Asia Pacific Rapid Diagnostic Tests Rdt Market
Tamaño del mercado en miles de millones de dólares
Tasa de crecimiento anual compuesta (CAGR) :
% 
 USD
15.78 Billion
USD
34.02 Billion
2025
2033
USD
15.78 Billion
USD
34.02 Billion
2025
2033
| 2026 –2033 | |
| USD 15.78 Billion | |
| USD 34.02 Billion | |
|
|
|
|
Segmentación del mercado de pruebas de diagnóstico rápido (RDT) de Asia-Pacífico, por tipo de producto (consumibles y kits, instrumentos y otros), modo (producto de prueba de diagnóstico rápido profesional y producto de prueba de diagnóstico rápido de venta libre [OTC]), tecnología (basada en PCR, ensayos de flujo continuo, ensayos inmunocromatográficos de flujo lateral, ensayo de aglutinación, microfluídica, tecnología de sustrato y otros), modalidad (prueba basada en laboratorio y prueba no basada en laboratorio), grupo de edad (adulto y pediátrico), tipo de prueba (determinación de confirmación, pruebas serológicas y secuenciación viral), enfoque (diagnóstico in vitro y diagnóstico molecular), muestra (hisopo, sangre, orina, saliva, esputo y otros), aplicación (pruebas de enfermedades infecciosas, monitoreo de glucosa, pruebas de cardiología, pruebas oncológicas, pruebas cardiometabólicas, pruebas de drogas de abuso, pruebas de embarazo y Pruebas de fertilidad, pruebas toxicológicas y otras), usuario final (hospitales y clínicas, laboratorios de diagnóstico, centros de atención domiciliaria, institutos de investigación y académicos, entre otros), canal de distribución (licitación directa, ventas minoristas y otros): tendencias de la industria y pronóstico hasta 2032
Tamaño del mercado de pruebas de diagnóstico rápido (RDT) en Asia-Pacífico
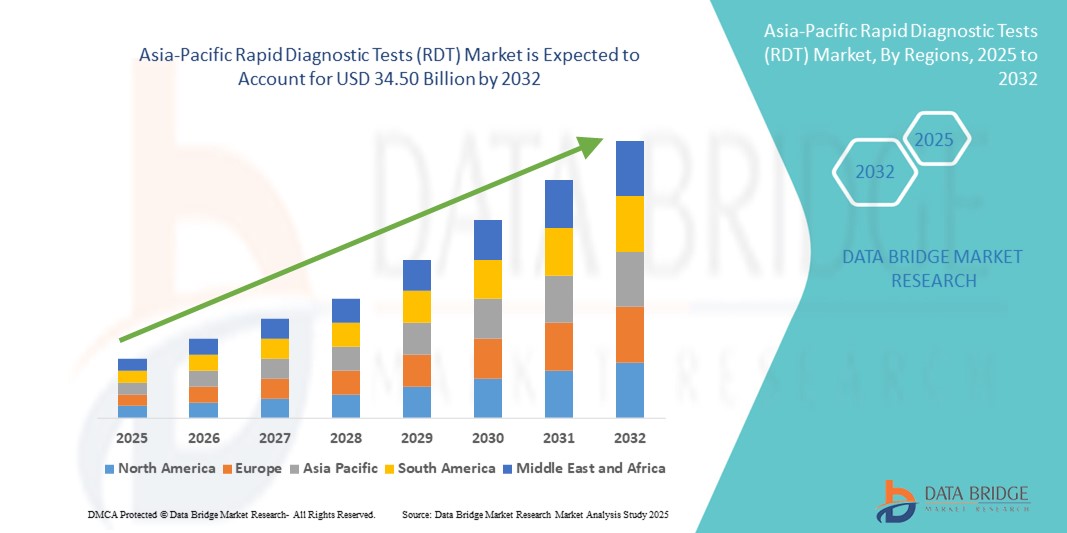
- El tamaño del mercado de pruebas de diagnóstico rápido (RDT) de Asia-Pacífico se valoró en USD 15.86 mil millones en 2024 y se espera que alcance los USD 34.50 mil millones para 2032 , con una CAGR del 10,20% durante el período de pronóstico.
- El crecimiento del mercado está impulsado en gran medida por la creciente prevalencia de enfermedades infecciosas , la creciente conciencia sobre la detección temprana de enfermedades y la expansión del acceso a la atención médica en las economías emergentes de la región, lo que lleva a una mayor adopción de diagnósticos rápidos y confiables en el punto de atención.
- Además, las iniciativas gubernamentales para fortalecer la vigilancia de enfermedades, junto con los avances en las tecnologías de RDT que ofrecen mayor precisión, portabilidad y asequibilidad, están posicionando estas pruebas como una herramienta esencial para el diagnóstico oportuno. Estos factores convergentes están acelerando la adopción de soluciones de RDT, impulsando así significativamente el crecimiento de la industria.
Análisis del mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico
- Las pruebas de diagnóstico rápido (PDR), diseñadas para brindar resultados rápidos y confiables para detectar enfermedades infecciosas y afecciones de salud, se están volviendo indispensables tanto en aplicaciones clínicas como de campo en Asia-Pacífico debido a su portabilidad, facilidad de uso y necesidades mínimas de infraestructura.
- La adopción de PDR está impulsada principalmente por la creciente prevalencia de enfermedades transmisibles, la creciente conciencia de la salud pública y la necesidad apremiante de un diagnóstico oportuno para permitir un tratamiento más rápido y el control de brotes, especialmente en poblaciones rurales y marginadas.
- China dominó el mercado de pruebas de diagnóstico rápido (RDT) con la mayor participación en los ingresos del 39,1% en 2024, respaldada por amplios programas de vigilancia de enfermedades, sólidas capacidades de fabricación nacional e iniciativas de atención médica respaldadas por el gobierno, con una adopción significativa en la detección a gran escala de enfermedades infecciosas.
- Se espera que India sea el país de más rápido crecimiento en el mercado de pruebas de diagnóstico rápido (RDT) durante el período de pronóstico, impulsado por su gran población, el aumento del gasto en atención médica y los esfuerzos a nivel nacional para integrar las pruebas rápidas en los sistemas de salud pública.
- El segmento de pruebas de enfermedades infecciosas dominó el mercado de pruebas de diagnóstico rápido (RDT) con una participación de mercado del 47,3% en 2024, impulsado por una fuerte demanda de pruebas rápidas de malaria, dengue, VIH y COVID-19, junto con mejoras tecnológicas continuas que mejoran la precisión y la asequibilidad de las pruebas.
Alcance del informe y segmentación del mercado de pruebas de diagnóstico rápido (RDT) en Asia-Pacífico
|
Atributos |
Análisis clave del mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico |
|
Segmentos cubiertos |
|
|
Países cubiertos |
Asia-Pacífico
|
|
Actores clave del mercado |
|
|
Oportunidades de mercado |
|
|
Conjuntos de información de datos de valor añadido |
Además de los conocimientos sobre escenarios de mercado, como valor de mercado, tasa de crecimiento, segmentación, cobertura geográfica y actores principales, los informes de mercado seleccionados por Data Bridge Market Research también incluyen análisis en profundidad de expertos, análisis de precios, análisis de participación de marca, encuesta de consumidores, análisis demográfico, análisis de la cadena de suministro, análisis de la cadena de valor, descripción general de materias primas/consumibles, criterios de selección de proveedores, análisis PESTLE, análisis de Porter y marco regulatorio. |
Tendencias del mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico
Los avances tecnológicos impulsan la precisión y la accesibilidad
- Una tendencia significativa y en auge en el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico es la incorporación de tecnologías de diagnóstico avanzadas, como el análisis de datos basado en IA , la microfluídica y la interpretación de resultados mediante teléfonos inteligentes, para mejorar la velocidad, la precisión y la accesibilidad de las pruebas. Este cambio permite una detección más precisa y oportuna de enfermedades, especialmente en zonas remotas y con recursos limitados.
- Por ejemplo, en marzo de 2024, Molbio Diagnostics, con sede en India, amplió su plataforma Truenat, impulsada por IA, ofreciendo pruebas moleculares rápidas, portátiles y de alta sensibilidad para la tuberculosis y la COVID-19, que pueden entregar resultados en una hora. De igual manera, Wondfo Biotech, de China, introdujo lectores de pruebas rápidas integrados en teléfonos inteligentes que permiten compartir los resultados en tiempo real con los profesionales sanitarios.
- La integración de IA en los dispositivos de RDT facilita la interpretación automatizada de las tiras reactivas, lo que reduce el error humano y mejora la consistencia del diagnóstico. Algunos sistemas portátiles ahora utilizan análisis basados en la nube para agregar datos sanitarios regionales, lo que facilita respuestas de salud pública más rápidas durante los brotes.
- La integración de las RDT con aplicaciones móviles también permite a los usuarios finales realizar un seguimiento del historial de pruebas, recibir asesoramiento médico y vincular los resultados a plataformas de telemedicina, creando un ecosistema de atención médica conectado.
- Esta tendencia hacia soluciones de RDT inteligentes, portátiles e integradas digitalmente está transformando la atención médica en Asia-Pacífico, facilitando el acceso a diagnósticos fiables para las poblaciones rurales y permitiendo una respuesta rápida a brotes de enfermedades. Por ello, empresas como SD Biosensor y Abbott están lanzando kits de RDT avanzados con funciones de conectividad mejoradas, dirigidos tanto a profesionales sanitarios como a usuarios domésticos.
- La demanda de RDT que combinen alta sensibilidad con portabilidad y conectividad digital está creciendo rápidamente en toda la región, impulsada por programas de salud gubernamentales, iniciativas de vigilancia de enfermedades infecciosas y una creciente preferencia por diagnósticos descentralizados.
Dinámica del mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico
Conductor
Aumento de la carga de enfermedades y ampliación del acceso a la atención sanitaria
- La creciente prevalencia de enfermedades infecciosas como la malaria, el dengue, el VIH, la tuberculosis y las enfermedades respiratorias, combinada con un énfasis cada vez mayor en la detección temprana y el control de enfermedades, es un impulsor importante del mercado de RDT de Asia y el Pacífico.
- Por ejemplo, en febrero de 2024, el Centro para el Control y la Prevención de Enfermedades de China (CDC de China) anunció una expansión de los programas de detección a nivel nacional de influenza y COVID-19, basándose en gran medida en kits de diagnóstico rápido para pruebas masivas.
- La ampliación del acceso a la atención sanitaria mediante iniciativas lideradas por el gobierno y programas apoyados por ONG está impulsando la adopción de PDR en zonas rurales y semiurbanas donde la infraestructura de laboratorio es limitada.
- La portabilidad, la facilidad de uso y los rápidos tiempos de respuesta de las RDT las hacen especialmente valiosas para los trabajadores de salud de campo, los equipos de respuesta a emergencias y las clínicas comunitarias.
- Además, el cambio hacia kits de autoprueba para enfermedades como el embarazo, el VIH y la COVID-19 está ampliando la base de consumidores, respaldada por la creciente disponibilidad en el comercio minorista y electrónico de productos RDT aprobados.
Restricción/Desafío
Barreras regulatorias y preocupaciones sobre garantía de calidad
- La variabilidad de los marcos regulatorios y la calidad de los productos en los países de Asia-Pacífico representan un desafío para la expansión del mercado. La falta de uniformidad en los estándares puede llevar a la circulación de kits de diagnóstico rápido (PDR) de baja calidad o falsificados, lo que socava la confianza entre los profesionales de la salud y los pacientes.
- Por ejemplo, en 2023, la autoridad reguladora de medicamentos de la India retiró del mercado varios lotes de pruebas rápidas de COVID-19 importadas debido a problemas de precisión, lo que pone de relieve la necesidad de un control de calidad más estricto.
- Además, si bien los avances tecnológicos están mejorando el rendimiento de las pruebas, la falta de procesos uniformes de certificación y aprobación en toda la región puede retrasar el ingreso al mercado de productos innovadores.
- Es fundamental abordar estas cuestiones mediante normas regulatorias armonizadas, una vigilancia posterior a la comercialización reforzada y campañas de concientización pública sobre la compra de productos certificados.
- Las limitaciones de costos siguen siendo otra barrera en las zonas de bajos ingresos, donde la asequibilidad puede limitar el acceso a pesar de los beneficios comprobados de las pruebas rápidas de diagnóstico (PDR) para la salud pública. El desarrollo de más soluciones de bajo costo y alta precisión, y el aprovechamiento de subsidios gubernamentales o financiación de donantes serán clave para superar este desafío.
Alcance del mercado de pruebas de diagnóstico rápido (RDT) en Asia-Pacífico
El mercado está segmentado según el tipo de producto, modo, tecnología, modalidad, grupo de edad, tipo de prueba, enfoque, muestra, aplicación, usuario final y canal de distribución.
- Por tipo de producto
Según el tipo de producto, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en consumibles y kits, instrumentos y otros. El segmento de consumibles y kits dominó el mercado con la mayor participación en los ingresos, con un 68,4 % en 2024, impulsado por las altas compras recurrentes para pruebas de enfermedades infecciosas, monitorización de glucosa y pruebas de embarazo. Los kits y reactivos de un solo uso gozan de una amplia preferencia por su comodidad y seguridad, lo que favorece su adopción en hospitales, clínicas y centros de atención domiciliaria. Los programas de salud pública a gran escala para enfermedades como la malaria, el dengue, la tuberculosis y la COVID-19 generan una demanda constante de consumibles. Además, estos kits son rentables, fáciles de transportar y no requieren infraestructura especializada. El segmento también se beneficia de las continuas innovaciones de productos que mejoran la sensibilidad, la especificidad y la facilidad de uso.
Se prevé que el segmento de instrumentos registre la tasa de crecimiento más rápida, del 14,9 %, entre 2025 y 2032, impulsada por la creciente adopción de analizadores compactos y portátiles y lectores compatibles con teléfonos inteligentes. Estos instrumentos proporcionan resultados más rápidos y precisos, especialmente para pruebas multiplex y diagnósticos moleculares. Los centros de salud, los laboratorios de diagnóstico y el personal sanitario de campo están adoptando cada vez más instrumentos para su uso en el punto de atención. Los instrumentos también permiten la integración con plataformas de salud digital para el seguimiento de datos en tiempo real y la monitorización remota. La creciente inversión en dispositivos portátiles y fáciles de usar para la atención sanitaria rural está impulsando un mayor crecimiento del mercado.
- Por modo
Según la moda, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en productos profesionales de diagnóstico rápido y productos de venta libre (OTC). El segmento de productos profesionales de PDR registró la mayor participación en los ingresos en 2024 debido a su amplio uso en hospitales, laboratorios de diagnóstico y campañas de detección impulsadas por los gobiernos. Estos productos son cruciales para el control de brotes y las iniciativas de pruebas masivas, ya que proporcionan resultados fiables y clínicamente validados. Las PDR profesionales también son preferidas para diagnósticos confirmatorios y procedimientos de prueba complejos. La capacitación y las medidas de control de calidad asociadas con las PDR profesionales mejoran su fiabilidad en entornos sanitarios. Los programas a gran escala para enfermedades infecciosas, como el VIH, la hepatitis y la malaria, mantienen el dominio del segmento.
Se prevé que el segmento de productos de PDR de venta libre crezca a la tasa de crecimiento anual compuesta (TCAC) más rápida, del 16,2 %, entre 2025 y 2032, impulsado por la creciente concienciación y preferencia de los consumidores por las pruebas de autodiagnóstico. Los kits de venta libre se utilizan cada vez más para afecciones como el embarazo, el VIH, la COVID-19 y la gripe, ya que ofrecen comodidad y resultados rápidos en casa. Su fácil disponibilidad en farmacias y plataformas en línea impulsa aún más su adopción. Las aprobaciones regulatorias para las pruebas de autodiagnóstico de autodiagnóstico aumentan la confianza de los consumidores en estos productos. Las pruebas en casa permiten a las personas controlar su salud de forma proactiva. El segmento se beneficia de la innovación continua en diseños intuitivos e interpretación digital de resultados.
- Por tecnología
En cuanto a la tecnología, el mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico se segmenta en ensayos basados en PCR, de flujo continuo, inmunocromatográficos de flujo lateral, de aglutinación, microfluídica, tecnología de sustratos, entre otros. El segmento de los inmunocromatográficos de flujo lateral dominó el mercado con una participación del 41,5 % en 2024, gracias a su bajo coste, facilidad de uso e idoneidad para el diagnóstico rápido en el punto de atención. Estos ensayos se utilizan ampliamente para la detección de enfermedades infecciosas en entornos profesionales y domésticos. Son portátiles, requieren una formación mínima y proporcionan resultados en cuestión de minutos. Su capacidad para utilizarse en zonas remotas y con recursos limitados refuerza su liderazgo en el mercado. Además, los fabricantes están mejorando la sensibilidad y la especificidad para aumentar la fiabilidad del diagnóstico.
Se proyecta que el segmento de la microfluídica registrará la tasa de crecimiento anual compuesta (TCAC) más rápida, del 17,8 %, entre 2025 y 2032, impulsada por la capacidad de ofrecer resultados de alta precisión con volúmenes de muestra mínimos. Las plataformas de microfluídica permiten realizar pruebas multiplex, la integración con dispositivos portátiles y la automatización del procesamiento de muestras. Estas plataformas se utilizan cada vez más en la detección de enfermedades infecciosas, la oncología y las pruebas cardiometabólicas. El aumento de las inversiones en investigación y desarrollo impulsa la innovación en tecnologías de laboratorio en chip. La microfluídica también optimiza las pruebas rápidas en entornos descentralizados, lo que la hace ideal para el diagnóstico de campo. La adopción de lectores de microfluídica integrados en teléfonos inteligentes está expandiendo aún más el segmento.
- Por modalidad
Según la modalidad, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en pruebas de laboratorio y pruebas no basadas en laboratorio. El segmento de pruebas no basadas en laboratorio registró la mayor participación en los ingresos en 2024, impulsado por la expansión de los diagnósticos en el punto de atención y las soluciones de atención médica domiciliaria. Su adopción es alta en zonas rurales y con recursos limitados, donde escasean los laboratorios centralizados. Las PDR no basadas en laboratorio ofrecen resultados rápidos, facilitan la toma de muestras y son fáciles de transportar para los programas de pruebas comunitarias. Estas pruebas se utilizan ampliamente para el seguimiento de enfermedades infecciosas, embarazo y enfermedades crónicas. Reducen la dependencia de la infraestructura clínica y permiten una intervención inmediata. Los programas gubernamentales y de ONG que promueven las pruebas descentralizadas también impulsan el crecimiento.
Se prevé un crecimiento constante del segmento de pruebas de laboratorio durante el período de pronóstico, especialmente para diagnósticos confirmatorios y pruebas moleculares complejas. Hospitales, centros de diagnóstico e institutos de investigación dependen de las pruebas rápidas de diagnóstico (PDR) de laboratorio para garantizar la precisión, la integración de datos y el control de calidad. Las pruebas de laboratorio son esenciales para la secuenciación viral, el análisis serológico y la detección de múltiples patógenos. Los avances tecnológicos, como los analizadores de alto rendimiento y la automatización, están mejorando la eficiencia de los laboratorios. Su adopción en laboratorios de referencia y en la investigación académica está en aumento.
- Por grupo de edad
Según el grupo de edad, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en adultos y niños. El segmento de adultos representó la mayor participación en 2024, principalmente debido al mayor volumen de pruebas para enfermedades crónicas, enfermedades infecciosas y detección preventiva. Los adultos se someten a pruebas frecuentes de diabetes, enfermedades cardiovasculares e infecciones de transmisión sexual. Los programas de salud laboral, los chequeos médicos obligatorios por las aseguradoras y las campañas de detección contribuyen a la alta demanda. La accesibilidad a PDR, tanto profesionales como de venta libre, para adultos impulsa aún más el crecimiento del segmento. La mayor concienciación sobre la detección temprana de enfermedades impulsa su adopción. La integración con plataformas de salud digital fomenta el autocontrol regular.
Se prevé un crecimiento más rápido del segmento pediátrico durante el período de pronóstico, impulsado por un mayor uso en programas de salud neonatal e infantil. Las pruebas de diagnóstico rápido (PDR) para la malaria, las infecciones respiratorias y las enfermedades congénitas se utilizan cada vez más en clínicas, hospitales y escuelas. Los kits fáciles de usar para la población pediátrica permiten la recolección rápida y no invasiva de muestras. Los programas gubernamentales de vacunación y cribado mejoran la penetración en el segmento. Este crecimiento también se ve impulsado por campañas de concienciación para padres. La detección temprana de enfermedades en niños mejora los resultados clínicos, lo que impulsa aún más la demanda.
- Por tipo de prueba
Según el tipo de prueba, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en confirmación, pruebas serológicas y secuenciación viral. El segmento de pruebas serológicas tuvo la mayor participación en 2024, gracias a su amplio uso en el cribado a gran escala de enfermedades infecciosas. Estas pruebas se aplican ampliamente en hospitales, laboratorios y programas de salud comunitaria. Los ensayos serológicos detectan anticuerpos, lo que ofrece información sobre la exposición a enfermedades y los niveles de inmunidad. Son esenciales para los estudios epidemiológicos y el monitoreo de la eficacia de las vacunas. La rentabilidad, la facilidad de uso y la rapidez de los resultados contribuyen a su dominio. Las mejoras continuas en la sensibilidad y la especificidad mejoran la fiabilidad.
Se proyecta que el segmento de secuenciación viral crecerá a su mayor tasa de crecimiento anual compuesto (TCAC) durante el período de pronóstico, debido a su papel en el seguimiento de mutaciones de patógenos y la orientación de las intervenciones de salud pública. La secuenciación es cada vez más crucial para la respuesta a brotes, la detección de variantes y la planificación de estrategias de vacunación. Su adopción en laboratorios de referencia y centros de investigación académica está en aumento. La integración con plataformas de secuenciación de nueva generación está mejorando la precisión. La financiación de organismos gubernamentales y de salud global está acelerando su implementación. La secuenciación viral facilita el diagnóstico de precisión y los enfoques de atención médica personalizados.
- Por enfoque
Según su enfoque, el mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico se segmenta en diagnóstico in vitro y diagnóstico molecular. El segmento de diagnóstico in vitro dominó en 2024 debido a su amplia aplicabilidad clínica y su fácil integración en los sistemas de salud existentes. Las PDR in vitro se utilizan ampliamente para enfermedades infecciosas, afecciones cardiometabólicas y pruebas de embarazo. Ofrecen plazos de entrega rápidos, protocolos sencillos y soluciones rentables. Hospitales, clínicas y programas de salud pública dependen ampliamente de estos diagnósticos. Su amplia disponibilidad y la aprobación regulatoria respaldan su adopción. La integración con plataformas digitales mejora la recopilación de datos y la vigilancia de enfermedades.
Se prevé que el segmento de diagnóstico molecular experimente el mayor crecimiento durante el período de pronóstico, impulsado por la creciente demanda de pruebas de alta precisión durante los brotes. Las pruebas de diagnóstico rápido (RDT) moleculares, como la PCR y las plataformas de amplificación isotérmica, permiten la detección de cargas virales bajas y la infección temprana. Las plataformas moleculares portátiles y rápidas se utilizan cada vez más en entornos de campo y domésticos. Las innovaciones tecnológicas en automatización y análisis asistido por IA mejoran la eficiencia. Los gobiernos están invirtiendo en diagnóstico molecular para la preparación ante pandemias. La creciente concienciación sobre el diagnóstico de precisión impulsa aún más el crecimiento.
- Por espécimen
En función de la muestra, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en hisopos, sangre, orina, saliva, esputo y otros. El segmento de las pruebas de hisopos dominó en 2024 debido a su uso generalizado en las pruebas de enfermedades respiratorias, como la COVID-19, la influenza y el VSR. Las PDR basadas en hisopos gozan de amplia aceptación por su precisión, rapidez y facilidad de recolección. Los centros de salud las utilizan para el control de brotes y el cribado rutinario. Los kits portátiles de recolección de hisopos se utilizan cada vez más para pruebas en casa y en campo. Su adopción también se ve respaldada por las aprobaciones regulatorias y los protocolos estandarizados. Las campañas de concienciación pública durante las pandemias han aumentado el conocimiento de las pruebas de hisopos.
Se prevé que el segmento de muestras de saliva experimente el mayor crecimiento durante el período de pronóstico, gracias a su naturaleza no invasiva y la facilidad de autotoma. Las pruebas rápidas de saliva (PDR) están ganando popularidad para enfermedades infecciosas, pruebas genéticas y monitoreo de carga viral. Eliminan la necesidad de personal capacitado y reducen el riesgo de exposición. La integración con kits de prueba caseros y lectores para teléfonos inteligentes está impulsando su adopción. Las pruebas de saliva también se están validando por su alta precisión en la detección de patógenos emergentes. El aumento de la investigación y el apoyo regulatorio impulsan aún más el crecimiento.
- Por aplicación
En función de su aplicación, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en pruebas de enfermedades infecciosas, monitorización de glucosa, pruebas cardiológicas, pruebas oncológicas, pruebas cardiometabólicas, pruebas de drogas de abuso, pruebas de embarazo y fertilidad, pruebas toxicológicas, entre otras. El segmento de pruebas de enfermedades infecciosas lideró el mercado con una participación del 47,3 % en 2024, impulsado por la alta demanda de diagnósticos rápidos de malaria, dengue, tuberculosis, VIH y COVID-19. Los programas de salud pública, el cribado hospitalario y las iniciativas de respuesta a brotes mantienen una sólida demanda. Los kits de prueba asequibles, rápidos y portátiles facilitan su adopción generalizada. Las innovaciones tecnológicas mejoran la sensibilidad, la especificidad y la usabilidad. Los gobiernos están priorizando la implementación de PDR en zonas rurales y desatendidas.
Se prevé una rápida expansión del segmento de pruebas oncológicas durante el período de pronóstico, a medida que las pruebas de diagnóstico rápido (PDR) cobran impulso para la detección temprana del cáncer, la monitorización de la respuesta al tratamiento y la detección de recurrencias. Cada vez se desarrollan más PDR multiplex para la elaboración de perfiles de marcadores tumorales, lo que permite la detección simultánea de múltiples biomarcadores de cáncer. La integración con dispositivos de punto de atención y plataformas de salud digital está mejorando la accesibilidad y la monitorización en tiempo real. Las crecientes campañas de concienciación e iniciativas de salud preventiva fomentan el cribado rutinario y la intervención temprana. Los institutos académicos y de investigación están invirtiendo en soluciones oncológicas basadas en PDR para mejorar la precisión diagnóstica.
- Por el usuario final
En función del usuario final, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en hospitales y clínicas, laboratorios de diagnóstico, centros de atención domiciliaria, institutos de investigación y académicos, entre otros. El segmento de hospitales y clínicas tuvo la mayor participación en 2024, sirviendo como centros principales para el diagnóstico, el inicio del tratamiento y las campañas de cribado masivo. Los hospitales dependen de PDR profesionales y de venta libre para la atención al paciente y la gestión de brotes. La creciente expansión hospitalaria, la modernización y la adopción de sistemas de salud digitales impulsan la demanda. Las medidas de capacitación y control de calidad mejoran la fiabilidad. Los programas gubernamentales y de ONG suelen suministrar PDR a granel a los hospitales. La disponibilidad de instrumentos avanzados facilita la realización de pruebas exhaustivas.
Se prevé que el segmento de atención domiciliaria experimente el mayor crecimiento durante el período de pronóstico, lo que refleja la creciente aceptación de las pruebas de autodiagnóstico y las soluciones de atención médica domiciliaria. Los consumidores prefieren cada vez más pruebas prácticas, rápidas y precisas para el embarazo, enfermedades infecciosas y afecciones crónicas. Los mercados en línea y las farmacias minoristas amplían la accesibilidad. Las pruebas rápidas de diagnóstico (PDR) compatibles con teléfonos inteligentes permiten la monitorización remota de resultados. Las PDR de atención domiciliaria reducen las visitas al hospital y facilitan la detección temprana de enfermedades. Las campañas de concienciación y las aprobaciones regulatorias para el uso de medicamentos de venta libre impulsan aún más su adopción.
- Por canal de distribución
Según el canal de distribución, el mercado de pruebas de diagnóstico rápido (PDR) de Asia-Pacífico se segmenta en licitación directa, venta minorista y otros. El segmento de licitación directa representó la mayor cuota de mercado en 2024, impulsado por las compras a gran escala para programas de salud pública, instituciones gubernamentales y hospitales. Las compras a gran escala garantizan la rentabilidad, la calidad estandarizada y un suministro constante. Los gobiernos y las ONG recurren a los acuerdos de licitación directa para las campañas de cribado masivo. Los contratos institucionales también proporcionan fuentes de ingresos recurrentes para los fabricantes de PDR. La eficiencia de la cadena de suministro y la adquisición centralizada impulsan este segmento.
Se prevé que el segmento de ventas minoristas experimente el mayor crecimiento durante el período de pronóstico, impulsado por la expansión de las cadenas de farmacias, las plataformas de comercio electrónico y la disponibilidad de kits de prueba rápida de venta libre. La creciente preferencia de los consumidores por las pruebas de autodiagnóstico impulsa la demanda minorista. El acceso rápido, la comodidad y los diseños intuitivos promueven su adopción. Los mercados en línea amplían el alcance a las poblaciones rurales y urbanas. Las campañas de marketing, el conocimiento del producto y las aprobaciones regulatorias impulsan aún más el crecimiento. Los avances tecnológicos en los kits de prueba caseros también impulsan la expansión minorista.
Análisis regional del mercado de pruebas de diagnóstico rápido (PDR) en Asia-Pacífico
- China dominó el mercado de pruebas de diagnóstico rápido (RDT) de Asia-Pacífico con la mayor participación en los ingresos del 39,1 % en 2024, respaldada por amplios programas de vigilancia de enfermedades, sólidas capacidades de fabricación nacional e iniciativas de atención médica respaldadas por el gobierno, con una importante adopción en la detección a gran escala de enfermedades infecciosas.
- El liderazgo del país en la adopción de RDT está respaldado por campañas de pruebas a gran escala para enfermedades infecciosas como COVID-19, influenza y tuberculosis, junto con un creciente despliegue de dispositivos de diagnóstico portátiles en entornos de atención médica rurales.
- Las crecientes inversiones en atención médica, un énfasis cada vez mayor en la detección temprana de enfermedades y los avances en tecnologías RDT integradas con IA y compatibles con teléfonos inteligentes están acelerando aún más el crecimiento del mercado, estableciendo a China como el centro principal tanto para la producción como para el consumo de soluciones de diagnóstico rápido en la región de Asia y el Pacífico.
Análisis del mercado de pruebas de diagnóstico rápido (RDT) en China
El mercado chino de pruebas de diagnóstico rápido (PDR) capturó la mayor participación en los ingresos, con un 39,1 %, en 2024 en Asia-Pacífico, gracias a los amplios programas de detección de enfermedades infecciosas del país, los rápidos avances tecnológicos y la sólida capacidad de fabricación nacional. Las iniciativas de vigilancia de enfermedades a gran escala como la COVID-19, la gripe, la tuberculosis y la hepatitis impulsan una demanda constante. La integración del análisis basado en IA y la interpretación de resultados a través de teléfonos inteligentes en los dispositivos de PDR está mejorando aún más la precisión y la velocidad del diagnóstico. Las inversiones gubernamentales en infraestructura sanitaria y el impulso hacia la accesibilidad a la atención médica en zonas rurales consolidan la posición de China como principal centro de producción y consumo de soluciones de PDR en la región.
Análisis del mercado de pruebas de diagnóstico rápido (PDR) en India
Se proyecta que el mercado de pruebas de diagnóstico rápido (PDR) de India se expandirá a la tasa de crecimiento anual compuesta (TCAC) más rápida durante el período de pronóstico, impulsado por las iniciativas nacionales de control de enfermedades, el aumento del gasto sanitario y la creciente adopción de soluciones de diagnóstico descentralizadas. La alta incidencia de enfermedades infecciosas en el país, como la malaria, el dengue y la tuberculosis, está impulsando la adquisición a gran escala de kits de PDR para uso profesional y comunitario. Este sólido crecimiento también se ve impulsado por la rápida expansión de los kits de autodiagnóstico domiciliario, disponibles a través de canales minoristas y de comercio electrónico. Además, el enfoque del gobierno en fortalecer la capacidad de diagnóstico en el marco del programa Ayushman Bharat está impulsando significativamente la penetración en el mercado.
Análisis del mercado de pruebas de diagnóstico rápido (PDR) en Japón
El mercado japonés de pruebas de diagnóstico rápido (PDR) experimenta un crecimiento constante, impulsado por la avanzada infraestructura sanitaria del país, el envejecimiento de la población y la atención a la detección temprana de enfermedades. Se observa una alta adopción de PDR para enfermedades infecciosas, afecciones cardiometabólicas y aplicaciones oncológicas, respaldada por la integración del seguimiento digital de resultados y las plataformas de telesalud. El sólido marco regulatorio japonés garantiza productos de diagnóstico de alta calidad, y los fabricantes nacionales innovan cada vez más con diseños portátiles y fáciles de usar para satisfacer las necesidades tanto de los profesionales clínicos como de los usuarios domésticos. El creciente uso de PDR no invasivas basadas en saliva también contribuye a la expansión del mercado.
Análisis del mercado de pruebas de diagnóstico rápido (PDR) en Corea del Sur
El mercado de pruebas de diagnóstico rápido (PDR) de Corea del Sur se encuentra en rápida expansión, impulsado por el sólido apoyo gubernamental al control de enfermedades infecciosas, un sector sanitario tecnológicamente avanzado y una importante capacidad de fabricación nacional. La rápida adopción de las PDR para las pruebas de COVID-19 en el país demostró su capacidad para implementar diagnósticos a gran escala de forma rápida y eficaz. Corea del Sur también está invirtiendo fuertemente en tecnologías de PDR moleculares y asistidas por IA para enfermedades como la gripe, la hepatitis y las infecciones de transmisión sexual. La creciente concienciación sobre la salud, sumada a la integración de dispositivos de PDR en los servicios de telemedicina, está impulsando aún más el crecimiento del mercado, especialmente en los centros urbanos.
Análisis del mercado de pruebas de diagnóstico rápido (PDR) en Australia
Se prevé que el mercado australiano de pruebas de diagnóstico rápido (PDR) experimente un crecimiento constante durante el período de pronóstico, impulsado por la creciente concienciación sobre la atención médica preventiva y la demanda de pruebas rápidas tanto en entornos clínicos como domiciliarios. Las campañas de salud impulsadas por el gobierno, dirigidas a infecciones de transmisión sexual, enfermedades respiratorias y enfermedades crónicas, están impulsando su adopción. La creciente aceptación de los kits de prueba autoadministrados, respaldada por sólidas redes de distribución de comercio electrónico, está ampliando el alcance del mercado. Además, la prioridad de Australia en los estándares de calidad y los sólidos vínculos con fabricantes globales de diagnóstico garantizan la disponibilidad de soluciones de PDR tecnológicamente avanzadas y precisas.
Cuota de mercado de pruebas de diagnóstico rápido (RDT) en Asia-Pacífico
La industria de pruebas de diagnóstico rápido (RDT) de Asia-Pacífico está liderada principalmente por empresas bien establecidas, entre las que se incluyen:
- Abbott (EE. UU.)
- F. Hoffmann-La Roche Ltd (Suiza)
- Siemens Healthineers AG (Alemania)
- BIOMÉRIEUX (Francia)
- Corporación QuidelOrtho (EE. UU.)
- SD Biosensor, Inc. (Corea del Sur)
- Access Bio, Inc. (EE. UU.)
- Mylab Discovery Solutions Pvt. Ltd. (India)
- Fujirebio Inc. (Japón)
- BD (EE. UU.)
- Bio-Rad Laboratories, Inc. (EE. UU.)
- Chembio Diagnostics, Inc. (EE. UU.)
- Oscar Medicare Pvt. Ltd. (India)
- CTK Biotech, Inc. (EE. UU.)
- Ellume Limited (Australia)
- Humasis Co., Ltd. (Corea del Sur)
- Wondfo Biotech Co., Ltd. (China)
- Lumos Diagnostics Holdings Ltd. (Australia)
- InBios International, Inc. (EE. UU.)
- Medsource Ozone Biomedicals Pvt. Ltd. (India)
¿Cuáles son los desarrollos recientes en el mercado de pruebas de diagnóstico rápido (RDT) de Asia-Pacífico?
- En agosto de 2025, Anbio (una empresa de biotecnología) amplió su arsenal de puntos de atención con el lanzamiento de una prueba rápida de chikungunya junto con una plataforma de PCR ultrarrápida para apoyar la respuesta al brote en la región de Asia y el Pacífico, abordando la necesidad de un diagnóstico rápido durante los picos virales y permitiendo intervenciones de tratamiento oportunas.
- En julio de 2025, la Organización Mundial de la Salud (OMS) precalificó el primer conjunto de tres pruebas de diagnóstico rápido (PDR) in vitro capaces de detectar simultáneamente el VIH, el virus de la hepatitis B (VHB) y la sífilis, tres infecciones importantes que plantean graves riesgos para la salud materna e infantil, lo que marca un hito significativo en el diagnóstico integrado y la mejora del acceso a herramientas de prueba críticas en entornos con recursos limitados.
- En junio de 2025, se desarrolló un nuevo prototipo de prueba de flujo lateral para permitir la detección temprana de una enfermedad fúngica mortal que surgió durante la era de la COVID-19, ofreciendo un método de detección rápido y rentable que podría implementarse ampliamente en los sistemas de atención médica de Asia y el Pacífico, especialmente en regiones que enfrentan altas cargas de infecciones fúngicas.
- En marzo de 2025, la Organización Mundial de la Salud emitió un Aviso Informativo (2025/1) advirtiendo que varias pruebas rápidas de diagnóstico de la malaria exhibieron líneas de prueba positivas débiles en pacientes con malaria confirmada, lo que generó inquietudes sobre la confiabilidad de las pruebas, los riesgos de falsos negativos y el control de calidad, lo que impulsó a las autoridades sanitarias de Asia y el Pacífico a revisar los protocolos de adquisición y uso.
- En febrero de 2025, Johns Hopkins Medicine presentó las iniciativas de su Centro de Diagnóstico Innovador, mostrando el desarrollo de nuevas pruebas rápidas de diagnóstico en el punto de atención para enfermedades infecciosas. Estos avances buscan ampliar el alcance del diagnóstico, reducir los plazos de entrega y mejorar la capacidad de realizar pruebas rápidas a nivel mundial, con especial atención a las aplicaciones en las regiones de Asia y el Pacífico con alta prevalencia de enfermedades infecciosas.
SKU-
Obtenga acceso en línea al informe sobre la primera nube de inteligencia de mercado del mundo
- Panel de análisis de datos interactivo
- Panel de análisis de empresas para oportunidades con alto potencial de crecimiento
- Acceso de analista de investigación para personalización y consultas
- Análisis de la competencia con panel interactivo
- Últimas noticias, actualizaciones y análisis de tendencias
- Aproveche el poder del análisis de referencia para un seguimiento integral de la competencia
Tabla de contenido
1 INTRODUCTION
1.1 OBJECTIVES OF THE STUDY
1.2 MARKET DEFINITION
1.3 OVERVIEW OF ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET
1.4 LIMITATIONS
1.5 MARKETS COVERED
2 MARKET SEGMENTATION
2.1 MARKETS COVERED
2.2 GEOGRAPHICAL SCOPE
2.3 YEARS CONSIDERED FOR THE STUDY
2.4 CURRENCY AND PRICING
2.5 DBMR TRIPOD DATA VALIDATION MODEL
2.6 MULTIVARIATE MODELLING
2.7 PRIMARY INTERVIEWS WITH KEY OPINION LEADERS
2.8 DBMR MARKET POSITION GRID
2.9 MARKET APPLICATION COVERAGE GRID
2.1 VENDOR SHARE ANALYSIS
2.11 SECONDARY SOURCES
2.12 ASSUMPTIONS
3 EXECUTIVE SUMMARY
4 PREMIUM INSIGHTS
4.1 PORTER’S FIVE FORCES
4.2 PESTEL ANALYSIS
4.2.1 POLITICAL FACTORS
4.2.2 ECONOMIC FACTORS
4.2.3 SOCIAL FACTORS
4.2.4 TECHNOLOGICAL FACTORS
4.2.5 ENVIRONMENTAL FACTORS
4.2.6 LEGAL FACTORS
4.2.7 QUALITY ASSURANCE & POST MARKET OBLIGATIONS
4.3 PATENT ANALYSIS
4.3.1 PATENT NUMBER AND EXPIRY
4.3.2 COUNTRY-LEVEL APPROVAL
4.3.3 LIST OF PRODUCTS REACHING EXPIRY IN NEXT 3 YEARS
4.3.4 DRUGS PATENT IN SPECIFIC COUNTRIES
4.4 COMPANY EVALUATION QUADRANT
4.5 SUPPLY CHAIN ECOSYSTEM
4.5.1 PROMINENT COMPANIES
4.5.2 SMALL & MEDIUM SIZE COMPANIES
4.5.3 END USERS
4.6 TOP TESTS RANKING & MARKET OPPORTUNITY SECTION
4.6.1 CHINA
4.6.2 JAPAN
4.6.3 INDIA
4.6.4 SOUTH KOREA
4.6.5 SINGAPORE
4.6.6 MALAYSIA
4.6.7 THAILAND
4.6.8 INDONESIA
4.6.9 PHILIPPINES
4.6.10 VIETNAM
4.6.11 REST OF ASIA–PACIFIC
4.7 CUSTOMER BUYING DRIVERS – QUALITY, ASP, SPEED, REPUTATION OTHERS
4.7.1 QUALITY
4.7.2 AVERAGE SELLING PRICE (ASP)
4.7.3 SPEED
4.7.4 REPUTATION
4.7.5 OTHER INFLUENTIAL FACTORS
4.8 INDUSTRY INSIGHTS
4.8.1 MICRO AND MACRO ECONOMIC FACTORS
4.8.2 MICROECONOMIC FACTORS
4.8.3 MACROECONOMIC FACTORS
4.8.4 PENETRATION AND GROWTH PROSPECT MAPPING
4.8.5 MARKET PENETRATION & GROWTH BY COUNTRY
4.8.6 BASED ON MODE (TYPE OF RAPID DIAGNOSTIC TEST)
4.8.7 BASED ON DIAGNOSTIC APPROACH
4.8.8 BASED ON END‑USER
4.9 KEY PRICING STRATEGIES
4.9.1 STRATEGIC PRICING APPROACHES FOR RDTS IN APAC
4.9.2 INTERVIEWS WITH SPECIALIST
4.9.3 MARKET PENETRATION AND TECHNOLOGY ADOPTION
4.1 GROWTH DRIVERS AND CHALLENGES
4.10.1 PRICING AND PROCUREMENT
4.10.2 END-USER PREFERENCES AND INSIGHTS
4.10.3 STRATEGIC IMPLICATIONS DERIVED FROM SPECIALIST INSIGHTS:
4.10.4 ANALYSIS AND RECOMMENDATION
4.10.5 MARKET ANALYSIS
4.10.6 STRATEGIC RECOMMENDATIONS
4.10.7 CONCLUSION
5 REGULATORY FRAMEWORK
5.1 REGULATORY APPROVAL PROCESS
5.2 GENERAL PROCESS STEPS (APAC):
5.3 VARIATIONS BY MARKET
5.4 GEOGRAPHIES’ EASE OF REGULATORY APPROVAL
5.5 REGULATORY APPROVAL PATHWAYS
5.6 STANDARD APPROVAL PATHWAY
5.7 RELIANCE / ABRIDGED APPROVAL PATHWAY
5.8 EXPEDITED / PRIORITY REVIEW PATHWAY
5.9 EMERGENCY USE AUTHORIZATION (EUA)
5.1 COUNTRY-SPECIFIC PATHWAY OVERVIEW
5.11 LICENSING AND REGISTRATION
5.12 INDIA — CDSCO LICENSING & REGISTRATION
5.13 SINGAPORE — HEALTH SCIENCES AUTHORITY (HSA)
5.14 AUSTRALIA — THERAPEUTIC GOODS ADMINISTRATION (TGA)
5.15 OTHER APAC MARKETS (SUMMARY)
5.16 POST-MARKETING SURVEILLANCE
5.17 GOOD MANUFACTURING PRACTICES (GMPS) GUIDELINES
6 EPIDEMIOLOGY
6.1 INCIDENCE OF ALL BY GENDER
6.2 TREATMENT RATE
6.3 MORTALITY RATE
6.4 DRUG ADHERENCE AND THERAPY SWITCH MODEL
6.5 PATIENT TREATMENT SUCCESS RATES
7 HEALTHCARE TARIFFS IMPACT ANALYSIS
7.1 OVERVIEW
7.2 TARIFF STRUCTURES.
7.2.1 ASIA-PACIFIC: GOVERNMENT-IMPOSED TARIFFS ON IMPORTED MEDICAL PRODUCTS
7.2.2 EMERGING MARKETS: CHALLENGES IN TARIFF IMPLEMENTATION
7.3 PHARMACEUTICAL TARIFFS AND TRADE BARRIERS
7.3.1 IMPORT DUTIES ON PRESCRIPTION DRUGS VS. GENERICS
7.3.2 IMPACT ON DRUG AFFORDABILITY AND ACCESS
7.3.3 KEY TRADE AGREEMENTS AFFECTING PHARMACEUTICAL TARIFFS
7.4 IMPACT OF HEALTHCARE TARIFFS ON PROVIDERS AND PATIENTS
7.4.1 COST BURDEN ON HOSPITALS AND HEALTHCARE FACILITIES
7.4.2 EFFECT ON PATIENT AFFORDABILITY AND INSURANCE COVERAGE
7.4.3 TARIFFS AND THEIR ROLE IN MEDICAL TOURISM
7.5 TRADE AGREEMENTS AND HEALTHCARE TARIFFS
7.5.1 WTO REGULATIONS ON HEALTHCARE TARIFFS
7.5.2 IMPACT OF TRADE WARS ON THE HEALTHCARE SUPPLY CHAIN
7.5.3 ROLE OF FREE TRADE AGREEMENTS (FTAS) IN REDUCING TARIFFS
7.6 IMPACT OF TARIFFS ON HEALTHCARE COSTS AND ACCESSIBILITY
7.7 IMPORTANCE OF TARIFFS IN THE HEALTHCARE SECTOR
8 MARKET OVERVIEW
8.1 DRIVERS
8.1.1 RISING DISEASE BURDEN AND EXPANDING HEALTHCARE ACCESS
8.1.2 INCREASING ADOPTION OF LATERAL FLOW ASSAY AND IMMUNOLOGY BASED RAPID TEST
8.1.3 INTEGRATION OF DIGITAL HEALTH PLATFORM WITH RAPID DIAGNOSTICS FOR RESULT TRACKING
8.1.4 INCREASING GOVERNMENT INITIATIVES AND FUNDING FOR HEALTHCARE SCREENING PROGRAMS
8.2 RESTRAINTS
8.2.1 HIGH COST OF ADVANCED RAPID DIAGNOSTIC TESTS (RDT)
8.2.2 SHORT SHELF LIFE AND STRINGENT STORAGE CONDITIONS OF (RDTS)
8.3 OPPORTUNITIES
8.3.1 TECHNOLOGICAL ADVANCEMENTS DRIVING ACCURACY AND ACCESSIBILITY
8.3.2 GROWTH POTENTIAL IN PANDEMIC PREPAREDNESS AND OUTBREAK SURVEILLANCE PROGRAM
8.3.3 GROWING OVER-THE-COUNTER (OTC) CONSUMER ADOPTION AND RETAIL DISTRIBUTION GROWTH
8.4 CHALLENEGS
8.4.1 REGULATORY BARRIERS AND QUALITY ASSURANCE CONCERNS
8.4.2 INCONSISTENT QUALITY STANDARDS AMONG LOW-COST TEST MANUFACTURERS
9 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE
9.1 OVERVIEW
9.2 PROFESSIONAL RAPID DIAGNOSTIC TEST – VISUAL READ
9.3 OTC (OVER-THE-COUNTER) RAPID DIAGNOSTIC TEST
9.4 PROFESSIONAL RAPID DIAGNOSTIC TEST – INSTRUMENT READ
9.5 PROFESSIONAL RAPID MOLECULAR DIAGNOSTIC TEST – INSTRUMENT READ
10 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY
10.1 OVERVIEW
10.2 LABORATORY BASED TEST
10.3 NON-LABORATORY BASED TEST
11 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP
11.1 OVERVIEW
11.2 ADULT
11.3 PEDIATRICS
12 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH
12.1 OVERVIEW
12.2 IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW
12.3 MOLECULAR DIAGNOSTIC (MD)
12.4 ASIA-PACIFIC IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
12.4.1 LATERAL FLOW IMMUNOASSAY (LFIA)
12.4.2 VISUAL READ RDTS (CASSETTE/STRIP FORM)
12.4.3 COLORIMETRIC
12.4.4 OTHERS
12.5 ASIA-PACIFIC MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
12.5.1 NAAT-BASED RAPID
12.5.2 RAPID PCR
12.5.3 ISOTHERMAL AMPLIFICATION
12.5.4 OTHERS
13 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA
13.1 OVERVIEW
13.2 INFECTIOUS DISEASE TESTING
13.3 ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.3.1 RESPIRATORY INFECTION TESTS
13.3.2 HEPATITIS
13.3.3 HIV
13.3.4 MALARIA
13.3.5 DENGUE
13.3.6 SEXUALLY TRANSMITTED DISEASES
13.3.7 GASTROINTESTINAL INFECTIONS
13.3.8 CHIKUNGUNYA
13.3.9 OTHERS
13.4 ASIA-PACIFIC RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.4.1 INFLUENZA A+B / A+B+C
13.4.2 RSV
13.4.3 STREP A
13.4.4 MYCOPLASMA
13.4.5 S. PNEUMONIA
13.4.6 LEGIONELLA
13.4.7 OTHERS
13.5 ASIA-PACIFIC SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.5.1 CHLAMYDIA
13.5.2 GONORRHEA
13.5.3 SYPHILIS
13.5.4 OTHERS
13.6 ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.6.1 H. PYLORI AB/AG
13.6.2 TYPHOID
13.6.3 ROTAVIRUS
13.6.4 C. DIFFICILE
13.6.5 E. COLI
13.6.6 NOROVIRUS
13.6.7 CAMPYLOBACTER
13.6.8 ADENOVIRUS
13.6.9 E71
13.6.10 OTHERS
13.7 CARDIOLOGY TESTING
13.8 ASIA-PACIFIC CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.8.1 HS-TROPONIN I
13.8.2 BNP / NT PROBNP
13.8.3 D DIMER
13.8.4 S-TROPONIN T
13.8.5 CK MB
13.8.6 HSCRP
13.8.7 MYOGLOBIN
13.8.8 PCT
13.8.9 ST2
13.8.10 HOMOCYSTEINE
13.8.11 GALECTIN 3
13.9 PREGNANCY & FERTILITY TESTING
13.1 ASIA-PACIFIC PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.10.1 HCG
13.10.2 LH
13.10.3 COMBINED FERTILITY PANELS
13.11 DRUGS OF ABUSE TESTING
13.12 ASIA-PACIFIC DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.12.1 MULTI-PANEL URINE TESTS
13.12.2 ORAL FLUID DRUG TESTS
13.13 ONCOLOGY TESTING
13.14 ASIA-PACIFIC ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.14.1 AFP
13.14.2 CEA
13.14.3 PSA
13.14.4 CA19 9
13.14.5 CA125
13.14.6 CA15 3
13.14.7 OTHERS
13.15 COAGULATION TESTING
13.16 ASIA-PACIFIC COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
13.16.1 PT
13.16.2 PTT
13.16.3 ACT
13.16.4 FIB
13.16.5 TT
13.17 OTHERS
14 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE
14.1 OVERVIEW
14.2 ECONOMY
14.3 MID-RANGE
14.4 PREMIUM
15 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER
15.1 OVERVIEW
15.2 HOSPITALS & CLINICS
15.3 ASIA-PACIFIC HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
15.3.1 PUBLIC
15.3.2 PRIVATE
15.4 DIAGNOSTIC LABORATORIES
15.5 HOME CARE SETTINGS
15.6 RESEARCH & ACADEMIC INSTITUTES
15.7 OTHERS
16 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE
16.1 OVERVIEW
16.2 GOVERNMENT SECTOR
16.3 PRIVATE SECTOR
17 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY COUNTRY
17.1 ASIA-PACIFIC
17.1.1 CHINA
17.1.2 JAPAN
17.1.3 INDIA
17.1.4 SOUTH KOREA
17.1.5 AUSTRALIA
17.1.6 INDONESIA
17.1.7 THAILAND
17.1.8 SINGAPORE
17.1.9 MALAYSIA
17.1.10 PHILIPPINES
17.1.11 VIETNAM
17.1.12 PAKISTAN
17.1.13 BANGLADESH
17.1.14 SRI LANKA
17.1.15 NEPAL
17.1.16 AFGHANISTAN
17.1.17 MALDIVES
17.1.18 BHUTAN
17.1.19 REST OF ASIA-PACIFIC
18 GERMANY SAFETY FOOTWEAR MARKET, COMPANY LANDSCAPE
18.1 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) COMPANY SHARE ANALYSIS: ASIA-PACIFIC
18.2 COMPANY SHARE ANALYSIS BY MODE
18.2.1 PROFESSIONAL RAPID DIAGNOSTIC TEST – VISUAL READ
18.2.2 PROFESSIONAL RAPID DIAGNOSTIC TEST – INSTRUMENT READ
18.2.3 PROFESSIONAL RAPID MOLECULAR DIAGNOSTIC TEST – INSTRUMENT READ
18.2.4 OTC (OVER-THE-COUNTER) RAPID DIAGNOSTIC TEST
19 SWOT ANALYSIS
20 COMPANY PROFILE
20.1 F. HOFFMANN-LA ROCHE LTD
20.1.1 COMPANY SNAPSHOT
20.1.2 REVENUE ANALYSIS
20.1.3 PRODUCT PORTFOLIO
20.1.4 RECENT DEVEOPMENT
20.2 ABBOTT
20.2.1 COMPANY SNAPSHOT
20.2.2 REVENUE ANALYSIS
20.2.3 PRODUCT PORTFOLIO
20.2.4 RECENT DEVEOPMENT
20.3 SYSMEX CORPORATION
20.3.1 COMPANY SNAPSHOT
20.3.2 REVENUE ANALYSIS
20.3.3 PRODUCT PORTFOLIO
20.3.4 RECENT DEVEOPMENT
20.4 DANAHER CORPORATION
20.4.1 COMPANY SNAPSHOT
20.4.2 REVENUE ANALYSIS
20.4.3 PRODUCT PORTFOLIO
20.4.4 RECENT DEVELOPMENT
20.5 THERMO FISHER SCIENTIFIC
20.5.1 COMPANY SNAPSHOT
20.5.2 REVENUE ANALYSIS
20.5.3 PRODUCT PORTFOLIO
20.5.4 RECENT DEVEOPMENT
20.6 ACCESS BIO
20.6.1 COMPANY SNAPSHOT
20.6.2 PRODUCT PORTFOLIO
20.6.3 RECENT DEVELOPMENT
20.7 BECTON, DICKINSON (BD)
20.7.1 COMPANY SNAPSHOT
20.7.2 REVENUE ANALYSIS
20.7.3 PRODUCT PORTFOLIO
20.7.4 RECENT DEVEOPMENT
20.8 BIOGENIX INC. PVT. LTD.
20.8.1 COMPANY SNAPSHOT
20.8.2 PRODUCT PORTFOLIO
20.8.3 RECENT DEVELOPMENT
20.9 BIOSCI HEALTHCARE
20.9.1 COMPANY SNAPSHOT
20.9.2 PRODUCT PORTFOLIO
20.9.3 RECENT DEVELOPMENT
20.1 BIOMERIEUX
20.10.1 COMPANY SNAPSHOT
20.10.2 REVENUE ANALYSIS
20.10.3 PRODUCT PORTFOLIO
20.10.4 RECENT DEVEOPMENT
20.11 BIO-RAD LABORATORIES, INC.
20.11.1 COMPANY SNAPSHOT
20.11.2 REVENUE ANALYSIS
20.11.3 PRODUCT PORTFOLIO
20.11.4 RECENT DEVELOPMENT
20.12 CHEMBIO DIAGNOSTICS, INC.
20.12.1 COMPANY SNAPSHOT
20.12.2 PRODUCT PORTFOLIO
20.12.3 RECENT DEVELOPMENT
20.13 FUJIREBIO
20.13.1 COMPANY SNAPSHOT
20.13.2 PRODUCT PORTFOLIO
20.13.3 RECENT DEVEOPMENT
20.14 GENBODY INC.
20.14.1 COMPANY SNAPSHOT
20.14.2 PRODUCT PORTFOLIO
20.14.3 RECENT DEVELOPMENT
20.15 INTEC PRODUCTS, INC.
20.15.1 COMPANY SNAPSHOT
20.15.2 PRODUCT PORTFOLIO
20.15.3 RECENT DEVELOPMENT
20.16 J. MITRA & CO. PVT. LTD.
20.16.1 COMPANY SNAPSHOT
20.16.2 PRODUCT PORTFOLIO
20.16.3 RECENT DEVELOPMENT
20.17 MERIL DIAGNOSTICS
20.17.1 COMPANY SNAPSHOT
20.17.2 PRODUCT PORTFOLIO
20.17.3 RECENT DEVELOPMENT
20.18 PRECISION BIOMED PVT LTD.
20.18.1 COMPANY SNAPSHOT
20.18.2 PRODUCT PORTFOLIO
20.18.3 RECENT DEVELOPMENT
20.19 QIAGEN
20.19.1 COMPANY SNAPSHOT
20.19.2 REVENUE ANALYSIS
20.19.3 PRODUCT PORTFOLIO
20.19.4 RECENT DEVEOPMENT
20.2 QUIDELORTHO CORPORATION
20.20.1 COMPANY SNAPSHOT
20.20.2 REVENUE ANALYSIS
20.20.3 PRODUCT PORTFOLIO
20.20.4 RECENT DEVELOPMENT
20.21 SD BIOSENSOR, INC.
20.21.1 COMPANY SNAPSHOT
20.21.2 REVENUE ANALYSIS
20.21.3 PRODUCT PORTFOLIO
20.21.4 RECENT DEVELOPMENT
20.22 SIEMENS HEALTHINEERS
20.22.1 COMPANY SNAPSHOT
20.22.2 REVENUE ANALYSIS
20.22.3 PRODUCT PORTFOLIO
20.22.4 RECENT DEVEOPMENT
20.23 TRINITY BIOTECH
20.23.1 COMPANY SNAPSHOT
20.23.2 REVENUE ANALYSIS
20.23.3 PRODUCT PORTFOLIO
20.23.4 RECENT DEVEOPMENT
20.24 WERFEN
20.24.1 COMPANY SNAPSHOT
20.24.2 PRODUCT PORTFOLIO
20.24.3 RECENT DEVELOPMENT
20.25 WONDFO
20.25.1 COMPANY SNAPSHOT
20.25.2 PRODUCT PORTFOLIO
20.25.3 RECENT DEVELOPMENT
21 QUESTIONNAIRE
22 RELATED REPORTS
Lista de Tablas
TABLE 1 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 2 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 3 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 4 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 5 ASIA-PACIFIC IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 6 ASIA-PACIFIC MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 7 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 8 ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 9 ASIA-PACIFIC RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 10 ASIA-PACIFIC SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 11 ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 12 ASIA-PACIFIC CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 13 ASIA-PACIFIC PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 14 ASIA-PACIFIC DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 15 ASIA-PACIFIC ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 16 ASIA-PACIFIC COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 17 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 18 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 19 ASIA-PACIFIC HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 20 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 21 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, 2018-2033 (USD THOUSAND)
TABLE 22 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY COUNTRY, 2018-2033 (USD THOUSAND)
TABLE 23 ASIA PACIFIC
TABLE 24 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 25 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 26 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 27 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 28 ASIA-PACIFIC IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 29 ASIA-PACIFIC MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 30 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 31 ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 32 ASIA-PACIFIC RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 33 ASIA-PACIFIC SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 34 ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 35 ASIA-PACIFIC CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 36 ASIA-PACIFIC PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 37 ASIA-PACIFIC DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 38 ASIA-PACIFIC ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 39 ASIA-PACIFIC COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 40 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 41 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 42 ASIA-PACIFIC HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 43 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 44 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 45 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 46 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 47 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 48 CHINA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 49 CHINA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 50 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 51 CHINA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 52 CHINA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 53 CHINA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 54 CHINA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 55 CHINA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 56 CHINA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 57 CHINA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 58 CHINA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 59 CHINA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 60 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 61 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 62 CHINA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 63 CHINA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 64 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 65 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 66 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 67 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 68 JAPAN IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 69 JAPAN MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 70 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 71 JAPAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 72 JAPAN RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 73 JAPAN SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 74 JAPAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 75 JAPAN CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 76 JAPAN PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 77 JAPAN DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 78 JAPAN ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 79 JAPAN COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 80 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 81 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 82 JAPAN HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 83 JAPAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 84 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 85 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 86 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 87 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 88 INDIA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 89 INDIA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 90 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 91 INDIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 92 INDIA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 93 INDIA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 94 INDIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 95 INDIA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 96 INDIA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 97 INDIA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 98 INDIA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 99 INDIA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 100 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 101 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 102 INDIA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 103 INDIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 104 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 105 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 106 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 107 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 108 SOUTH KOREA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 109 SOUTH KOREA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 110 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 111 SOUTH KOREA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 112 SOUTH KOREA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 113 SOUTH KOREA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 114 SOUTH KOREA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 115 SOUTH KOREA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 116 SOUTH KOREA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 117 SOUTH KOREA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 118 SOUTH KOREA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 119 SOUTH KOREA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 120 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 121 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 122 SOUTH KOREA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 123 SOUTH KOREA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 124 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 125 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 126 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 127 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 128 AUSTRALIA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 129 AUSTRALIA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 130 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 131 AUSTRALIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 132 AUSTRALIA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 133 AUSTRALIA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 134 AUSTRALIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 135 AUSTRALIA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 136 AUSTRALIA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 137 AUSTRALIA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 138 AUSTRALIA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 139 AUSTRALIA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 140 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 141 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 142 AUSTRALIA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 143 AUSTRALIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 144 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 145 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 146 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 147 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 148 INDONESIA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 149 INDONESIA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 150 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 151 INDONESIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 152 INDONESIA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 153 INDONESIA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 154 INDONESIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 155 INDONESIA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 156 INDONESIA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 157 INDONESIA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 158 INDONESIA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 159 INDONESIA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 160 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 161 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 162 INDONESIA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 163 INDONESIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 164 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 165 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 166 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 167 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 168 THAILAND IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 169 THAILAND MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 170 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 171 THAILAND INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 172 THAILAND RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 173 THAILAND SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 174 THAILAND INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 175 THAILAND CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 176 THAILAND PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 177 THAILAND DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 178 THAILAND ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 179 THAILAND COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 180 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 181 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 182 THAILAND HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 183 THAILAND RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 184 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 185 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 186 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 187 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 188 SINGAPORE IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 189 SINGAPORE MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 190 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 191 SINGAPORE INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 192 SINGAPORE RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 193 SINGAPORE SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 194 SINGAPORE INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 195 SINGAPORE CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 196 SINGAPORE PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 197 SINGAPORE DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 198 SINGAPORE ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 199 SINGAPORE COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 200 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 201 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 202 SINGAPORE HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 203 SINGAPORE RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 204 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 205 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 206 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 207 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 208 MALAYSIA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 209 MALAYSIA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 210 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 211 MALAYSIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 212 MALAYSIA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 213 MALAYSIA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 214 MALAYSIA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 215 MALAYSIA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 216 MALAYSIA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 217 MALAYSIA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 218 MALAYSIA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 219 MALAYSIA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 220 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 221 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 222 MALAYSIA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 223 MALAYSIA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 224 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 225 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 226 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 227 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 228 PHILIPPINES IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 229 PHILIPPINES MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 230 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 231 PHILIPPINES INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 232 PHILIPPINES RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 233 PHILIPPINES SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 234 PHILIPPINES INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 235 PHILIPPINES CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 236 PHILIPPINES PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 237 PHILIPPINES DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 238 PHILIPPINES ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 239 PHILIPPINES COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 240 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 241 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 242 PHILIPPINES HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 243 PHILIPPINES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 244 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 245 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 246 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 247 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 248 VIETNAM IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 249 VIETNAM MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 250 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 251 VIETNAM INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 252 VIETNAM RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 253 VIETNAM SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 254 VIETNAM INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 255 VIETNAM CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 256 VIETNAM PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 257 VIETNAM DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 258 VIETNAM ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 259 VIETNAM COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 260 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 261 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 262 VIETNAM HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 263 VIETNAM RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 264 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 265 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 266 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 267 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 268 PAKISTAN IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 269 PAKISTAN MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 270 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 271 PAKISTAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 272 PAKISTAN RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 273 PAKISTAN SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 274 PAKISTAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 275 PAKISTAN CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 276 PAKISTAN PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 277 PAKISTAN DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 278 PAKISTAN ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 279 PAKISTAN COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 280 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 281 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 282 PAKISTAN HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 283 PAKISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 284 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 285 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 286 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 287 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 288 BANGLADESH IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 289 BANGLADESH MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 290 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 291 BANGLADESH INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 292 BANGLADESH RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 293 BANGLADESH SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 294 BANGLADESH INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 295 BANGLADESH CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 296 BANGLADESH PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 297 BANGLADESH DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 298 BANGLADESH ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 299 BANGLADESH COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 300 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 301 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 302 BANGLADESH HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 303 BANGLADESH RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 304 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 305 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 306 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 307 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 308 SRI LANKA IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 309 SRI LANKA MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 310 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 311 SRI LANKA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 312 SRI LANKA RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 313 SRI LANKA SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 314 SRI LANKA INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 315 SRI LANKA CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 316 SRI LANKA PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 317 SRI LANKA DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 318 SRI LANKA ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 319 SRI LANKA COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 320 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 321 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 322 SRI LANKA HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 323 SRI LANKA RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 324 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 325 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 326 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 327 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 328 NEPAL IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 329 NEPAL MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 330 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 331 NEPAL INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 332 NEPAL RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 333 NEPAL SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 334 NEPAL INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 335 NEPAL CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 336 NEPAL PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 337 NEPAL DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 338 NEPAL ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 339 NEPAL COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 340 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 341 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 342 NEPAL HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 343 NEPAL RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 344 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 345 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 346 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 347 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 348 AFGHANISTAN IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 349 AFGHANISTAN MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 350 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 351 AFGHANISTAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 352 AFGHANISTAN RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 353 AFGHANISTAN SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 354 AFGHANISTAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 355 AFGHANISTAN CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 356 AFGHANISTAN PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 357 AFGHANISTAN DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 358 AFGHANISTAN ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 359 AFGHANISTAN COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 360 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 361 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 362 AFGHANISTAN HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 363 AFGHANISTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 364 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 365 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 366 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 367 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 368 MALDIVES IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 369 MALDIVES MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 370 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 371 MALDIVES INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 372 MALDIVES RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 373 MALDIVES SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 374 MALDIVES INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 375 MALDIVES CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 376 MALDIVES PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 377 MALDIVES DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 378 MALDIVES ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 379 MALDIVES COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 380 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 381 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 382 MALDIVES HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 383 MALDIVES RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 384 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 385 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 386 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 387 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 388 BHUTAN IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 389 BHUTAN MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 390 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 391 BHUTAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 392 BHUTAN RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 393 BHUTAN SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 394 BHUTAN INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 395 BHUTAN CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 396 BHUTAN PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 397 BHUTAN DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 398 BHUTAN ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 399 BHUTAN COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 400 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 401 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 402 BHUTAN HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 403 BHUTAN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
TABLE 404 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2018-2033 (USD THOUSAND)
TABLE 405 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2018-2033 (USD THOUSAND)
TABLE 406 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2018-2033 (USD THOUSAND)
TABLE 407 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2018-2033 (USD THOUSAND)
TABLE 408 REST OF ASIA-PACIFIC IN-VITRO DIAGNOSTIC (IVD) – IMMUNOASSAY / LATERAL FLOW IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 409 REST OF ASIA-PACIFIC MOLECULAR DIAGNOSTIC (MD) IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 410 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2018-2033 (USD THOUSAND)
TABLE 411 REST OF ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 412 REST OF ASIA-PACIFIC RESPIRATORY INFECTION TESTS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 413 REST OF ASIA-PACIFIC SEXUALLY TRANSMITTED DISEASES IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 414 REST OF ASIA-PACIFIC INFECTIOUS DISEASE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 415 REST OF ASIA-PACIFIC CARDIOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 416 REST OF ASIA-PACIFIC PREGNANCY & FERTILITY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 417 REST OF ASIA-PACIFIC DRUGS OF ABUSE TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 418 REST OF ASIA-PACIFIC ONCOLOGY TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 419 REST OF ASIA-PACIFIC COAGULATION TESTING IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 420 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2018-2033 (USD THOUSAND)
TABLE 421 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2018-2033 (USD THOUSAND)
TABLE 422 REST OF ASIA-PACIFIC HOSPITALS & CLINICS IN RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY TYPE, 2018-2033 (USD THOUSAND)
TABLE 423 REST OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2018-2033 (USD THOUSAND)
Lista de figuras
FIGURE 1 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET : SEGMENTATION
FIGURE 2 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET : DATA TRIANGULATION
FIGURE 3 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: DROC ANALYSIS
FIGURE 4 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: GLOBAL VS REGIONAL ANALYSIS
FIGURE 5 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET : COMPANY RESEARCH ANALYSIS
FIGURE 6 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: INTERVIEW DEMOGRAPHICS
FIGURE 7 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: DBMR MARKET POSITION GRID
FIGURE 8 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: MARKET APPLICATION COVERAGE GRID
FIGURE 9 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET : VENDOR SHARE ANALYSIS
FIGURE 10 EXECUTIVE SUMMARY
FIGURE 11 STRATEGIC DECISIONS
FIGURE 12 ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: SEGMENTATION
FIGURE 13 FOUR SEGMENTS COMPRISE THE ASIA PACIFIC RAPID DIAGNOSTICS TESTS (RDT) MARKET, BY MODE (2025)
FIGURE 14 RISING DISEASE BURDEN AND EXPANDING HEALTHCARE ACCESS EXPECTED TO DRIVE THE ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET IN THE FORECAST PERIOD OF 2026 TO 2033
FIGURE 15 PROFESSIONAL RAPID DIAGNOSYIC TEST-VISUAL READ SEGMENT IS EXPECTED TO ACCOUNT FOR THE LARGEST SHARE OF THE ASIA PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET IN 2026 & 2033
FIGURE 16 PESTEL ANALYSIS
FIGURE 17 NUMBER OF PATENT
FIGURE 18 TOP ENTITIES BASED ON R&D GLANCE FOR ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET
FIGURE 19 DRIVERS, RESTRINTS, OPPORTUNITIES AND CHALLENGES OF ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET
FIGURE 20 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODE, 2025
FIGURE 21 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY MODE, 2026-2033 (USD THOUSAND)
FIGURE 22 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY MODE, CAGR (2026-2033)
FIGURE 23 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY MODE, LIFELINE CURVE
FIGURE 24 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY MODALITY, 2025
FIGURE 25 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY MODALITY, 2026-2033 (USD THOUSAND)
FIGURE 26 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY MODALITY, CAGR (2026-2033)
FIGURE 27 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY MODALITY, LIFELINE CURVE
FIGURE 28 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY AGE GROUP, 2025
FIGURE 29 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY AGE GROUP, 2026-2033 (USD THOUSAND)
FIGURE 30 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY AGE GROUP, CAGR (2026-2033)
FIGURE 31 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY AGE GROUP, LIFELINE CURVE
FIGURE 32 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY DIAGNOSTIC APPROACH, 2025
FIGURE 33 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY DIAGNOSSTIC APPROACH, 2026-2033 (USD THOUSAND)
FIGURE 34 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY DIAGNOSTIC APPROACH, CAGR (2026-2033)
FIGURE 35 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY DIAGNOSTIC APPROACH, LIFELINE CURVE
FIGURE 36 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY APPLICATION AREA, 2025
FIGURE 37 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY APPLICATION AREA, 2026-2033 (USD THOUSAND)
FIGURE 38 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY APPLICATION AREA, CAGR (2026-2033)
FIGURE 39 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY APPLICATION AREA, LIFELINE CURVE
FIGURE 40 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY PRICE RANGE, 2025
FIGURE 41 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY PRICE RANGE, 2026-2033 (USD THOUSAND)
FIGURE 42 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY PRICE RANGE, CAGR (2026-2033)
FIGURE 43 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY PRICE RANGE, LIFELINE CURVE
FIGURE 44 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY END USER, 2025
FIGURE 45 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY END USER, 2026-2033 (USD THOUSAND)
FIGURE 46 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY END USER, CAGR (2026-2033)
FIGURE 47 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY END USER, LIFELINE CURVE
FIGURE 48 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, BY BUSINESS TYPE, 2025
FIGURE 49 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY BUSINESS TYPE, 2026-2033 (USD THOUSAND)
FIGURE 50 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY BUSINESS TYPE, CAGR (2026-2033)
FIGURE 51 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: BY BUSINESS TYPE, LIFELINE CURVE
FIGURE 52 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET, SNAPSHOT (2025)
FIGURE 53 ASIA-PACIFIC RAPID DIAGNOSTIC TESTS (RDT) MARKET: COMPANY SHARE 2025 (%)

Metodología de investigación
La recopilación de datos y el análisis del año base se realizan utilizando módulos de recopilación de datos con muestras de gran tamaño. La etapa incluye la obtención de información de mercado o datos relacionados a través de varias fuentes y estrategias. Incluye el examen y la planificación de todos los datos adquiridos del pasado con antelación. Asimismo, abarca el examen de las inconsistencias de información observadas en diferentes fuentes de información. Los datos de mercado se analizan y estiman utilizando modelos estadísticos y coherentes de mercado. Además, el análisis de la participación de mercado y el análisis de tendencias clave son los principales factores de éxito en el informe de mercado. Para obtener más información, solicite una llamada de un analista o envíe su consulta.
La metodología de investigación clave utilizada por el equipo de investigación de DBMR es la triangulación de datos, que implica la extracción de datos, el análisis del impacto de las variables de datos en el mercado y la validación primaria (experto en la industria). Los modelos de datos incluyen cuadrícula de posicionamiento de proveedores, análisis de línea de tiempo de mercado, descripción general y guía del mercado, cuadrícula de posicionamiento de la empresa, análisis de patentes, análisis de precios, análisis de participación de mercado de la empresa, estándares de medición, análisis global versus regional y de participación de proveedores. Para obtener más información sobre la metodología de investigación, envíe una consulta para hablar con nuestros expertos de la industria.
Personalización disponible
Data Bridge Market Research es líder en investigación formativa avanzada. Nos enorgullecemos de brindar servicios a nuestros clientes existentes y nuevos con datos y análisis que coinciden y se adaptan a sus objetivos. El informe se puede personalizar para incluir análisis de tendencias de precios de marcas objetivo, comprensión del mercado de países adicionales (solicite la lista de países), datos de resultados de ensayos clínicos, revisión de literatura, análisis de mercado renovado y base de productos. El análisis de mercado de competidores objetivo se puede analizar desde análisis basados en tecnología hasta estrategias de cartera de mercado. Podemos agregar tantos competidores sobre los que necesite datos en el formato y estilo de datos que esté buscando. Nuestro equipo de analistas también puede proporcionarle datos en archivos de Excel sin procesar, tablas dinámicas (libro de datos) o puede ayudarlo a crear presentaciones a partir de los conjuntos de datos disponibles en el informe.