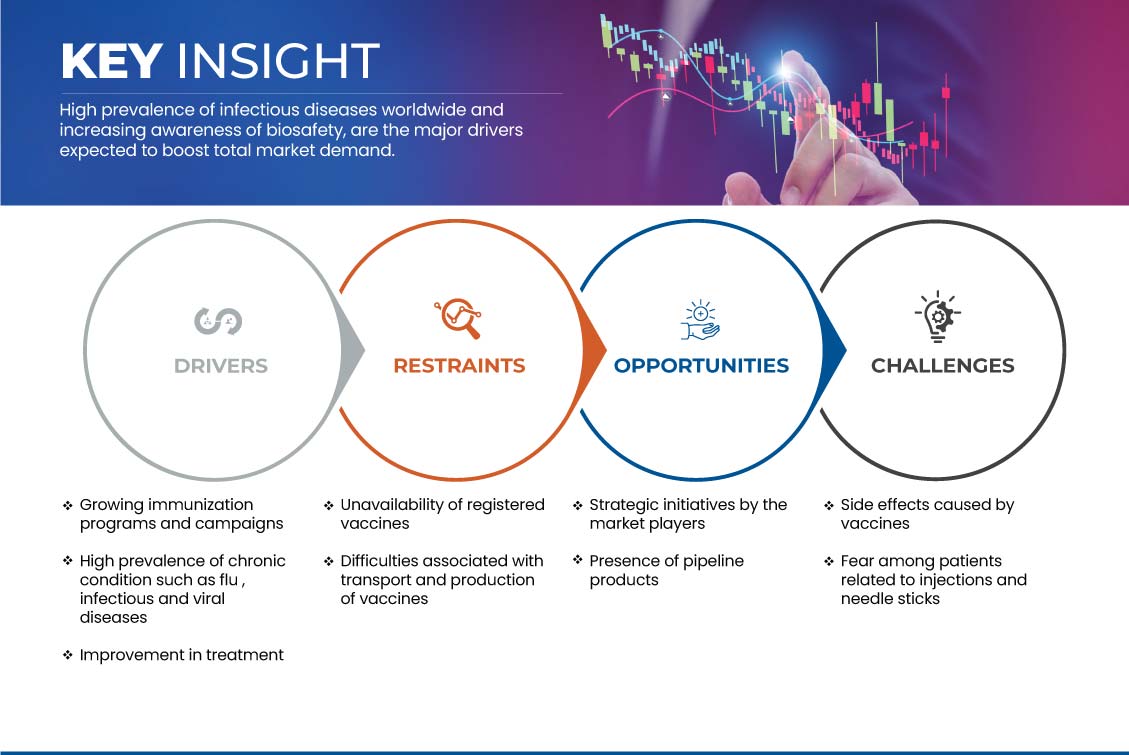
CONDUCTORES
CRECIMIENTO DE LOS PROGRAMAS Y CAMPAÑAS DE VACUNACIÓN
Los programas y campañas de inmunización están aumentando en todo el mundo debido al aumento del número de enfermedades crónicas. Como la hepatitis, la difteria, la tos ferina y la poliomielitis, entre otras enfermedades infecciosas, están prevaleciendo en el medio ambiente, existe una necesidad urgente de aumentar la concienciación sobre la vacunación, lo que se puede lograr mediante el lanzamiento de varias campañas y programas. Se ha informado de que el número de programas de inmunización está aumentando con el aumento de las enfermedades infecciosas. La cobertura de inmunización también está aumentando en todo el mundo, con la intención de luchar contra las enfermedades debilitantes.
Sin embargo, alrededor de 20 millones de personas siguen sin vacunarse o están subvacunadas, lo que aumenta la demanda de vacunación. La prevalencia de enfermedades crónicas está aumentando en todo el mundo, por lo que la necesidad de vacunarse es grande. Esto significa que se espera que el creciente programa de inmunización y la campaña hagan crecer el mercado de las vacunas.
ALTA PREVALENCIA DE ENFERMEDADES CRÓNICAS COMO GRIPE, ENFERMEDADES INFECCIOSAS Y VIRALES
La prevalencia de enfermedades infecciosas está aumentando en todo el mundo, y se ha visto que la gripe y las enfermedades infecciosas bacterianas están aumentando rápidamente. Esta tasa creciente de enfermedades infecciosas ha creado la necesidad de prevención de enfermedades que se pueden prevenir mediante la vacunación o la inmunización. Como las enfermedades están aumentando significativamente, existe una necesidad urgente de vacunación masiva. Las vacunaciones masivas requieren muchas enfermedades, por lo que se espera que proporcionen al mercado de vacunas un crecimiento lucrativo.
A medida que aumentan los casos de la enfermedad, también aumenta la atención en la vacunación masiva para evitar que la población crezca demasiado. Muchas personas que no recibieron la vacuna a una edad más temprana están inmunizadas en todo el mundo. Por lo tanto, para satisfacer estas necesidades, la demanda de nuevas vacunas está aumentando y, por lo tanto, se espera que actúen como un impulsor del mercado de vacunas de Asia y el Pacífico.
RESTRICCIÓN
NO DISPONIBILIDAD DE VACUNAS REGISTRADAS
Las estrictas aprobaciones regulatorias y los lentos procedimientos de desarrollo de las vacunas son algunos de los factores que son responsables de la falta de disponibilidad de vacunas registradas. Las autoridades regulatorias encuentran dificultades en la evaluación, autorización, control y vigilancia de las vacunas. El suministro mundial de vacunas se retrasa debido a estas regulaciones.
Por lo tanto, los procesos de regulación y formación de documentos de las empresas fabricantes en diferentes países pueden actuar como un freno y obstaculizar el crecimiento del mercado de vacunas.
OPORTUNIDAD
INICIATIVAS ESTRATÉGICAS DE LOS AGENTES DEL MERCADO
Los actores del mercado adoptan diversas iniciativas estratégicas en el mercado de vacunas que implican expansión, colaboración y adquisición. Estas iniciativas les permiten aumentar la cartera de productos de la empresa, lo que conduce a la expansión del mercado y al aumento de la demanda de productos entre los clientes, lo que en última instancia permite a los actores del mercado obtener los máximos ingresos.
A medida que aumenta la demanda de vacunas efectivas y novedosas en todo el mundo, estas iniciativas estratégicas adoptadas por los principales actores del mercado apuntan a mejorar las operaciones comerciales y obtener más rentabilidad en el mercado.
Varias iniciativas estratégicas adoptadas por los actores del mercado les permitieron expandir su presencia en el mercado de vacunas y obtener un mayor crecimiento del mercado. Por lo tanto, los actores del mercado que operan en el sector de vacunas están adoptando varias iniciativas estratégicas que se espera que actúen como una oportunidad para el crecimiento del mercado de vacunas.
DESAFÍO
EFECTOS SECUNDARIOS CAUSADOS POR LAS VACUNAS
La vacuna es un producto médico que ayuda a prevenir distintas enfermedades. Sin embargo, a veces, el uso de las vacunas puede provocar efectos secundarios. Algunos de ellos son leves, como enrojecimiento, dolor o hinchazón en el lugar de la inyección. Sin embargo, los efectos secundarios adversos de las vacunas son poco frecuentes, pero pueden poner en peligro la vida.
Los efectos secundarios potencialmente mortales pueden generar temor en la población. Además, afectan la credibilidad de los fabricantes de vacunas, lo que afecta las ventas de productos. Esto sugiere que los efectos secundarios causados por las vacunas pueden obstaculizar el crecimiento del mercado de vacunas.
Acontecimientos recientes
- En octubre de 2022, Indonesia lanzó su primera vacuna contra la COVID-19 de fabricación nacional. La vacuna IndoVac ha sido desarrollada conjuntamente por la empresa farmacéutica estatal indonesia Bio Farma y el Baylor College of Medicine, un centro independiente de ciencias de la salud en Houston, Texas.
- En noviembre de 2020, Merck Sharp & Dohme Corp., una subsidiaria de Merck & Co., Inc., firmó un acuerdo para adquirir OncoImmune, una empresa biofarmacéutica en etapa clínica. OncoImmune Company está muy centrada en el desarrollo de opciones de tratamiento para la COVID-19. A través de este acuerdo, la empresa espera desarrollar nuevas vacunas candidatas
- En septiembre de 2020, Sanofi y GSK firmaron un acuerdo con el gobierno de Canadá para suministrar 72 millones de dosis de vacunas contra la COVID-19. Existe una gran demanda de vacunas contra la COVID-19, y la demanda está aumentando con la pandemia en aumento. Este acuerdo permitió a la empresa asegurar el potencial futuro
Panorama del mercado de vacunas en Asia y el Pacífico
El mercado de vacunas de Asia-Pacífico está segmentado por composición, tipo, clase, edad de administración, enfermedades, vía de administración, usuario final y canal de distribución. El crecimiento entre estos segmentos le ayudará a analizar segmentos de crecimiento reducido en las industrias y brindará a los usuarios una valiosa descripción general del mercado y conocimientos del mercado para tomar decisiones estratégicas para identificar las principales aplicaciones del mercado.
COMPOSICIÓN
- Vacunas combinadas
- Vacunas mono
Sobre la base de su composición, el mercado de vacunas de Asia y el Pacífico está segmentado en vacunas combinadas y monovacunas.
TIPO
- Vacunas de subunidades, recombinantes, polisacáridas y conjugadas
- Vacunas vivas atenuadas
- Vacunas inactivadas
- Vacunas toxoides
Según el tipo, el mercado de vacunas de Asia y el Pacífico está segmentado en vacunas de subunidades, recombinantes, polisacáridas y conjugadas, vacunas vivas atenuadas, vacunas inactivadas y vacunas toxoides.
AMABLE
- Vacuna de rutina
- Vacuna recomendada
- Vacuna requerida
Según el tipo, el mercado de vacunas de Asia y el Pacífico se segmenta en vacuna de rutina, vacuna recomendada y vacuna requerida.
EDAD DE ADMINISTRACIÓN
- Vacuna pediátrica
- Vacuna para adultos
En función de la edad de administración, el mercado de vacunas de Asia y el Pacífico se segmenta en vacunas pediátricas y vacunas para adultos.
ENFERMEDADES
- Enfermedad neumocócica
- Sarampión, paperas y varicela
- DPT
- Hepatitis
- Influenza
- Tifoidea
- Meningococo
- Varicela
- Rabia
- Encefalitis japonesa
- Fiebre amarilla
- Otros
Sobre la base de las enfermedades, el mercado de vacunas de Asia y el Pacífico está segmentado en enfermedad neumocócica, sarampión, paperas y varicela, DPT, hepatitis, influenza, fiebre tifoidea, meningococo, rabia, encefalitis japonesa, fiebre amarilla y otras.
VÍA DE ADMINISTRACIÓN
- Inyectable
- Nasal
- Oral
Según la vía de administración, el mercado de vacunas de Asia y el Pacífico está segmentado en inyectable, oral y nasal.
USUARIO FINAL
- Hospitales comunitarios
- Hospitales
- Centros especializados
- Clínicas
- Otros
Sobre la base del usuario final, el mercado de vacunas de Asia y el Pacífico está segmentado en hospitales comunitarios, hospitales, centros especializados, clínicas y otros.
CANAL DE DISTRIBUCIÓN
- Farmacia hospitalaria
- Farmacia minorista
- Farmacia en línea
Sobre la base del canal de distribución, el mercado de vacunas de Asia-Pacífico está segmentado en farmacias hospitalarias, farmacias minoristas y farmacias en línea.
Análisis y perspectivas regionales del mercado de vacunas de Asia y el Pacífico
Se analiza el mercado de vacunas de Asia y el Pacífico y se proporcionan información y tendencias del tamaño del mercado por país, composición, tipo, clase, edad de administración, enfermedades, vía de administración, usuario final y canal de distribución, como se mencionó anteriormente.
Los países cubiertos en este informe de mercado son Japón, China, Corea del Sur, India, Australia, Singapur, Tailandia, Malasia, Indonesia, Filipinas, Vietnam y el resto de Asia-Pacífico.
Se espera que Japón domine el mercado de vacunas de Asia y el Pacífico en términos de participación de mercado e ingresos y que siga fortaleciendo su dominio durante el período de pronóstico. Esto se debe a una creciente preferencia por los controles de salud preventivos.
La sección de países del informe también proporciona factores individuales que impactan en el mercado y cambios en las regulaciones del mercado que afectan las tendencias actuales y futuras del mercado. Los puntos de datos, como las ventas nuevas y de reemplazo, la demografía del país, la epidemiología de las enfermedades y los aranceles de importación y exportación, son algunos de los principales indicadores utilizados para pronosticar el escenario del mercado para países individuales. Además, la presencia y disponibilidad de marcas globales y sus desafíos enfrentados debido a la competencia de las marcas locales y nacionales y el impacto de los canales de venta se consideran al proporcionar un análisis de pronóstico de los datos del país.
Análisis del panorama competitivo y de la cuota de mercado de las vacunas en Asia-Pacífico
El panorama competitivo del mercado de vacunas proporciona detalles sobre un competidor. Los detalles incluyen una descripción general de la empresa, las finanzas de la empresa, los ingresos generados, el potencial de mercado, la inversión en investigación y desarrollo, las nuevas iniciativas de mercado, la presencia global, los sitios e instalaciones de producción, las capacidades de producción, las fortalezas y debilidades de la empresa, el lanzamiento de productos, la amplitud y variedad de productos y el dominio de las aplicaciones. Los puntos de datos anteriores solo están relacionados con el enfoque de las empresas en el mercado de vacunas.
Algunos de los principales actores que operan en el mercado de vacunas de Asia y el Pacífico son Bharat Biotech, Biological E Limited, Bio Farma, Serum Institute of India Pvt. Ltd., Takeda Pharmaceutical Company Limited, Merck Sharp & Dohme Corp. (una subsidiaria de Merck & Co., Inc.), Abbott, AstraZeneca, Sanofi, Pfizer Inc., Janssen Global Services, LLC (una subsidiaria de Johnson & Johnson Services, Inc.), F. Hoffmann-La Roche Ltd, Panacea Biotec Ltd y BAXTER VACCINES (una subsidiaria de Baxter), entre otros.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF THE ASIA-PACIFIC VACCINES MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 COMPOSITION LIFELINE CURVE
2.8 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.9 DBMR MARKET POSITION GRID
2.1 SECONDARY SOURCES
2.11 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHT
4.1 PESTEL MODEL
4.2 PORTER'S FIVE FORCES
4.3 EPIDEMIOLOGY
4.4 INDUSTRIAL INSIGHTS:
4.5 PIPELINE ANALYSIS
4.6 ASIA-PACIFIC VACCINES MARKET: SUPPLY CHAIN MANAGEMENT OF VACCINES
4.6.1 COLD CHAIN STORAGE:
4.6.2 PROCESS OF LOGISTICS
5 REGULATORY FRAMEWORK
5.1 JAPAN
5.2 CHINA
5.3 SOUTH KOREA
5.4 INDIA
5.5 AUSTRALIA
5.6 SINGAPORE
5.7 THAILAND
5.8 MALAYSIA
5.9 INDONESIA
5.1 VIETNAM
5.11 PHILIPPINES
5.12 REST OF ASIA-PACIFIC
5.12.1 TAIWAN
5.12.2 CAMBODIA
6 MARKET OVERVIEW
6.1 DRIVERS
6.1.1 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS
6.1.2 HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU, INFECTIOUS AND VIRAL DISEASES
6.1.3 IMPROVEMENT IN TREATMENT
6.1.4 LAUNCH OF NEWER VACCINES
6.1.5 INCREASING GOVERNMENT SUPPORT
6.2 RESTRAINTS
6.2.1 UNAVAILABILITY OF REGISTERED VACCINES
6.2.2 DIFFICULTIES ASSOCIATED WITH THE TRANSPORT AND PRODUCTION OF VACCINES
6.3 OPPORTUNITIES
6.3.1 STRATEGIC INITIATIVES BY THE MARKET PLAYERS
6.3.2 PRESENCE OF PIPELINE PRODUCTS
6.3.3 RISE IN EXPENDITURE IN THE HEALTHCARE SECTOR
6.3.4 INCREASING AWARENESS FOR VACCINATION
6.4 CHALLENGES
6.4.1 SIDE EFFECTS CAUSED BY VACCINES
6.4.2 FEAR AMONG PATIENTS RELATED TO INJECTIONS AND NEEDLE STICKS
6.4.3 PRODUCT RECALL
7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION
7.1 OVERVIEW
7.2 COMBINATION VACCINES
7.3 MONO VACCINES
8 ASIA-PACIFIC VACCINES MARKET, BY TYPE
8.1 OVERVIEW
8.2 SUBUNIT, RECOMBINANT, POLYSACCHARIDE, AND CONJUGATE VACCINES
8.2.1 PNEUMOCOCCAL DISEASE
8.2.2 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
8.2.3 HPV (HUMAN PAPILLOMA VIRUS)
8.2.4 HEPATITIS B
8.2.5 MENINGOCOCCAL
8.2.6 SHINGLES
8.2.7 WHOOPING COUGH
8.2.8 OTHERS
8.3 LIVE-ATTENUTAED VACCINES
8.3.1 ROTAVIRUS
8.3.2 MEASLES
8.3.3 MUMPS
8.3.4 RUBELLA
8.3.5 SMALLPOX
8.3.6 YELLOW FEVER
8.3.7 OTHERS
8.4 INACTIVATED VACCINES
8.4.1 FLU (SHOT ONLY)
8.4.2 POLIO (SHOT ONLY)
8.4.3 HEPATITIS A
8.4.4 RABIES
8.4.5 OTHERS
8.5 TOXOID VACCINES
8.5.1 DIPHTHERIA, TETANUS & PERTUSSIS (DTP)
8.5.2 OTHERS
9 ASIA-PACIFIC VACCINES MARKET, BY KIND
9.1 OVERVIEW
9.2 ROUTINE VACCINES
9.2.1 PNEUMOCOCCAL DISEASES
9.3 DIPTHERIA, TETANUS & PERTUSIS(DPT)
9.3.1 HIB (HAEMOPHILUS INFLUENZA TYPE B) DISEASE
9.3.2 MEASLES
9.3.3 MUMPS
9.3.4 HEPATITIS B
9.3.5 RUBELLA
9.3.6 POLIO
9.3.7 OTHERS
9.4 RECOMMENDED VACCINE
9.4.1 TYPHOID FEVER VACCINE
9.5 HEPATITIS A
9.5.1 RABIES
9.5.2 JAPANESE ENCEPHALITIS
9.5.3 TICK-BORNE ENCEPHALITIS
9.5.4 CHOLERA
9.5.5 OTHERS
9.6 REQUIRED VACCINE
9.6.1 MENINGOCOCCAL
9.7 YELLOW FEVER
9.7.1 OTHERS
10 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION
10.1 OVERVIEW
10.2 PEDIATRIC VACCINE
10.2.1 PNEUMOCOCCAL DISEASES
10.3 MEASLES, MUMPS & RUBELLA
10.3.1 DIPTHERIA, TETANUS & PERTUSIS (DPT)
10.3.2 ROTAVIRUS
10.3.3 MENINGOCOCCAL
10.3.4 VARICELLA
10.3.5 POLIO
10.3.6 TUBERCULOSIS
10.3.7 MALARIA
10.3.8 OTHERS
10.4 ADULT VACCINE
10.4.1 INFLUENZA
10.5 HPV (HUMAN PAPILLOMA VIRUS)
10.5.1 TYPHOID
10.5.2 HEPATITIS B
10.5.3 JAPANESE ENCEPHALITIS
10.5.4 YELLOW FEVER
10.5.5 CANCER
10.5.6 OTHERS
11 ASIA-PACIFIC VACCINES MARKET, BY DISEASES
11.1 OVERVIEW
11.2 PNEUMOCCOCAL DISEASE
11.3 MEASLES, MUMPS & RUBELLA
11.4 DPT
11.5 HEPATITIS
11.6 INFLUENZA
11.7 TYPHOID
11.8 MENINGOCOCCAL
11.9 VARICELLA
11.1 RABIES
11.11 JAPANESE ENCEPHALITIS
11.12 YELLOW FEVER
11.13 OTHERS
12 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION
12.1 OVERVIEW
12.2 INJECTABLE
12.2.1 INTRAMUSCULAR
12.2.2 SUBCUTANEOUS
12.2.3 INTRADERMAL
12.3 ORAL
12.4 NASAL
13 ASIA-PACIFIC VACCINES MARKET, BY END USER
13.1 OVERVIEW
13.2 COMMUNITY HOSPITALS
13.3 HOSPITALS
13.4 SPECIALTY CENTERS
13.5 CLINICS
13.6 OTHERS
14 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL
14.1 OVERVIEW
14.2 HOSPITAL PHARMACY
14.3 RETAIL PHARMACY
14.4 ONLINE PHARMACY
15 ASIA-PACIFIC VACCINE MARKET
15.1 ASIA-PACIFIC
15.1.1 JAPAN
15.1.2 CHINA
15.1.3 AUSTRALIA
15.1.4 INDIA
15.1.5 SOUTH KOREA
15.1.6 SINGAPORE
15.1.7 MALAYSIA
15.1.8 THAILAND
15.1.9 INDONESIA
15.1.10 PHILIPPINES
15.1.11 VIETNAM
15.1.12 REST OF ASIA PACIFIC
16 ASIA-PACIFIC VACCINES MARKET: COMPANY LANDSCAPE
16.1 COMPANY SHARE ANALYSIS: ASIA-PACIFIC
16.2 DISEASE SHARE ANALYSIS: PFIZER, INC.
16.3 COUNTRY SHARE ANALYSIS: PFIZER, INC.
16.4 DISEASE SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.5 COUNTRY SHARE ANALYSIS: MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
16.6 DISEASE SHARE ANALYSIS: GLAXOSMITHKLINE PLC
16.7 COUNTRY SHARE ANALYSIS: GLAXOSMITHKLINE PLC.
16.8 DISEASE SHARE ANALYSIS: SANOFI
16.9 COUNTRY SHARE ANALYSIS: SANOFI
16.1 DISEASE SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
16.11 COUNTRY SHARE ANALYSIS: SERUM INSTITUTE OF INDIA PVT. LTD.
17 SWOT ANALYSIS
18 COMPANY PROFILE
18.1 PFIZER INC.
18.1.1 COMPANY SNAPSHOT
18.1.2 REVENUE ANALYSIS
18.1.3 PRODUCT PORTFOLIO
18.1.4 RECENT DEVELOPMENTS
18.2 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.)
18.2.1 COMPANY SNAPSHOT
18.2.2 REVENUE ANALYSIS
18.2.3 COMPANY WEBSITE AND PRESS RELEASES
18.2.4 PRODUCT PORTFOLIO
18.2.5 RECENT DEVELOPMENTS
18.3 GLAXOSMITHKLINE PLC.
18.3.1 COMPANY SNAPSHOT
18.3.2 REVENUE ANALYSIS
18.3.3 PRODUCT PORTFOLIO
18.3.4 RECENT DEVELOPMENTS
18.4 SANOFI
18.4.1 COMPANY SNAPSHOT
18.4.2 REVENUE ANALYSIS
18.4.3 PRODUCT PORTFOLIO
18.4.4 RECENT DEVELOPMENTS
18.5 SERUM INSTITUTE OF INDIA PVT. LTD.
18.5.1 COMPANY SNAPSHOT
18.5.2 PRODUCT PORTFOLIO
18.5.3 RECENT DEVELOPMENTS
18.6 ABBOTT
18.6.1 COMPANY SNAPSHOT
18.6.2 REVENUE ANALYSIS
18.6.3 PRODUCT PORTFOLIO
18.6.4 RECENT DEVELOPMENTS
18.7 ASTRAZENECA (2022)
18.7.1 COMPANY SNAPSHOT
18.7.2 REVENUE ANALYSIS
18.7.3 PRODUCT PORTFOLIO
18.7.4 RECENT DEVELOPMENTS
18.8 ALK
18.8.1 COMPANY SNAPSHOT
18.8.2 REVENUE ANALYSIS
18.8.3 PRODUCT PORTFOLIO
18.8.4 RECENT DEVELOPMENTS
18.9 BAXTER VACCINES (A SUBSIDIARY OF BAXTER)
18.9.1 COMPANY SNAPSHOT
18.9.2 REVENUE ANALYSIS
18.9.3 PRODUCT PORTFOLIO
18.9.4 RECENT DEVELOPMENT
18.1 BHARAT BIOTECH
18.10.1 COMPANY SNAPSHOT
18.10.2 PRODUCT PORTFOLIO
18.10.3 RECENT DEVELOPMENTS
18.11 BIO FARMA
18.11.1 COMPANY SNAPSHOT
18.11.2 PRODUCT PORTFOLIO
18.11.3 RECENT DEVELOPMENTS
18.12 BIOLOGICAL E LIMITED
18.12.1 COMPANY SNAPSHOT
18.12.2 PRODUCT PORTFOLIO
18.12.3 RECENT DEVELOPMENTS
18.13 DAIICHI SANKYO COMPANY, LIMITED
18.13.1 COMPANY SNAPSHOT
18.13.2 REVENUE ANALYSIS
18.13.3 PRODUCT PORTFOLIO
18.13.4 RECENT DEVELOPMENTS
18.14 F. HOFFMANN-LA ROCHE LTD
18.14.1 COMPANY SNAPSHOT
18.14.2 REVENUE ANALYSIS
18.14.3 PRODUCT PORTFOLIO
18.14.4 RECENT DEVELOPMENT
18.15 JANSSEN GLOBAL SERVICES, LLC (A SUBSIDIARY OF JOHNSON & JOHNSON SERVICES, INC.)
18.15.1 COMPANY SNAPSHOT
18.15.2 REVENUE ANALYSIS
18.15.3 PRODUCT PORTFOLIO
18.15.4 RECENT DEVELOPMENTS
18.16 LANZHOU BIOLOGICAL PRODUCTS RESEARCH INSTITUTE CO., LTD.,
18.16.1 COMPANY SNAPSHOT
18.16.2 PRODUCT PORTFOLIO
18.16.3 RECENT DEVELOPMENTS
18.17 PANACEA BIOTEC LTD
18.17.1 COMPANY SNAPSHOT
18.17.2 REVENUE ANALYSIS
18.17.3 PRODUCT PORTFOLIO
18.17.4 RECENT DEVELOPMENTS
18.18 SEQIRUS (A SUBSIDIARY OF CSL LIMITED)
18.18.1 COMPANY SNAPSHOT
18.18.2 REVENUE ANALYSIS
18.18.3 PRODUCT PORTFOLIO
18.18.4 RECENT DEVELOPMENTS
18.19 TAKEDA PHARMACEUTICAL COMPANY LIMITED
18.19.1 COMPANY SNAPSHOT
18.19.2 REVENUE ANALYSIS
18.19.3 COMPANY WEBSITE AND PRESS RELEASES
18.19.4 PRODUCT PORTFOLIO
18.19.5 RECENT DEVELOPMENTS
19 QUESTIONNAIRE
20 RELATED REPORTS
Lista de Tablas
TABLE 1 ASIA-PACIFIC VACCINES MARKET, PIPELINE ANALYSIS
TABLE 2 RECOMMENDED TEMPERATURE AND STORAGE LENGTH AT VARIOUS LEVELS OF THE COLD CHAIN.
TABLE 3 LOGISTICS PROCESS ACROSS DIFFERENT REGIONS.
TABLE 4 LAWS AND REGULATIONS IN TAIWAN
TABLE 5 VACCINES UNDER CLINICAL TRIAL
TABLE 6 THE SIDE EFFECTS RELATED TO THE VACCINES
TABLE 7 ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 8 ASIA-PACIFIC VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 9 ASIA-PACIFIC SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 10 ASIA-PACIFIC LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 11 ASIA-PACIFIC INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 12 ASIA-PACIFIC TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 13 ASIA-PACIFIC VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 14 ASIA-PACIFIC ROUTINE VACCINES IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 15 ASIA-PACIFIC RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 16 ASIA-PACIFIC REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 17 ASIA-PACIFIC VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 18 ASIA-PACIFIC PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 19 ASIA-PACIFIC ADULT VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 20 ASIA-PACIFIC VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 21 ASIA-PACIFIC VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 22 ASIA-PACIFIC INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2022-2030 (USD MILLION)
TABLE 23 ASIA-PACIFIC VACCINES MARKET, BY END USER, 2022-2030 (USD MILLION)
TABLE 24 ASIA-PACIFIC VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 25 ASIA-PACIFIC KNEE CARTILAGE REPAIR MARKET, BY COUNTRY, 2021-2030 (USD MILLION)
TABLE 26 JAPAN VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 27 JAPAN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 28 JAPAN SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 29 JAPAN LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 30 JAPAN INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 31 JAPAN TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 32 JAPAN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 33 JAPAN ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 34 JAPAN RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 35 JAPAN REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 36 JAPAN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 37 JAPAN PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 38 JAPAN ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 39 JAPAN VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 40 JAPAN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 41 JAPAN INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 42 JAPAN VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 43 JAPAN VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 44 CHINA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 45 CHINA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 46 CHINA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 47 CHINA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 48 CHINA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 49 CHINA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 50 CHINA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 51 CHINA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 52 CHINA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 53 CHINA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 54 CHINA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 55 CHINA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 56 CHINA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 57 CHINA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 58 CHINA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 59 CHINA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 60 CHINA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 61 CHINA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 62 AUSTRALIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 63 AUSTRALIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 64 AUSTRALIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 65 AUSTRALIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 66 AUSTRALIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 67 AUSTRALIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 68 AUSTRALIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 69 AUSTRALIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 70 AUSTRALIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 71 AUSTRALIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 72 AUSTRALIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 73 AUSTRALIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 74 AUSTRALIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 75 AUSTRALIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 76 AUSTRALIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 77 AUSTRALIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 78 AUSTRALIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 79 INDIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 80 INDIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 81 INDIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 82 INDIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 83 INDIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 84 INDIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 85 INDIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 86 INDIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 87 INDIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 88 INDIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 89 INDIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 90 INDIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 91 INDIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 92 INDIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 93 INDIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 94 INDIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 95 INDIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 96 INDIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 97 SOUTH KOREA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 98 SOUTH KOREA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 99 SOUTH KOREA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 100 SOUTH KOREA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 101 SOUTH KOREA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 102 SOUTH KOREA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 103 SOUTH KOREA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 104 SOUTH KOREA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 105 SOUTH KOREA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 106 SOUTH KOREA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 107 SOUTH KOREA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 108 SOUTH KOREA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 109 SOUTH KOREA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 110 SOUTH KOREA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 111 SOUTH KOREA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 112 SOUTH KOREA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 113 SOUTH KOREA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 114 SINGAPORE VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 115 SINGAPORE SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 116 SINGAPORE LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 117 SINGAPORE INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 118 SINGAPORE TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 119 SINGAPORE VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 120 SINGAPORE ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 121 SINGAPORE RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 122 SINGAPORE REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 123 SINGAPORE VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 124 SINGAPORE PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 125 SINGAPORE ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 126 SINGAPORE VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 127 SINGAPORE VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 128 SINGAPORE INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 129 SINGAPORE VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 130 SINGAPORE VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 131 MALAYSIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 132 MALAYSIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 133 MALAYSIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 134 MALAYSIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 135 MALAYSIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 136 MALAYSIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 137 MALAYSIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 138 MALAYSIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 139 MALAYSIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 140 MALAYSIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 141 MALAYSIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 142 MALAYSIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 143 MALAYSIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 144 MALAYSIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 145 MALAYSIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 146 MALAYSIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 147 MALAYSIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 148 MALAYSIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 149 THAILAND VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 150 THAILAND VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 151 THAILAND SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 152 THAILAND LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 153 THAILAND INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 154 THAILAND TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 155 THAILAND VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 156 THAILAND RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 157 THAILAND REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 158 THAILAND VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 159 THAILAND PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 160 THAILAND ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 161 THAILAND VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 162 THAILAND VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 163 THAILAND INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 164 THAILAND VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 165 THAILAND VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 166 INDONESIA VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 167 INDONESIA VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 168 INDONESIA SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 169 INDONESIA LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 170 INDONESIA INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 171 INDONESIA TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 172 INDONESIA VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 173 INDONESIA ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 174 INDONESIA RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 175 INDONESIA REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 176 INDONESIA VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 177 INDONESIA PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 178 INDONESIA ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 179 INDONESIA VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 180 INDONESIA VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 181 INDONESIA INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 182 INDONESIA VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 183 INDONESIA VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 184 PHILIPPINES VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 185 PHILIPPINES VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 186 PHILIPPINES SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 187 PHILIPPINES LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 188 PHILIPPINES INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 189 PHILIPPINES TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 190 PHILIPPINES VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 191 PHILIPPINES ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 192 PHILIPPINES RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 193 PHILIPPINES REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 194 PHILIPPINES VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 195 PHILIPPINES PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 196 PHILIPPINES ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 197 PHILIPPINES VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 198 PHILIPPINES VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 199 PHILIPPINES INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 200 PHILIPPINES VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 201 PHILIPPINES VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 202 VIETNAM VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
TABLE 203 VIETNAM VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 204 VIETNAM SUBUNIT, RECOMBINANT, POLYSACCHARIDE & CONJUGATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 205 VIETNAM LIVE-ATTENUATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 206 VIETNAM INACTIVATED VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 207 VIETNAM TOXOID VACCINES IN VACCINES MARKET, BY TYPE, 2021-2030 (USD MILLION)
TABLE 208 VIETNAM VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 209 VIETNAM ROUTINE VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 210 VIETNAM RECOMMENDED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 211 VIETNAM REQUIRED VACCINE IN VACCINES MARKET, BY KIND, 2021-2030 (USD MILLION)
TABLE 212 VIETNAM VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 213 VIETNAM PEDIATRIC VACCINE IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 214 VIETNAM ADULT IN VACCINES MARKET, BY AGE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 215 VIETNAM VACCINES MARKET, BY DISEASE, 2021-2030 (USD MILLION)
TABLE 216 VIETNAM VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 217 VIETNAM INJECTABLE IN VACCINES MARKET, BY ROUTE OF ADMINISTRATION, 2021-2030 (USD MILLION)
TABLE 218 VIETNAM VACCINES MARKET, BY END USER, 2021-2030 (USD MILLION)
TABLE 219 VIETNAM VACCINES MARKET, BY DISTRIBUTION CHANNEL, 2021-2030 (USD MILLION)
TABLE 220 REST OF ASIA-PACIFIC VACCINES MARKET, BY COMPOSITION, 2021-2030 (USD MILLION)
Lista de figuras
FIGURE 1 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 2 ASIA-PACIFIC VACCINES MARKET: DATA TRIANGULATION
FIGURE 3 ASIA-PACIFIC VACCINES MARKET: DROC ANALYSIS
FIGURE 4 ASIA-PACIFIC VACCINES MARKET: ASIA-PACIFIC VS COUNTRY MARKET ANALYSIS
FIGURE 5 ASIA-PACIFIC VACCINES MARKET: COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA-PACIFIC VACCINES MARKET: MULTIVARIATE MODELLING
FIGURE 7 ASIA-PACIFIC VACCINES MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 8 ASIA-PACIFIC VACCINES MARKET: DBMR MARKET POSITION GRID
FIGURE 9 ASIA-PACIFIC VACCINES MARKET: SEGMENTATION
FIGURE 10 GROWING IMMUNIZATION PROGRAMS AND CAMPAIGNS AND THE HIGH PREVALENCE OF CHRONIC CONDITIONS SUCH AS FLU AND BACTERIAL INFECTIOUS DISEASES ARE DRIVING THE ASIA-PACIFIC VACCINES MARKET IN THE FORECAST PERIOD OF 2023 TO 2030
FIGURE 11 THE COMBINATION VACCINES SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA-PACIFIC VACCINES MARKET IN 2023 & 2030
FIGURE 12 FDA REGULATORY REVIEW PROCESS OF VACCINES
FIGURE 13 PROCESS OF SPECIAL APPROVAL ON VACCINES DURING THE 2019 H1N1PDM PANDEMIC
FIGURE 14 REGULATION OVERVIEW FOR THERAPEUTICS IN SINGAPORE
FIGURE 15 DRIVERS, RESTRAINTS, OPPORTUNITIES AND CHALLENGES OF THE ASIA-PACIFIC VACCINES MARKET
FIGURE 16 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2022
FIGURE 17 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, 2023-2030 (USD MILLION)
FIGURE 18 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, CAGR (2023-2030)
FIGURE 19 ASIA-PACIFIC VACCINES MARKET: BY COMPOSITION, LIFELINE CURVE
FIGURE 20 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2022
FIGURE 21 ASIA-PACIFIC VACCINES MARKET: BY TYPE, 2023-2030 (USD MILLION)
FIGURE 22 ASIA-PACIFIC VACCINES MARKET: BY TYPE, CAGR (2023-2030)
FIGURE 23 ASIA-PACIFIC VACCINES MARKET: BY TYPE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2022
FIGURE 25 ASIA-PACIFIC VACCINES MARKET: BY KIND, 2023-2030 (USD MILLION)
FIGURE 26 ASIA-PACIFIC VACCINES MARKET: BY KIND, CAGR (2023-2030)
FIGURE 27 ASIA-PACIFIC VACCINES MARKET: BY KIND, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2022
FIGURE 29 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 30 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 31 ASIA-PACIFIC VACCINES MARKET: BY AGE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2022
FIGURE 33 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, 2023-2030 (USD MILLION)
FIGURE 34 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, CAGR (2023-2030)
FIGURE 35 ASIA-PACIFIC VACCINES MARKET: BY DISEASES, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2022
FIGURE 37 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, 2023-2030 (USD MILLION)
FIGURE 38 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, CAGR (2023-2030)
FIGURE 39 ASIA-PACIFIC VACCINES MARKET: BY ROUTE OF ADMINISTRATION, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2022
FIGURE 41 ASIA-PACIFIC VACCINES MARKET: BY END USER, 2023-2030 (USD MILLION)
FIGURE 42 ASIA-PACIFIC VACCINES MARKET: BY END USER, CAGR (2023-2030)
FIGURE 43 ASIA-PACIFIC VACCINES MARKET: BY END USER, LIFELINE CURVE
FIGURE 44 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2022
FIGURE 45 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, 2023-2030 (USD MILLION)
FIGURE 46 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, CAGR (2023-2030)
FIGURE 47 ASIA-PACIFIC VACCINES MARKET: BY DISTRIBUTION CHANNEL, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC VACCINE MARKET: SNAPSHOT (2022)
FIGURE 49 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022)
FIGURE 50 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2023 & 2030)
FIGURE 51 ASIA-PACIFIC VACCINE MARKET: BY COUNTRY (2022 & 2030)
FIGURE 52 ASIA-PACIFIC VACCINE MARKET: BY COMPOSITION (2023-2030)
FIGURE 53 ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 54 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 55 PFIZER, INC., ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 56 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 57 MERCK SHARP & DOHME CORP. (A SUBSIDIARY OF MERCK & CO., INC.) ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 58 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 59 GLAXOSMITHKLINE PLC, ASIA-PACIFIC VACCINES MARKET: COMPANY SHARE 2022 (%)
FIGURE 60 SANOFI, ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 61 SANOFI ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)
FIGURE 62 SERUM INSTITUTE OF INDIA PVT. LTD., ASIA-PACIFIC VACCINES MARKET: DISEASE SHARE 2022 (%)
FIGURE 63 SERUM INSTITUTE OF INDIA PVT. LTD. ASIA-PACIFIC VACCINES MARKET: COUNTRY SHARE 2022 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.